Abstract
Douglas-fir (Pseudotsuga menziesii) is a valuable conifer timber species. It has a thick bark with a high proportion of cork in the rhytidome that allows considering its recovery. This study focuses on the characterization of the bark features and their variation with cambial age along the stem using samples of 20 trees from two sites in Portugal at harvest for the sawmilling industry. The morphology and anatomical features of bark were examined including a detailed analysis of the arrangement of tissues, cell biometry, tissue proportion of the phloem, and the development of the rhytidome. Bark structure varied within the tree with cambial age at various height levels, and differences concerned mostly the rhytidome and periderm development, tissue morphology and disarray in the non-conducting phloem. A relationship between cell dimension, proportion of tissues in the phloem and age was observed; the effect of stem height position was statistically significant for sieve cell length, fiber–sclereid length and wall thickness with a decrease from the base to the top. The rhytidome thickness increased with cambial age: At the stem base (45–50 years of cambial age), the bark includes a rhytidome of about 3 cm thickness corresponding to 84% of the bark, with 5–8 periderms, containing nearly 50% of cork. The cork cells were thin-walled and oriented in radial rows, and the occurrence of thick-walled lignified cells was associated with the increments of the phellem layer. In the youngest periderm, the occurrence of phellem cells with empty lumens and thin suberized walls started at 25–30 years of cambial age. The results show that trees with 45–50 years of age and their logs up to 5 m of height may be suitable for bark and cork exploitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) is native to North America, where it is planted and exploited as a timber species. Today, it is also an important economic species in Europe due to its fast growth rate, high reproduction capacity and adaptation, low number of pests and diseases, and good-quality wood (Da Ronch et al. 2016). The bark characteristics contribute to the species success and adaptation by its enhanced protective and defense functions, for example, shielding from water loss, adverse external temperatures and fire, as well as from pathogen entry and mechanical damage (Morris and Jansen 2016).
Tree barks as residue from forestry and primary wood processing are a valuable raw material for biorefineries due to their structural and chemical diversity (Le Normand et al. 2014; Pereira and Knapic 2017). Recent research has looked into the characterization of barks in view of their valorization as a material and for chemical compounds with high added value, which can be used further in chemical source or pharmaceutical industry (e.g., Miranda et al. 2012, 2013a; Baptista et al. 2013; Mota et al. 2016).
One of the striking features of Douglas-fir bark is its substantial proportion of cork in the rhytidome (Ferreira et al. 2015, 2016; Cardoso et al. 2017). Cork is a cellular material with a unique set of properties that allows its use as sealant, insulator, damper and protective surfacing, for example, as the world-known wine corks that are at the basis of its economic importance as raw material (Pereira 2015). The main source of cork is Quercus suber (Pereira 2007), but other species also contain appreciable proportions of cork (Leite and Pereira 2017), for example, Quercus variabilis (Miranda et al. 2013b), Quercus cerris (Şen et al. 2010, 2011), Plathymenia reticulata (Mota et al. 2016), Betula pendula (Pinto et al. 2009; Ferreira et al. 2017).
Potential use of Douglas-fir bark as a raw material has already been analyzed and presented in earlier papers published in the 1950s of last century (Kurth and Kiefer 1950; Hergert and Kurth 1952; Grillos and Smith 1959; Hall 1971; Krahmer and Wellons 1973; Ross and Corden 1974; Litvay 1976; Litvay and Krahmer 1977; Dougal 1981). The first description of Douglas-fir bark structure based on light microscope observations was presented in 1954 (Chang 1954), and detailed illustrations using transmission electron micrographs were reported in 1981 (Dougal 1981).
Although bark formation is a known process involving the activity of the vascular cambium and the phellogen (Angyalossy et al. 2016), several aspects of bark development have been scarcely studied, namely the timing of the formation of different bark tissues, especially of periderms, or the age-related variation in structural features, i.e., secondary changes in older bark tissue and the rhytidome accumulation.
For Douglas-fir, the origin and development of the bark tissues in different stages of development were studied (Grillos 1956) as well as the variation in the bark structure and development with tree growth and environmental factors (Ross and Krahmer 1971).
Age-related structural changes in bark have been reported for some other species, for example, Quercus robur, Ulmus glabra, Populus tremula and Betula pendula (Trockenbrodt 1994), Eucalyptus globulus (Quilhó et al. 1999, 2000), and Quercus faginea (Quilhó et al. 2013). In recent years, the development and secondary changes in bark tissues of Quercus petraea were also studied by Gricar et al. (2015). In general, the structural changes in the bark are related to age and give rise to a typical within-tree pattern of axial variation that is characterized by an increase in sclerification and cell dilatation from top to bottom of the tree, as well as by rhytidome accumulation (Pereira et al. 2010).
This paper reports the results of a detailed analysis of the bark structure and development within the stem of Douglas-fir trees growing in two sites in Portugal in relation to cambial age, concerning the phloem and rhytidome formation. Specific goals of this study were to investigate the age-related trends in the bark structure of Douglas-fir and evaluate the contribution of individual tissues; to estimate the age at which rhytidome was first observed and the formation rate of the successive periderms, and to evaluate the relationship between cork proportion and cambial age. These results will be relevant for a potential industrial exploitation of Douglas-fir bark with a valorization of its cork component, including designing management orientations at forest and primary conversion levels.
Materials and methods
The study was performed on the bark of 20 Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) trees that were randomly selected in two state-owned stands in northern and central Portugal at the time of harvest for the timber industry. The stands resulted from the afforestation programs carried out by the state in the middle of last century targeting forest densities around 1000-1200 trees ha−1.
One stand (here named Cabreira) is located in the Forest Perimeter of Serra da Cabreira, Cabeceiras de Basto (40°21′28.5″N, 07°27′07.2″W, 850 m of altitude), where the annual rainfall ranges from 1600 to 2000 mm, with 50% of precipitation during the winter season, and a mean annual temperature from 7.5 to 10 °C; it is a mixed stand, with Douglas-fir as the dominant species but including also Pinus pinaster, Pinus sylvestris, Cupressus lusitanica, Chamaecyparis spp., and Betula pendula. The other stand (here named Estrela) is a pure stand, located in the Forest Perimeter of Sarzedo, in the region of Serra da Estrela, Covilhã (41°35′18.0″N, 8°01′00.6″W, 930 m of altitude), where the annual rainfall ranges from 1200 to 1400 mm, with 42% of precipitation during the winter, and a mean annual temperature from 7.5 to 10 °C.
The selected trees (ten trees per site) had an average diameter at breast height of 61 cm in both sites and a total height of 29 m and 35 m at Cabreira and Estrela, respectively. The trees were felled after the end of the growth season (September–January) and bucked into 2.5-m-long logs following the sawmill’s requirements. Tree age (as given by ring counting at stem base) ranged from 43 to 50 years and 39 to 64 years at Cabreira and Estrela, respectively.
Cross-sectional disks with approximately 10 cm thickness were taken from each tree along the stem at six height levels at intervals of approximately 5 m of stem height that corresponded to a difference of tree age between height levels of ca. 6 years. The sampling was made carefully in order to maintain the total bark layer in each stem disk. The stem disks were air-dried indoor under well-ventilated conditions. The surface was smoothed by sanding prior to observations and measurements. Ring counting was made along two opposing radii on each observed cross section.
Images of the cross-sectional disks were acquired with an image analysis system that included a digital seven mega pixels in macro-stand solution set on an acquisition Kaiser RS1 Board with a controlled illumination apparatus, connected to a computer using AnalySIS® image processing software (AnalySIS Soft Imaging System GmbH, Münster, Germany, version 3.2). The determination of the radial thickness of phloem, periderm or rhytidome in the bark was made on two opposite radii as well as the proportion of cork and phloem delimitating in the rhytidome the areas of each tissue. Counting of the number of periderms in the rhytidome and measurement of the thickness of the phloem and cork layers in the rhytidome were made in three randomly selected directions on the same images with the software Leica Qwin Plus.
Analysis of bark anatomical features was made at the six stem height levels of each tree from Cabreira; small bark specimens were cut, impregnated with DP 1500 polyethylene glycol, and transverse and longitudinal microscopic sections of approximately 17 μm thickness were prepared with a Leica SM 2400 microtome using Tesafilm 106/4106 adhesive for sample retrieval (Barbosa et al. 2010). The sections were stained with a double staining of chrysoidine and astra blue. Sudan 4 was used for selective staining of suberin. The sections were mounted on Kaiser glycerine, and after 24 h drying, they were submerged in xylol during 30 min to remove the Tesafilm, dehydrated with 96% and 100% ethanol, and mounted on Eukitt. Individual specimens of bark samples were also macerated in a mixture (1:1) of 30% hydrogen peroxide and acetic acid in a 60 °C oven for 48 h and stained with astra blue.
The parameters measured and the number of measurements for each specimen at each tree height level were as follows: length, width and cell wall thickness of 40 fiber–sclereids, measured in the macerated material; length and tangential diameter of 30 sieve cells measured in the macerated material and in the transverse section, respectively; and height (in µm and number of cells) of 30 rays determined in the tangential section. All measurements were taken using a microscope and a semiautomatic image analyzer system (Leica Application Suite).
The proportion of tissue types was calculated in the transverse section on five randomly selected areas from cambium to periderm using the image analysis system coupled to a microscope; a grid with 48 points was placed over each image, and tissue types (sieve cells, axial parenchyma, fibro-sclereids, sclereids and rays) were counted and converted into a percentage of the total area (Quilhó et al. 2000). The axial parenchyma cells and the sieve cells were quantified together, since the sieve cells were only distinguished in conducting phloem by their radial alignment and axial diameter. In transverse section, the collapse of the sieve cells in non-conducting phloem did not allow their recognition.
The light microscopic observations were made using a Leica DM LA microscope, and photomicrographs were taken with a Nikon Microphot-FXA. Additionally, small cubes of bark with approximately 5 mm of edge were cut with a sharp razor blade at each of the six stem height levels, and their surfaces were examined with a scanning electron microscope Hitachi TM 3030 Plus at 5 kV with different magnifications, and the images of cubical crystals and starch grains were recorded in digital format.
Significant differences in stand characteristics were assessed with paired T tests. Analyses of variance (ANOVA) were performed for bark anatomical features to assess the effects of site, trees, stem height levels and their interactions. Statistical calculations were carried out with IMB SPSS Statistics v19 software.
The terminology follows IAWA list of microscopic bark features (Angyalossy et al. 2016).
Results
Bark structure
The external appearance of Douglas-fir bark varied within the tree along the stem: It was thick, rough with deep fissures and reddish brown in color in the older portions at the stem base and lower levels and rather smooth and grayish at the top of the tree (Fig. 1).
Figure 2 illustrates the layers of phloem, periderm and rhytidome that comprised the Douglas-fir bark and could be macroscopically observed in cross sections. In the lower part of the stem, the rhytidome was substantial with numerous and sinuous periderms (Fig. 2a–c), varying from thin lines to large bands, that isolated patches of phloem tissue visible to the naked eye. The light cream-colored periderm (Pr) tissues differed clearly from the brown phloem tissue (Phm) in the rhytidome (Rt). In contrast, at the top of the tree, only one single and more or less continuous periderm was present (Fig. 2e, f). With age, at the stem base and lower stem levels, the tree develops in the phloem fairly thick cork layers and many short fiber–sclereids (Fig. 2), where the bark has flaky patches of cork bound together by sclerenchyma fractured in furrows, while it is rather smooth and only slightly fissured in the younger bark at the stem top levels.
The bark structure and radial dimensions at the different stem height levels were similar in all the studied Douglas-fir trees (Table 1) with small significant differences (p < 0.05) between sites, height levels and trees. The largest bark thickness was at the stem base (on average 32.7 mm), decreasing to the top (on average 5.5 mm). The proportion of the rhytidome in terms of its radial thickness proportion in relation to the total bark thickness was highest at the stem base and lowest at the top (on average 84% vs. 9%). The rhytidome thickness increased with the cambial age (Fig. 3) from approximately 5 and 4 mm at 25 and 27 years to 29 and 27 mm at 45 and 50 years, respectively, for Cabreira and Estrela (Fig. 3). The phloem thickness increased until 18 and 25 years for Cabreira and Estrela, respectively, and declined then until approximately 35 years and increased subsequently.
The number of periderms contained in the rhytidome was highest in the lower part of the stem (five and eight in Cabreira and Estrela, respectively) and decreased to the upper part of the trees (Fig. 4a). The number of periderms increased with cambial age, in particular after 30 and 35 years at Cabreira and Estrela, respectively (Fig. 4b). In Cabreira, the first periderm that was formed in the phloem tissue appeared at a cambial age of 25 years and died between 30 and 37 years; in Estrela, the first periderm was formed and replaced earlier, because at a cambial age of about 21 years, the trees had already one periderm (Table 2).
The proportion of cork in the bark of Douglas-fir trees was in relation to the development of the rhytidome; i.e., it increased with cambial age and decreased along the stem height. No significant difference (p > 0.05) was found between sites (Fig. 4c, d): Cork represented nearly 50% of the rhytidome at a cambial age of 50 years and 15% at 30 years. The proportion of phloem varied correspondingly with an opposite trend (Fig. 4c, d).
The bark structure was similar in all the trees sampled from both sites; the site did not influence the proportion of cork but affected the thickness of total bark, phloem and rhytidome, and the rhytidome proportion.
Bark anatomical features
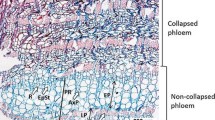
The Douglas-fir bark anatomical structure was analyzed by light and scanning electron microscopy. The bark includes a secondary phloem comprising the conducting and non-conducting phloem, and a rhytidome that includes the inner and sequent periderms interspersed with layers of phloem tissue (Fig. 5).
The conducting phloem, located near the vascular cambium, was composed of living sieve cells with turgid Strasburger cells, axial and radial parenchyma; no typical phloem fibers were found, and instead, short and very thick-walled cells with minute lumen, like fiber–sclereids, were present (Fig. 6). The transition of conducting to non-conducting phloem started early, i.e., close to the cambial region, and was marked by the collapse of sieve cells; the parenchyma cells and sclerenchymatic tissue (fiber–sclereids and sclereids) became the prominent tissue in the non-conducting phloem. Annual increments could be noticed in the phloem by tangentially compressed cells (Fig. 6a).
Anatomical features of Douglas-fir bark in transverse (a), radial (b), and tangential (c) sections. a Sieve cells (S), increment growth (arrows), axial parenchyma cells (P), rays (R), fiber–sclereids (FSC). Annual increments were noticeable in the phloem (arrows). b Sieve cells (S) with sieve areas (arrow), axial parenchyma (P) and fiber–sclereids (FSC); c Uniseriate rays (R) and fusiform ray with a transverse resin canal (arrow) (bar: a 125 µm and b, c 50 µm)
The sieve cells were arranged in regular rows of about 3–10 cells and interspersed by parenchyma cells and fiber–sclereids (Fig. 6a). The sieve cells were elongated with unlignified thin walls and sieve areas on the radial walls; the sieve areas were mostly oval to elliptical, in single rows or sometimes arranged in pairs, including numerous and distinct pores (Fig. 6b).
The axial parenchyma cells were thin-walled, approximately circular and somewhat rectangular near the cambium, mostly arranged in 2–3 discontinuous tangential lines; they were sometimes difficult to distinguish from sieve cells due to the similar size and outline (Fig. 6a). Abundant cubical crystals and ergastic contents filled the cell lumen, as well as starch grains (Fig. 7a).
The rays were of two types: uniseriate and homocellular, and fusiform rays containing resin ducts with distinct border formed by thin-walled epithelial cells (Fig. 6c). Strasburger cells appeared in the margins of the rays in the conducting phloem. Ray cells contained dark brown substances.
On transverse sections, the fiber–sclereids were mostly solitary and diffusely distributed (Figs. 6, 7), with occasional groups of 2–3 cells; they appeared early, i.e., about 6–8 cells in the first-formed phloem tissue (Figs. 5e, 6a), more or less oval in cross section and becoming more irregular toward the periderm, with very thick and polylamellated walls and a very narrow lumen. Figure 6b shows the fiber–sclereids in tangential view. After maceration (Fig. 8), these cells appeared elongated with pointed ends, and some were branched and forked; branched sclereids (brachysclereids) occurred in the external portion of phloem, mainly in the cortex.
Two different forms of sclerenchyma were identified in the present work: fiber–sclereids and sclereids (Fig. 8). The latter, mostly brachysclereids, were short and ramified (Fig. 8b) and developed mainly in the non-conducting phloem, cortex and periderm. In the present work and given the lack of ontogenetic studies for the precise classification of cell type, the term fiber–sclereids was preferably used for the cells with an elongated form and thick wall (Fig. 8a). The first sclerenchyma cells appeared early in the bark of Douglas-fir, and thick cells were found after 6–8 cells in the first-formed phloem tissue (Figs. 5e, 6a).
The periderm (Pr) is made up of phellem or cork (Phlm), phellogen and phelloderm (Fig. 9). The phellem layer comprised a variable number of mainly thin-walled and somewhat rectangular cells in radially oriented rows (early cork cells), although thick-walled lignified cells also occurred (late cork cells) and were associated with the annual phellem increments (Fig. 9d, e); small crystals were observed inside phellem cells. The phelloderm comprised radially aligned thin- to thick-walled cells. The rhytidome included a variable number of periderms according to the cambial age (Fig. 2), forming layers with outward curving edges that merged with older periderms; the phloem layers were thus isolated in a scale-type pattern, and the outer bark formed a scale bark.
Different stages of periderm development in Douglas-fir bark in transverse section. a An initial periderm (Pr) and beneath the cortical cells (cx) observed in the top of the tree. b, c Degrees of periderm (Pr) development including a layer of phellem cell (phlm); differentiation of phelloderm cell (1–2 cells) (star) by the phellogen (arrow) in the base of the tree. d Annual increments in the phellem (arrows). e Phellem or cork cells (star) (bar: 50 µm)
The cork tissue comprised extensive areas of crushed phellem cells that formed a very compressed and compact structure alternated by patches of uncompressed cells with the typical arrangement of cork cells. As a result of the different intensities of compression, the cork cell walls showed different degrees of folding, from heavy buckling to only little undulation (Fig. 9d). These aspects could be recognized along the different heights of the stem, although they were more visible at the bottom where the proportion of phellem was higher. Late cork cells with lignified thick walls were observed that were associated with annual increments in the phellem (Fig. 9d, e).
Bark structure varied within the tree with cambial age at the various height levels, mostly regarding rhytidome and periderm development (Figs. 2, 9). Qualitative changes in the non-conducting phloem were also observed, mostly regarding tissue disarray (Fig. 4), mainly due to the enlargement of the axial parenchyma cells. The cortex (primary phloem) was maintained until the replacement of the first periderm (Fig. 9) at 30–37 years of cambial age.
The rhytidome thickness and its number of periderms increase with age, i.e., from top to stem base (Figs. 3, 4), and consequently, the amount of cork is higher in the bottom part of the tree: the cork represented nearly 50% of the rhytidome at a cambial age of 50 years (Fig. 4c, d). The occurrence of phellem cells with empty lumens and thin suberized walls started at approximately 25–30 years of cambial age, but a substantial proportion of cork was found at older ages of about 50 years.
Age variation in bark anatomy
The bark structural features showed changes that increased gradually from the upper stem levels of the trees downwards with most structural differences occurring at the base and lower levels of the tree stem, i.e., at older ages (Fig. 4c, d). At these levels, corresponding to cambial ages of over 50 years, there was a pronounced disarray of the phloem tissues from the beginning of the non-conducting phloem outwards, mainly due to the enlargement of the axial parenchyma cells and the increase in number and diameter of sclerenchyma cells. The radial alignment of the sieve cells that was evident near the cambium was subsequently lost toward the outside by distortion through cell collapse (Fig. 5c, d). In the non-conducting phloem and toward the periderm, the axial parenchyma cells expanded and tended to lose the initial alignment (Fig. 5c). In contrast, the rays that extended linearly through the conducting phloem became gradually distorted and slightly dilated (Fig. 5d). In the young levels near the top, a cortex was observed beneath the inner periderm (Fig. 9a); the cortex was preserved until approximately 26 years of cambial age.
The periderms showed age-related differences, namely regarding their number, which increased with age (Fig. 4a, b), the number of cells in each phellem layer, their outline and content. At the top, at cambial age of 10 years, only one periderm was present with a few phellem cells (5–6 cells in a row), rounded and filled with heavily stained materials (Fig. 9a). The occurrence of phellem cells with empty lumens and thin suberized walls, as represented in Fig. 9b–e, started at approximately 25–30 years of cambial age; at the tree base, corresponding to cambial ages of over 40 years, a broad phellem layer (up to 10 cells) could be observed. After approximately 30 years, annual increments could be noticed in the phellem layer: the initial cells of the phellem were large, thin-walled and free of contents, followed by a few crushed cells, occasionally thick-walled and with heavy deposits (Fig. 9d). Each phellogen mother cell produced usually two or three phelloderm cells often containing dark substances.
Cell biometry and tissue proportion
Cell sizes and amount of tissues varied within and between trees, as shown in Table 3.
Sieve cells were on average 20 µm in diameter and 2293 µm in length (Table 3); only cell length showed significant differences between trees (p < 0.05). Younger barks had in general thinner and shorter sieve cells, although the within-tree variation did not show a regular gradient. The cell length slightly increased from stem base to approximately 5 m, followed by a decreasing trend to the upper part of the stem. The effect of height position was statistically significant (p < 0.05) for sieve cell length. The sieve cells were about two times longer than fiber–sclereids (Table 3).
The diameter of sieve cells did not vary significantly along the tree, contrary to what occurred for length with the longest sieve cells found at the stem base (Table 3).
Fiber–sclereids were on average 77 µm in diameter, 1277 µm in length and 32 µm in wall thickness (Table 3) with significant between-tree differences (p < 0.05) of length and wall thickness. The axial variation in fiber–sclereid width and wall thickness showed a decreasing pattern from the base to the top with some fluctuations, and length increased initially and decreased at the top. The fiber–sclereid length and wall thickness varied significantly within the tree (p < 0.05) but with little dimensional variation. Differentiation of parenchyma cells into fibro-sclereids included elongation and wall thickening and additional deposited cell wall layers, thereby developing a multilayered cell wall (Figs. 6, 9).
Height of phloem rays averaged 184 µm (Table 3), with significant differences between trees and height levels (p < 0.05), and was mostly uniseriate and eight cells high on average. Ray height showed a decreasing trend from the base to the top of the trees.
The proportion of the different tissues in the phloem was determined at each height level (Table 3). The axial parenchyma together with sieve cells represented the major tissue followed by the sclerenchyma tissue (fiber–sclereids and sclereids) ranging between 62–76 and 15–29%, respectively; rays corresponded to 9–13% of the phloem. The different types of cells varied within the tree but not always in the same manner and extent. The axial parenchyma and sieve cells were more abundant in young barks, and their proportion increased toward the top; in contrast, fiber–sclereids and sclereids showed the highest proportion at stem base corresponding to higher cambial age. The proportion of the radial parenchyma tended to decrease toward the top. The proportion of the different cell types in the transverse section (Table 3) shows a very high value of axial parenchyma and sieve cells (68%) compared to, for example, rays (10%) or fiber–sclereids (23%). The proportion of fiber–sclereids was higher toward the stem base.
Discussion
Bark structure and anatomical features
The bark of Douglas-fir is characterized by the formation of a rhytidome, and the periderms include substantial layers of cork cells (Fig. 2). These striking features of Douglas-fir bark were reported in earlier studies (Kurth and Kiefer 1950; Hergert and Kurth 1952; Ross and Krahmer 1971) and more recently regarding the characterization of the cork layers (Ferreira et al. 2015, 2016; Cardoso et al. 2017).
The observed variation in the bark external appearance along the stem of Douglas-fir (Fig. 1) is related to the specific structural features and their development with tree age (Junikka 1994). The occurrence of flaky patches of cork held together by sclerenchyma fractured in furrows in the bark of mature trees and of a rather smooth and only slightly fissured bark at the stem top levels has already been reported in the literature (Chang 1954; Ross and Krahmer 1971).
The observed Douglas-fir bark anatomical structure is generally in accordance with the first descriptions made in the literature, although a different terminology was then used to name the sclerenchyma cells, for example, fibers (Chang 1954; Patel 1975), sclereids-like phloem fibers (Einspahr et al. 1978), sclereids phloem fibers (Parameswaran 1980), or sclereids (Grillos 1956; den Outer 1967; Ross and Corden 1973; Ross and Krahmer 1971). The presence of sclereids, mostly brachysclereids, results from the modification of parenchyma cells (Angyalossy et al. 2016) and gives mechanical support to the phloem tissue (den Outer 1967). Sclerenchyma usually includes fibers, fiber–sclereids, and sclereids (Angyalossy et al. 2016), which have a different origin: Fibers arise from cambial cells, while fiber–sclereids and sclereids are formed by a secondary sclerosis of parenchyma cells (Evert 2006). The early appearance of the first sclerenchyma cells observed is in accordance with previous reports of an early formation at 15 cells away from cambium (Chang 1954), or 1–30 cells away from the cambium (Grillos 1956).
Cell biometry and tissue proportion
Sieve cells, fiber–sclereids and ray dimensions, and their proportion in the phloem varied within the tree (Table 3). This is a combined age effect on the structure of cambial cells and their derivatives, i.e., phloem mother cells and their products (Evert 2006) with influence of exogenous and endogenous regulators (Fromm 2013), and of phloem adjustment to tree radial growth. The cell dimensions (Table 3) fitted in general with the scarce available information on Douglas-fir bark.
Sieve cell length was in the range of values reported, for example, 2.5–3.7 mm (Chang 1954), 3–4 mm (Einspahr et al. 1978), and 3.0 mm (Patel 1975), and tangential diameter similar or smaller to, for example, 20 µm (Patel 1975) and 50 µm (Chang 1954). Sieve cells four times longer than sclerenchymatic cells have previously been reported (Ross and Krahmer 1971). The longest sieve cells were found at the stem base, but the variation in sieve cell diameter along the tree was small, as already observed in many other species (Iqbal and Ghouse 1983; Trockenbrodt 1994), and may reflect an increasing length of cambial fusiform initials with increasing age (Larson 1994). However, there are contradictory reports on the axial variation, for example, an increase in cell length to the top of mature Douglas-fir trees (Ross and Krahmer 1971) as well as irregularly axial variations, probably explained by the combined effect of cambial initial length, extent of anticlinal divisions and apical intrusive growth (Ross and Krahmer 1971; Ghouse and Iqbal 1977; Trockenbrodt 1994; Quilhó et al. 2000).
The dimensions of fiber–sclereids (Table 3) were in accordance with the reported range of values, for example, for diameter 50–100 µm (Einspahr et al. 1978), 50–92 µm (Grillos 1956), 50 µm (Chang 1954; Dougal 1981), 54–56 µm (Ross and Krahmer 1971), and 44–95 µm (Patel 1975) and for length 1 mm (Dougal 1981), 0.5–2.0 mm (Ross and Krahmer 1971), 0.6–1.5 mm (Chang 1954), 1–1.5 mm (Einspahr et al. 1978), 0.6–4.0 mm (Grillos 1956), and 0.65–1.65 mm (Patel 1975). The small variation in fiber–sclereids dimension within the tree was also reported in other studies (Ross and Krahmer 1971).
Ray dimensions (Table 3) were also in accordance with reported values, for example, 4–30 cells high (Quilhó et al. 2013) and 8–15 cells and 200–250 µm high (Grillos and Smith 1959). The decreasing pattern of variation in ray height toward the top of the tree is in relation to the progressive increase in size of cambial initials with age (Ridoutt and Sands 1993; Trockenbrodt 1994).
The major proportion of axial parenchyma and sieve cells compared to rays or fiber–sclereids (68% vs. 10–23%, Table 3) is according to the literature reporting that the secondary phloem in Pinaceae consists of up to 90% sieve cells (den Outer 1967). The higher proportion of fiber–sclereids at the stem base agrees with the intense lignification and sclerification of the cell walls that constitute the main structural features related to tree age (Trockenbrodt 1994; Quilhó et al. 1999). Rays also increased downwards like in other species (Quilhó et al. 2000).
Age-related changes
Bark structure varied within the tree with cambial age at the various height levels. The differences concerned mostly the rhytidome and periderm development (Figs. 2, 3, 4), tissue morphology, and disarray in the non-conducting phloem (Fig. 5). At the base of the trees, at older ages, where the bark thickness was largest, the rhytidome was substantial with numerous periderms separated by patches of phloem tissue (Figs. 2, 3). In contrast, at the younger ages, at the top of the trees, the bark thickness was lowest and only one single periderm was present (Figs. 2, 3).
The qualitative changes in the non-conducting phloem are a consequence of a dilatation process resulting in the bark radial increase by parenchyma cell division and expansion (Angyalossy et al. 2016) to adjust bark to the tree secondary growth. These structural changes are common in conifers, for example, Pinus pinaster (Parameswaran 1980) and Pinus pinea (Nunes et al. 1999), as well as in angiosperm tree species (Quilhó et al. 1999, 2013; Şen et al. 2011). Alteration in ray cells also occurred, but tangential cell division and their enlargement were of small magnitude, and they did not develop funnel-shaped dilatation growth as in other conifers, i.e., Phyllocladus trichomanoides (Chang 1954).
The maintaining of cortex until the replacement of the first periderm at 30–37 years of cambial age is in accordance with earlier observations made for Douglas-fir (Gartner 1996) that recognized a first periderm immediately underneath the surface at a cambial age between 12 and 43 years and that the cork formation occurs at a relatively early age (Hergert and Kurth 1952).
In consequence of the development of the rhytidome with cambial age, as seen by the variations along the stem height, there is an increasing proportion of cork in the bark of older trees in comparison with that of younger trees (Fig. 4). These present results support the findings that indicated for Douglas-fir bark a substantial proportion of cork from 25 to almost 50% (Kurth 1953; Ross and Krahmer 1971; Krahmer and Wellons 1973; Cardoso and Pereira 2017). The present results also are in accordance with Kurth and Kiefer (1950), Hergert and Kurth (1952) and Ross and Krahmer (1971) who considered that Douglas-fir bark presents a great variety in thickness and cork content according to site quality, tree age, and axial position on the tree.
Recently, the structure of the cork tissue in the bark of Douglas-fir was described in relation to topological arrangement, geometry, and dimensions of the cells (Cardoso et al. 2017). The present research confirms the features of the Douglas-fir rhytidome, i.e., a substantial proportion of cork, the thin cork layers, their discontinuous distribution with interspersed phloem tissues (Fig. 2), and the substantial compression of cork cells forming a compact mass of crushed cells. The cork cells with empty lumens and thin suberized walls appeared approximately at 25–30 years of cambial age (Fig. 4). Annual increments with early cork and late cork cells could be noticed (Fig. 9), as described for cork of other species, like, for example, Q. suber (Pereira 2007), Q. cerris (Şen et al. 2011), and Q. variabilis (Miranda et al. 2013b).
The results obtained here on the variation in bark structure within the tree and the proportion of cork in relation to cambial age show that the valorization of Douglas-fir bark by means of a targeted exploitation of cork is possible for mature trees. The butt log until 5 m of stem height is the preferable raw material, because the substantial proportion of cork is found at older ages of about 50 years.
Conclusion
The bark anatomy of Douglas-fir trees grown in Portugal was characterized in detail for the first time including an analysis of tissue structure and proportion, cell biometry, and the development of the rhytidome with cambial age along the stem. Bark structure varied within the tree with cambial age regarding rhytidome and periderm development, tissue morphology, and disarray in the non-conducting phloem.
Douglas-fir has a thick bark that develops with age a rhytidome with several periderms containing a substantial proportion of cork. The proportion of cork in the bark increases with age and becomes interesting from a yield perspective for mature trees and older cambial ages, namely over 35 years of age, with a 50% cork content in the rhytidome at 50 years. Bark valorization is therefore advantageous to be included within the Douglas-fir timber exploitation and to consider the species as a potential cork provider for the cork industry. The integration of the Douglas-fir bark valorization in the current exploitation for sawmill processing means that it will be the lower part of the stem, for example the logs up to 5 m of height that should be directed for debarking and bark processing to recover the cork component.
References
Angyalossy V, Pace MR, Evert RF, Marcati CR, Oskolski AA, Terrazas T, Kotina E, Lens F, Mazzoni-Viveiros SC, Angeles G et al (2016) IAWA list of microscopic bark features. IAWA J 37:517–615
Baptista I, Miranda I, Quilhó T, Gominho J, Pereira H (2013) Characterisation and fractioning of Tectona grandis bark in view of its valorisation as a biorefinery raw-material. Ind Crops Prod 50:166–175
Barbosa ACF, Marcelo RP, Witovisk L, Angyalossy V (2010) A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA J 31:373–383
Cardoso S, Pereira H (2017) Characterization of Douglas-fir grown in Portugal: heartwood, sapwood, bark, ring width and taper. Eur J For Res 136:597–607
Cardoso S, Ferreira J, Quilhó T, Pereira H (2017) Cork of Douglas-fir bark: impact of structural and anatomical features on usage. Ind Crops Prod 99:135–141
Chang YP (1954) Bark structure of North American conifer. United States Department of Agriculture, Washington DC
Da Ronch F, Caudullo G, de Rigo D (2016) Pseudotsuga menziesii in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European atlas of forest tree species. Publications Office of the European Union, Luxembourg, pp 146–147
den Outer RW (1967) Histological investigation of secondary phloem of gymnosperms. H. Veenman & Zonen NV, Wageningen
Dougal EF (1981) Ultrastructure of parenchyma and sclereids in Douglas-fir [Pseudotsuga menziesii (Mirb.) Franco] bark. Dissertation, Oregon State University
Einspahr DW, Harder ML, Parham RA (1978) Bark and wood properties of pulpwood species as related to separation and segregation of chip/bark mixtures. Project 3212, report ten : a progress report to members of the Institute of Paper Chemistry. Institute of Paper Chemistry, pp 1–113
Evert RF (2006) Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. Wiley, New York
Ferreira JPA, Miranda I, Gominho J, Pereira H (2015) Selective fractioning of Pseudotsuga menziesii bark and chemical characterization in view of an integrated valorization. Ind Crops Prod 74:998–1007
Ferreira J, Miranda I, Gominho J, Pereira H (2016) Chemical characterization of cork and phloem from Douglas fir outer bark. Holzforschung 70:475–483
Ferreira JPA, Quilhó T, Pereira H (2017) Characterization of Betula pendula outer bark regarding cork and phloem components at chemical and structural levels in view of biorefinery integration. J Wood Chem Technol 37:10–25
Fromm J (2013) Xylem development in trees: from cambial divisions to mature wood cells. In: Fromm J (ed) Cellular aspects of wood formation. Springer, Berlin, pp 3–39
Gartner BL (1996) Does photosynthetic bark have a role in the production of core vs. outer wood? Wood Fiber Sci 28:53–61
Ghouse AKM, Iqbal M (1977) Trends of size variation in phloem fibres and sieve-tube cells within the bark of some arid-zone trees. Flora 166:517–521
Gricar J, Jagodic S, Prislan P (2015) Structure and subsequent seasonal changes in the bark of sessile oak (Quercus petraea). Trees 29:747–757
Grillos SJ (1956) Structure and development of the bark of Douglas-fir, Pseudotsuga menziesii (Mirb.) Franco. Dissertation, Oregon State College
Grillos SJ, Smith FH (1959) The secondary phloem of Douglas-fir. For Sci 5:377–388
Hall JA (1971) Utilization of Douglas-fir bark. Pacific Northwest Forest and Range Experiment Station, Portland
Hergert HL, Kurth EF (1952) The chemical nature of the cork from Douglas-fir bark. TAPPI 35:59–66
Iqbal M, Ghouse AKM (1983) An analytical study on cell size variation in some arid zone trees of India: Acacia Nilotica and Prosopis Spicigera. IAWA J 4:46–52
Junikka L (1994) Survey of English macroscopic bark terminology. IAWA J 15:3–45
Krahmer R, Wellons J (1973) Some anatomical and chemical characteristics of Douglas-fir cork. Wood Sci 6:97–105
Kurth EF (1953) Chemicals from Douglas-fir bark. Oregon Forest Products Laboratory, Corvallis
Kurth EF, Kiefer HJ (1950) Wax from Douglas-fir bark. TAPPI 33:183–186
Larson PR (1994) The vascular cambium, development and structure. Springer, New York
Le Normand M, Moriana R, Ek M (2014) Isolation and characterization of cellulose nanocrystals from spruce bark in a biorefinery perspective. Carbohydr Polym 111:979–987
Leite C, Pereira H (2017) Cork-containing barks—a review. Front Mater 3:63
Litvay JD (1976) Anatomical and chemical characteristics of the Douglas-fir (Pseudotsuga menziesii (mirb.) Franko) phellem cell. Dissertation, Oregon State University
Litvay JD, Krahmer RL (1977) Wall layering in Douglas-fir cork cells. Wood Sci 9:167–173
Miranda I, Gominho J, Mirra I, Pereira H (2012) Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind Crops Prod 36:395–400
Miranda I, Gominho J, Mirra I, Pereira H (2013a) Fractioning and chemical characterization of barks of Betula pendula and Eucalyptus globulus. Ind Crops Prod 41:299–305
Miranda I, Gominho J, Pereira H (2013b) Cellular structure and chemical composition of cork from the Chinese cork oak (Quercus variabilis). J Wood Sci 59:1–9
Morris H, Jansen S (2016) Bark. Yearbook 2016. International Dendrology Society, Mexico, pp 51–61
Mota GS, Sartori CJ, Ferreira J, Miranda I, Quilhó T, Mori FA, Pereira H (2016) Cellular structure and chemical composition of cork from Plathymenia reticulata occurring in the Brazilian Cerrado. Ind Crops Prod 90:65–75
Nunes E, Quilhó T, Pereira H (1999) Anatomy and chemical composition of Pinus pinea L. bark. Ann Sci 56:479–484
Parameswaran N (1980) Some remarks on the nomenclature of fibres, sclereids and fibre–sclereids in the secondary phloem of trees. IAWA J 1:130–132
Patel RN (1975) Bark anatomy of radiata pine, corsican pine, and Douglas fir grown in New Zealand. N Z J Bot 13:149–167
Pereira H (2007) Cork: biology, production and uses. Elsevier Publications, Amsterdam
Pereira H (2015) The rationale behind cork properties: a review of structure and chemistry. BioResources 10:6095–6206
Pereira H, Knapic S (2017) Bark and cork. In: Performance of bio-based building materials. Elsevier Publications, Amsterdam, pp 78–96
Pereira H, Miranda I, Tavares F, Gominho J, Quilhó T, Graça J, Rodrigues JC, Shatalov A, Knapic S (2010) Qualidade e utilização tecnológica do eucalipto (Eucalyptus globulus) [Quality and technological use of eucalyptus (Eucalyptus globulus)]. Centro de Estudos Florestais, Lisboa, p 377 (in Portuguese)
Pinto PCRO, Sousa AF, Silvestre AJD, Neto CP, Gandini A, Eckerman C, Holmbom B (2009) Quercus suber and Betula pendula outer barks as renewable sources of oleochemicals: a comparative study. Ind Crops Prod 29:126–132
Quilhó T, Pereira H, Richter HG (1999) Variability of bark structure in plantation-grown Eucalyptus globulus. IAWA J 20:171–180
Quilhó T, Pereira H, Richter HG (2000) Within-tree variation in phloem cell dimensions and proportions in Eucalyptus globulus. IAWA J 21:31–40
Quilhó T, Sousa V, Tavares F, Pereira H (2013) Bark anatomy and cell size variation in Quercus faginea. Turk J Bot 37:561–570
Ridoutt BG, Sands R (1993) Within-tree variation in cambial anatomy and xylem cell differentiation in Eucalyptus globulus. Trees 8:18–22
Ross WD, Corden ME (1973) Microscopic and histochemical changes in Douglas fir bark: accompanying fungal invasion. Wood Fiber Sci 5:129–138
Ross WD, Corden ME (1974) Selective degradation of lignin and condensed tannins of Douglas-fir bark sclereids by fungi. Wood Fiber Sci 6:1–12
Ross WD, Krahmer RL (1971) Some sources of variation in structural characteristics of Douglas-fir bark. Wood Fiber Sci 3:35–46
Şen A, Miranda I, Santos S, Graça J, Pereira H (2010) The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind Crops Prod 31:417–422
Şen A, Quilhó T, Pereira H (2011) Bark anatomy of Quercus cerris L. var. cerris from Turkey. Turk J Bot 35:45–55
Trockenbrodt M (1994) Quantitative changes of some anatomical characters during bark development in Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. IAWA J 15:387–398
Acknowledgements
We thank Instituto da Conservação da Natureza e das Florestas (ICNF) for helping in tree selection and the sawmills Albano Leite da Silva, LDA and VilaMadeiras - Comércio de Madeiras, LDA, for allowing the sampling at the time of tree harvest. Centro de Estudos Florestais (CEF) is a research unit funded by Fundação para a Ciência e a Tecnologia (FCT) (AGR/UID00239/2013). The first author acknowledges a FCT doctoral fellowship (PD/BD/52404/2013) under the Sustainable Forests and Products (SUSFOR) doctoral program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardoso, S., Quilhó, T. & Pereira, H. Influence of cambial age on the bark structure of Douglas-fir. Wood Sci Technol 53, 191–210 (2019). https://doi.org/10.1007/s00226-018-1055-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-018-1055-5