Abstract
During 2003–2005, we examined the effect of seasonal drought on water status, gas exchange, δ13C, chlorophyll fluorescence and spectral reflectance in six woody species in a valley savanna near the Yuanjiang River (the upper Red River) in southwestern China. Three different phenological types of these woody species were compared, i.e., an evergreen species, Cyclobalanopsis helferiana, two winter-deciduous (WD) species, Buchanania latifolia and Symplocos racemosa, and three drought-deciduous (DD) species, Terminthia paniculata, Wendlandia tinctoria and Woodfordia fruticosa. We aimed to test the following three hypotheses: (1) the evergreen and WD species employ a drought avoidance strategy, whereas DD species employ a drought tolerance strategy; (2) the evergreen and WD species have a more economical water use strategy than the DD species and (3) the evergreen and WD species have a stronger photoprotection capacity through thermal dissipation than the DD species. At the end of a prolonged drought, the predawn leaf water potential (Ψpd) in C. helferiana and S. racemosa dropped to ca. −0.8 MPa, whereas the Ψpd in B. latifolia remained close to zero and DD species were leafless. In the rainy seasons, maximal photosynthetic rates of the evergreen (18.4 μmol m−2 s−1) and W. fruticosa (18.0 μmol m−2 s−1) were higher than those of the other four species (12.2−13.8 μmol m−2 s−1). The evergreen and WD species responded to drought by closing stomata and thus maintained a constant relative water content (RWC), which is a typical drought avoidance strategy; however, it is at the expense of carbon gain. DD species maintained a high photosynthetic capacity with a decrease in both stomatal conductance and RWC until the driest period, and then shifted from the drought tolerance strategy to the avoidance mechanism by shoot dieback. There was no significant difference in the means of δ13C across the phenological groups. The evergreen and WD species had stronger heat dissipation than the DD species in dry seasons. All species increased leaf spectral reflectance, probably because of degradation of chlorophyll as indicated by the leaf reflectance index, which should reduce light harvesting. All species showed a strong increase in the ratio of red to green spectral reflectance of leaves during dry seasons, indicating the accumulation of anthocyanin, which may contribute to screening sunlight and scavenging reactive oxygen species. Different responses to drought of savanna woody species with different leaf phenologies may facilitate the partitioning of resource use and hence their co-existence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High longitudinally oriented mountain ranges, as the eastern extensions of the Himalayan mountains, cover a large part of Yunnan Province and the southern Sichuan Province in southwestern China. These mountains block the humid air from the Bay of Bengal and cause a rain shadow effect. Most of the river valleys between these mountains have a dry and hot/warm climate, with a distinct dry season that usually lasts for half a year, commencing with a chilly period from November to February followed by a warm dry period from March to April. The total area of these dry and hot/warm valleys is estimated to be about 8 × 104 km2. The vegetation in these valleys is classified as valley savanna (Wu 1995; Jin and Ou 2000).
In savannas, water is the main factor limiting photosynthesis and plant growth. Water deficit causes stomatal and non-stomatal limitations to photosynthesis (Tezara et al. 1999; Lawlor and Cornic 2002). When plants sense soil drought, abscisic acid is produced, mainly in the roots, and transported to the leaves, inducing stomatal closure (Bray 1997; Steudle 2000). A high leaf-to-air vapor pressure deficit (LAVPD) due to low air humidity and high temperature also induces stomatal closure (Eamus et al. 1999). Stomatal closure leads to a concomitant reduction in the intercellular CO2 concentration (C i) and hence a reduction in photosynthesis. Water deficit results in higher water tension in xylem vessels and tracheids, which consequently induces embolisms that also induce stomatal closure (Domec et al. 2006). Non-stomatal limitation can be partitioned into diffusional (mesophyll resistance to CO2 conductance) and non-diffusional processes (decrease in carboxylation capacity; Centritto et al. 2003; Niinemets et al. 2005). However, the latter is only important during severe droughts (Maroco et al. 2002; Bota et al. 2004).
The dry seasons in savanna areas are often associated with sunny weather, when photosynthesis may be suppressed. Light absorbed by chloroplasts is hardly affected by water deficit, resulting in excess light energy that can cause photoinhibition (Powles 1984). Photoinhibition is associated with sustained heat dissipation, photosystem II (PSII) core rearrangement and degradation, as well as sustained depression in maximum light harvesting efficiency (F v/F m; Demmig-Adams and Adams 2006). Many physiological mechanisms are involved in the protection of photosynthetic apparatuses against photodamage under drought stress. Excess light energy absorbed by PSII can be largely dissipated through non-photochemical quenching (NPQ; Horton et al. 1996; Niyogi 1999). Increasing leaf spectral reflectance and decreasing chlorophyll content also reduces light harvesting in drought stressed plants (Carter and Knapp 2001). Drought-induced accumulation of anthocyanin in leaves can enhance the screening of sunlight and the scavenging of reactive oxygen species, which are produced under stress conditions and are toxic to cell membranes (Gould et al. 2002; Tattini et al. 2004).
Two contrasting strategies that plants use to cope with drought have been identified, i.e. drought avoidance and drought tolerance. Drought-avoidance strategies are accomplished by developing deep roots to access deep soil water and by reducing water loss through stomatal closure (Gulías et al. 2002). Drought tolerance strategies permit continued gas exchange at low relative water availability through physiological regulation, such as osmoregulation and developing tissue structures resistant to water deficit (Tyree et al. 2002). The vegetation in the savanna valleys of southwestern China is dominated by deciduous woody species, with scattered occurrence of evergreen woody species (Jin and Ou 2000). The deciduous species can be classified into winter-deciduous (WD) and drought-deciduous (DD) species; the former start to shed leaves at the beginning of winter, when soil moisture is still quite high. However, they can flush leaves and bloom flowers at the end of the dry season. In contrast, DD species only shed leaves in the late dry season, when the water deficit is severe. Other studies have reported that some evergreen savanna species possess deep root systems (Eamus 1999) and have smaller vessels than co-occurring deciduous woody species, allowing them to be more resistant to drought-induced embolism (Choat et al. 2005).
In this study, we monitored seasonal changes in water status, gas exchange and chlorophyll fluorescence in six woody species with three types of leaf phenology in a valley savanna over 3 years. Carbon isotope composition was measured as a proxy of long-term water use efficiency (WUE; Farquhar et al. 1989). Leaf spectral reflectance was measured to examine seasonal changes in pigments. We tested the following three hypotheses: (1) evergreen species employed a drought avoidance strategy whereas the DD species employed a drought tolerance strategy to cope with drought. Because WD species flush leaves in the driest periods of a year, their performance might be similar to that of evergreen species; (2) evergreen and WD species have a more economic water use strategy (higher WUE) than DD species and (3) evergreen and WD species have a stronger photoprotection capacity through heat dissipation than DD species because they must endure more severe drought in a 1-year cycle.
Materials and methods
Study site
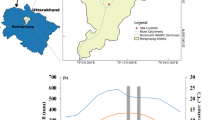
The study site was located in a valley (23°41′N, 101°59′E, 770 m asl) of the Yuanjiang River, 10 km north of Yuanjiang City, Yunnan, southwestern China. According to the weather station located just outside this city (396 m asl), the mean annual temperature is 23.8°C (1961–2005; Fig. 1), the average minimum air temperature of the coldest month (January) is 11.9°C and the average maximum air temperature of the hottest month (May) is 34.6°C. Mean annual rainfall is 802 mm, 79% of which falls between May and October. The mean annual rainfall is similar to that in the savanna area of northern Sudan (ca. 600–900 mm; De Bie et al. 1998), and higher than in the savanna of South Africa (ca. 480 mm; Shackleton 1999); however, it is much lower than in savannas of coastal northern Australia (ca. 1,650 mm; Prior et al. 1997; Williams et al. 1997) and central Brazil (ca. 1,500 mm; Domec et al. 2006). The seasonality index of rainfall (Walsh and Lawler 1981) is 0.72. The incident photosynthetic photon flux density (PPFD) at midday reaches about 2,300 μmol m−2 s−1 in summer and 1,900 μmol m−2 s−1 in winter. The soil is a typical US ttorrox with a soil profile of pH 6.1, 1.063% organic matter, 0.104% nitrogen, 0.038% phosphorus and 2.564% potassium at a 0–20 cm depth.
The total rainfall in three rainy seasons (2003–2005) was 552, 533 and 612 mm, respectively. Rainfall in the dry season (November to April) between 2002 and 2003 was 179 mm, which was similar to the long-term mean value of precipitation in the dry season (168 mm; 1961–2005). However, precipitation in the other two dry seasons (2003–2004 and 2004–2005) was only 133 and 130 mm. The dry season of 2005 was about 1 month longer than the other two dry seasons. Rainfall in the driest 3-month period in the three dry seasons was 53, 32 and 23 mm, respectively.
A secondary shrub vegetation was growing in the study site, which was dominated by deciduous woody species, including WD species such as Buchanania latifolia Roxb., Lannea coromandelica (Houtt.) Merr., Symplocos racemosa Roxb. and Phyllanthus emblica L., and DD species such as Polyalthia cerasoides (Roxb.) Benth. et Hook. f. ex Bedd., Terminthia paniculata (Wall. ex G. Don) C. Y. Wu et T. L. Ming, Wendlandia tinctoria subsp. intermedia (F. C. How) W. C. Chen and Woodfordia fruticosa (L.) Kurz. A few evergreen species were scattered in the vegetation, such as Carissa spinarum L., Cyclobalanopsis helferiana (A. DC.) Oerst., Olea ferruginea Royle, Pistacia weinmannifolia J. Poiss. ex Franch., and Euphorbia antiquorum L., with occasional occurrence of a CAM species. Indigenous grasses, Bothriochloa pertusa (L.) A. Camus and Heteropogon contortus (L.) P. Beauv. ex Roem. et Schult., and the exotic invasive herbs, Eupatorium coelesticum L. and Eupatorium odoratum L., were present in the understory and open sites.
Species included in the study
Six woody species were chosen for the study, five tree species, B. latifolia, C. helferiana, S. racemosa, T. paniculata and W. tinctoria, and one shrub species, W. fruticosa (Table 1). These species are mainly distributed throughout southwestern China and southwestern Asia, and W. fruticosa is also distributed throughout tropical Africa. Among these, only C. helferiana is evergreen and flushes leaves twice a year in the rainy season. WD species start to shed their leaves at the start of winter and are completely leafless during the coldest period of the year (January). They also flush leaves once in the late dry season (usually in early March). DD species flush leaves successively from the start of the rainy season onwards, and gradually commence dropping leaves in the late rainy season. DD species were leafless for less than 2 months in 2003 and 2004, and for nearly 3.5 months in 2005. At the peak of the dry season, almost all of their leaves had fallen, with some top shoots dying back. C. helferiana has a typical tap root system and B. latifolia has a thin tap root system. The other four species have shallow roots with long lateral roots. Some saplings of B. latifolia and many seedlings of C. helferiana and T. paniculata occurred under or around their respective parent trees.
All measurements were made on photosynthetically mature and sun-exposed canopy leaves from five to six adult individuals of each species, with heights of 1.5–2 m. To avoid destructive and frequent sampling to the study plants, leaf samples to measure relative water content (RWC) were collected from the other trees at the same height that leaves were taken for photosynthesis measurements. The same trees were used throughout the study period.
Water status
From March 2003 to February 2004, we monitored monthly changes in RWC determined as: (fresh weight − dry weight)/(turgid weight − dry weight) × 100%. At the end of each month, five to six fresh leaves of each species were collected and sealed in plastic bags, stored in a cold box, and then immediately transported to the laboratory for determination of leaf fresh weight. Leaves were put in distilled water in darkness overnight and then their turgid weights were determined. Leaf dry weight was determined after leaves were dried to a constant weight at 80°C. The predawn (Ψpd) and midday (Ψmd) leaf water potentials were measured on four to six leaves with a pressure chamber (SKPM 1400, Skye Instruments Ltd, Powys, UK) at the end of the prolonged dry season (May) and in the late rainy season (September) in 2005.
Gas exchange
Between 08.30 and 10.30 h on ten separate days from March 2003 to September 2005, light-saturated gas exchange was measured on three to seven leaves per species with a portable infrared gas analyzer (LI-6400, LI-COR, Nebraska, USA). The photosynthetic photon flux density (PPFD) of 1,000 μmol m−2 s−1 on the cuvette surface was provided by a LED light source, which was photosynthetically saturated for all species. CO2 concentration inside the leaf chamber was maintained at 380 μmol mol−1 through the CO2 controlling system of the LI-6400 attached with a tiny CO2 cylinder. During measurements, the relative air humidity was 37–51 and 50–82%, and the LAVPD was 1.7–2.6 and 0.9–2.2 kPa in the dry and rainy seasons, respectively. During most measurements, the ambient temperature was 24–28°C. When ambient air temperature reached 29–32°C, the leaf temperature inside the chamber was controlled at 25°C to reduce the influence of overheating of the sensor head/IRGA on leaf photosynthetic capacity. The maximum CO2 assimilation rates (A max), stomatal conductance (gs) and intercellular to ambient CO2 concentration (C i/C a) were obtained from these measurements.
We examined the stomatal effect on photosynthesis by analyzing the relationship between the C i/C a and A max in accordance with techniques used by Lauer and Boyer (1992) and Eamus et al. (1999). A decreased assimilation rate with a reduced C i/C a ratio indicates stomatal limitation to photosynthesis, whereas a low assimilation rate with a relatively high C i/C a ratio indicates non-stomatal limitation.
Water use efficiency
It has been well established that the δ13C of leaves is correlated with the efficiency of their long-term water use (Farquhar et al. 1989). We used δ13C to assess long-term WUE. Leaves from each of the same five to six individual adult trees measured for gas exchange, and leaves from another five species were used for δ13C analysis. Leaves were washed with deionized water, dried at 60°C and then pulverized to a fine powder through a sieve with holes of 0.25 mm in diameter. δ13C was analyzed with a elemental analyzer (Flash EA 1112, Thermo Electron Corporation, Massachusetts, USA) interfaced to an isotope ratio mass spectrometer (Thermo Finnigan MAT DELTAplusXP, Thermo Electron Corporation, Massachusetts, USA), at the Institute of Botany, Chinese Academy of Sciences.
δ13C was calculated as: (R sample − R standard)/R standard × 1,000‰ where R sample and R standard were the ratios of 13C/12C in the sample and in the Pee Dee Belemnite standard, respectively. Standard glycine and cellulose measurements for every 15–20 samples were used to check the accuracy of the analysis. The accuracy of the spectrometric analysis of the laboratory standard was ±0.19‰. The leaves for δ13C analysis were collected in March and August 2003.
Chlorophyll fluorescence
Predawn F v/F m was measured on nine to ten leaves per species on the same day as the gas exchange measurements using a portable chlorophyll fluorescence monitoring system (FMS2, Hansatech, Norfolk, UK).
Diurnal chlorophyll fluorescence was measured on three clear days in the two dry seasons (4–5 March 2003 for the evergreen and the three DD species; 23–24 April 2003 for the two WD species; and 1 February 2004 for all species) and on 1 day (10 October) in the rainy season in 2003. Steady-state chlorophyll fluorescence (F s) and maximal fluorescence in the light-adapted state (F′m) were determined every 1–2 h at incident sunlight, which was measured with a quantum sensor attached to the leaf clip. The quantum yield of PSII (ΦPSII) in light was calculated as ΦPSII = (F′m − F s)/F′m (Genty et al. 1989). NPQ, indicating dissipation of the absorbed light energy as heat, was calculated as NPQ = (F m − F′m)/F′m (Bilger and Björkman 1990). The predawn maximal fluorescence (F m) was used to determine NPQ throughout the day.
Spectral reflectance
The spectral reflectance of leaves from 306 to 1,138 nm was measured on 15 leaves per species at predawn at the peak of the rainy season in 2003 (August) and the middle of the dry season in 2004 (February), using a spectral analysis system (UniSpec, PP Systems, Massachusetts, USA) with a 2.3 mm diameter (0.042 cm2) fiber optic cable and an internal 7 W halogen lamp attached. The measurement covered 256 visible spectral bands at a 3.3 nm interval. Individual leaves were held in a black plastic polyvinyl chloride leaf clip at a 60° angle relative to the fiber optic cable. Because certain spectral characteristics are known to change rapidly with incident irradiance (Gamon et al. 1997), the predawn reflectance was measured to standardize the measurements across different measurement days. Reflectance at each spectrum wavelength (at 3.3 nm intervals) was divided by the measurement of the reflectance standard.
The chlorophyll normalized difference index (Chl NDI) was calculated as (R 750 − R 705)/(R 750 + R 705) (Richardson et al. 2002) and used as an indication for leaf chlorophyll content. The ratio of red (600–700 nm) to green (500–600 nm) radiation reflectance (R RED/R GREEN) was used as an indication for anthocyanin activity (Gamon and Surfus 1999). We also calculated the mean value of leaf reflectance of photosynthetically active radiation (R PAR, 400–700 nm).
Statistics
We examined the relationships of ΦPSII and NPQ with PPFD, and of A max with gs and C i/C a using linear regression. The seasonal differences in the means of spectral reflectance indices and δ13C within the same species were analyzed with independent-samples t test. Differences in the mean δ13C across phenological groups were analyzed with one-way ANOVA.
Results
Water status
The RWC of the evergreen and WD species changed little between the rainy and dry seasons (16, 7 and 10% decrease for C. helferiana, B. latifolia, and S. racemosa, respectively), whereas the RWC of DD species decreased more sharply in the dry season (by 17–32%; Fig. 2). At the end of the prolonged drought in 2005, the Ψpd in C. helferiana and S. racemosa dropped to −0.8 MPa, whereas the Ψpd in B. latifolia remained close to zero (Table 2). The Ψmd was usually much lower than the Ψpd for the species studied except for T. paniculata in the rainy season.
Gas exchange
Amax in all species decreased in the dry seasons (Fig. 3). In the normal dry seasons in 2003 and 2004, Amax in the evergreen and S. racemosa decreased by 37–75% compared with the rainy season, whereas at the end of the prolonged drought in 2005, Amax in these two species decreased by more than 90% (Amax was ca. 1.3 μmol m−2 s−1). Amax in B. latifolia remained relatively high in both normal and severe dry seasons. Amax in the three DD species in the normal dry seasons, which were leafless in the late period of the prolonged dry season in 2005, decreased by less than 28%.
Maximal photosynthetic rates (A max) and maximum light harvesting efficiency (F v/F m) in the species studied over three wet seasons (closed bars) and three dry seasons (open bars) during 2003–2005. There was rain between May 31 and June 4 in 2005. Genera names only are given. Data are means ± SE, n = 3–9
The regression slopes between A max and gs for the evergreen and S. racemosa in the dry seasons were steeper than those in the rainy seasons (Fig. 4), indicating much stronger stomatal control. The regression lines for the DD species were higher in the rainy seasons than in the dry seasons, indicating non-stomatal limitation to photosynthesis in the dry seasons.
The relationships between maximal photosynthetic rate (A max) and stomatal conductance to vapor (gs), and between A max and the ratio of intercellular to ambient CO2 concentration (C i/C a) for the species studied. Genera names only are given. Data are pooled from wet (closed circles) and dry seasons (open circles) during 2003–2005
Amax in C. helferiana, B. latifolia and S. racemosa was positively correlated with Ci/Ca, with lower values in the dry seasons (Fig. 4), indicating stomatal limitation to photosynthesis. In contrast, Amax were not correlated with Ci/Ca for the three DD species, and their Ci/Ca values remained high in the dry seasons; however, their Amax were lower. This confirmed that non-stomatal limitation of photosynthesis occurred in these DD species, which was also indicated by the regressions between Amax and gs presented above.
Water use efficiency
The δ13C between the dry and rainy seasons was not significantly different for all species (P > 0.05). There was no significant difference in the mean δ13C values across phenological groups, with δ13C ranging from −26.3 to −29.3‰ (Table 3).
Chlorophyll fluorescence
During the study period, only at the end of the prolonged dry season in 2005 was the predawn F v/F m in C. helferiana and S. racemosa significantly lower than 0.8 (0.67–0.69, Fig. 3; one-sample t test, P < 0.05). Even during this period, predawn F v/F m in B. latifolia was still higher than 0.8, whereas the other deciduous species were leafless.
The ΦPSII was negatively correlated with the incident PPFD for all species (Fig. 5). For the evergreen and the two WD species, the slopes or intercepts of the regression lines between the ΦPSII and PPFD in the dry seasons were lower than those in the rainy seasons. In contrast, for two DD species, W. tinctoria and W. fruticosa, the regression lines in the dry season in early 2003 and the rainy season in 2003 largely overlapped and were higher than those in the dry season in early 2004. However, for the other DD species, T. paniculata, the regression lines in the dry seasons were higher than those in the rainy seasons.
The relationship between quantum yield of photosystem II (ΦPSII) and the incident photosynthetic photon flux density (PPFD) for the species studied. Measurements were made in the dry season in early 2003 (open circles, long-dashed regression line), the rainy season in 2003 (filled circles, solid regression line), and the dry season in early 2004 (triangles, dotted regression line). Genera names only are given. Data are pooled from measurements of nine to ten leaves from five to six individuals of each species
NPQ was positively correlated with the incident PPFD for all species (Fig. 6). For the evergreen and the two WD species, the regression slopes between NPQ and PPFD in the dry seasons were higher than those in the rainy seasons. The DD species T. paniculata and W. fruticosa had higher NPQ on a given PPFD in the dry season in early 2004 than in the dry season in early 2003 and in the rainy season 2003.
The relationship between non-photochemical quenching (NPQ) and incident photosynthetic photon flux density (PPFD) for the species studied. Measurements were made in the dry season in early 2003 (open circles, long-dashed regression line), the rainy season in 2003 (filled circles, solid regression line), and the dry season in early 2004 (triangles, dotted regression line), respectively. Genera names only are given. Data are pooled from measurements of nine to ten leaves from five to six individuals of each species
Spectral reflectance
Except for C. helferiana and W. fruticosa, the species studied exhibited a significantly reduced Chl NDI in the dry season (Table 4). All species displayed a significantly increased R RED/R GREEN in the dry season compared with the rainy season. The R PAR increased significantly in the dry season for all species.
Discussion
At the end of the prolonged drought in 2005, the Ψpd remained fairly high in the three species with leaves at that time (Table 2). The lowest Ψpd value of ca. −0.8 MPa in the present study was close to that of savanna (cerrado) woody species in central Brazil (e.g., Bucci et al. 2005; Franco et al. 2005); however, it was much higher than that of some northern Australian savanna trees (Ψpd < −1.5 MPa; Prior et al. 1997). B. latifolia even maintained high water potentials during the prolonged drought in 2005 (Table 2). This would be mainly because of its deep tap roots (Table 1).
In the rainy seasons, the species studied had quite high A max (Fig. 3), which are within the range of other savanna woody species (Prado and De Moraes 1997; Eamus and Prichard 1998; Eamus et al. 1999; Prior et al. 2004; Franco et al. 2005). The savanna species of the present study, especially C. helferiana and W. fruticosa, have a highly developed mesophyll structure, e.g., a high ratio of palisade to spongy mesophyll thickness (Song and Cao 2005), which would facilitate light absorption and CO2 diffusion into the mesophyll, thus enhancing the photosynthetic light use efficiency and a high photosynthetic rate when water is not limited (Terashima et al. 2001; Evans and Vogelmann 2003).
The relationships of A max with gs and C i/C a indicated stomatal limitation of photosynthesis in the evergreen and the two WD species, and non-stomatal limitation in the three DD species in the dry seasons (Fig. 4). Because of strong stomatal regulation in the dry seasons (Fig. 4), WD species maintained quite high RWCs and the evergreen species maintained stable RWCs (Fig. 2). This is a typical characteristic of drought avoidance plants (Gulías et al. 2002), confirming our first hypothesis set at the start of the study.
DD species shift their drought resistance mechanisms from drought tolerance in the early dry season to drought avoidance in the late dry season. The RWCs in DD species decreased from the start of the dry season (Fig. 2), whereas the leaves of DD species maintained fairly high gas exchange until the driest period (Fig. 3). This is a typical drought tolerance strategy (Gulías et al. 2002; Tyree et al. 2002), which benefits DD species by enabling them to accumulate the assimilates as much of the limited water available as possible. At the same time, the disadvantage of this strategy is that maintaining high gas exchange will unavoidably lead to excess water loss. In the driest period, some of the top shoots of the DD species died back and all of the remaining leaves dropped off, probably because of hydraulic dysfunction (Prior and Eamus 2000; Davis et al. 2002; Kondoh et al. 2006). Avoiding water loss by sacrificing some top shoots will maintain the water balance for the whole plant in DD species (Munné-Bosch and Alegre 2004; Rice et al. 2004).
In contrast to the evergreen and DD species, WD species in the present study, as well as some trees in savannas reported by other studies, can flush leaves before the rainy season (Table 1; Brodribb et al. 2002; Rivera et al. 2002; Bowman and Prior 2005; Franco et al. 2005; Chapotin et al. 2006). This is beneficial for these plants, enabling them to elongate the photosynthetic period, recover earlier and to minimize leaf nutrient leaching by rainfall in the early rainy season. Furthermore, B. latifolia has a deep root system that enables it to tap underground water in the spring when temperatures are favorable; thus it is able to flush leaves early. S. racemosa has a typical shallow root system with very long lateral roots, allowing it to absorb water in moist microsites over a long distance and to recover more quickly after the first rains (Cao 2000; Sternberg et al. 2004).
In the present study, we found that there were no significant differences in δ13C across the three phenological groups (Table 3), rejecting our second hypothesis that evergreen and WD species have a higher WUE. However, δ13C of the present savanna woody plants was higher (−26.51 to −29.28‰; Table 3) than that of tropical rainforest trees (−32.01 to −33.01‰; Qu et al. 2001) in the same region where precipitation is nearly twice that of the present savanna site, indicating that savanna plants have a higher long-term WUE than rainforest plants.
The ΦPSII decreased for all species in the dry seasons, which was consistent with the suppression of photosynthesis. An exception was T. paniculata, which had an even higher ΦPSII in the dry seasons than in the rainy seasons (Fig. 5). This was probably because T. paniculata had a high photosynthetic capacity (Fig. 3) associated with a high leaf water potential in the dry seasons (Table 2). The DD species had a higher ΦPSII than the other species in the dry seasons (Fig. 5). This was consistent with their high photosynthetic activities during most periods of the dry seasons (Fig. 3), resulting in less excess light energy in their chloroplasts.
In the dry seasons, the evergreen and WD species exhibited higher NPQ than DD species (Fig. 6), which confirms our third hypothesis. NPQ is related to the thermal dissipation of excess light energy through de-epoxidation of the xanthophyll cycle (Long et al. 1994; Horton et al. 1996; Gilmore 1997). In another study, we examined the activity of xanthophyll cycle pigments in two of the study species (T. paniculata and W. fruticosa) and found that NPQ correlated with the pool size and de-epoxidation state of the xanthophyll cycle. The ΦPSII (Fig. 5) and F v/F m (Fig. 3) in the normal dry seasons can be completely relaxed overnight. This indicates no sustained photoinhibition occurred in the present savanna plants in normal dry seasons.
Sustained photoinhibiton was only detected in C. helferiana and S. racemosa during the prolonged drought in 2005. During this drought period, after rainfall, the A max of these two species recovered to the level observed in the normal dry season (Fig. 3). However, the F v/F m remained depressed, and zeaxanthin was retained even at predawn (Zhu et al., unpublished data). This indicates that sustained photoinhibition takes a longer time to recover in these species.
In the dry seasons, the reduction in chlorophyll content, indicated by the spectral reflectance indices in all species in the present study, could explain the increase in the R PAR (Table 4). The increase in the R PAR and the degeneration of chlorophyll can reduce light harvesting, which should contribute to photoprotection (Long et al. 1994). Elevated anthocyanin activity in all species in the dry season, indicated by the strong increase of the R RED/R GREEN (Table 4), also strengthens protection for the plants by enhancing the screening of sunlight and the scavenging of reactive oxygen species (Gould et al. 2002).
The low temperatures in winter may be one of the reasons for the reduction in A max for the savanna species studied. Plants that live in hot and dry habitats must have developed a strong tolerance of high temperature (Valladares and Pearcy 1997; Knight and Ackerly 2003a, b). This is supported by TL50 (50% ion leakage after heat treatment; method refers to Chen et al. 1982; Yeh and Lin 2003) being as high as 51–52°C in plant species we studied (Zhu et al., unpublished data). However, these plants may be intolerant to cold temperatures. It has been reported that the regular winter temperatures of the present region can strongly suppress the photosynthesis of chilling-sensitive tropical plants supplied with sufficient water (Cao et al. 2006). However, WD species can avoid this low winter temperature by being leafless during this season.
In summary, the six woody species we studied showed somewhat different photosynthetic responses to annual drought, and species with the same phenological leaf type displayed similar physiological responses to water deficit. To cope with dry weather, the evergreen and WD species avoid drought by closing stomata to maintain constant RWCs; however, this is at the expense of carbon gain. The DD species maintained a high photosynthetic capacity with a decrease in gs and RWCs until the driest period, when they shifted their drought tolerance strategy to avoidance mechanisms by shoot dieback. The plants of three different types of leaf phenology exhibited similar WUE. The evergreen and WD species had stronger heat dissipation than the DD species in dry seasons. Different responses to drought of the savanna plant species may facilitate the partitioning of resource use and thus their co-existence.
References
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Bota J, Medrano H, Flexas J (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162:671–681
Bowman DMJS, Prior LD (2005) Why do evergreen trees dominate the Australian seasonal tropics? Aust J Bot 53:379–399
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2:48–54
Brodribb TJ, Holbrook NM, Gutiérrez MV (2002) Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ 25:1435–1444
Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Campanello P, Scholz FG (2005) Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in neotropical savanna trees. Trees 19:296–304
Cao KF (2000) Water relations and gas exchange of tropical saplings after a prolonged drought in a Bornean heath forest, with reference to root architecture. J Trop Ecol 16:101–116
Cao KF, Guo YH, Cai ZQ (2006) Photosynthesis and antioxidant activity in breadfruit, jackfruit and mangosteen in southern Yunnan, China. J Hortic Sci Biotech 81:168–172
Carter GA, Knapp AK (2001) Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am J Bot 88:677–684
Centritto M, Loreto F, Chartzoulakis K (2003) The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ 26:585–594
Chapotin SM, Razanameharizaka JH, Holbrook NM (2006) Baobab trees (Adansonia) in Madagascar use stored water to flush new leaves but not to support stomatal opening before the rainy season. New Phytol 169:549–559
Chen HH, Shen ZY, Li PH (1982) Adaptability of crop plants to high temperature stress. Crop Sci 22:719–725
Choat B, Ball MC, Luly JG, Holtum JAM (2005) Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees 19:305–311
Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC (2002) Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. Am J Bot 89:820–828
De Bie S, Ketner P, Paasse M, Geerling C (1998) Woody plant phenology in the West Africa savanna. J Biogeogr 25:883–900
Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172:11–21
Domec JC, Scholz FG, Bucci SJ, Meinzer FC, Goldstein G, Villalobos-Vega R (2006) Diurnal and seasonal variation in root xylem embolism in Neotropical savanna woody species: impact on stomatal control of plant water status. Plant Cell Environ 29:26–35
Eamus D (1999) Ecophysiological traits of deciduous and evergreen woody species in the seasonally dry tropics. Trends Ecol Evol 14:11–16
Eamus D, Prichard H (1998) A cost-benefit analysis of leaves of four Australian savanna species. Tree Physiol 18:537–545
Eamus D, Myers B, Duff G, Williams D (1999) Seasonal changes in photosynthesis of eight savanna tree species. Tree Physiol 19:665–671
Evans JR, Vogelmann TC (2003) Profiles of 14C fixation through spinach leaves in relation to light absorption and photosynthetic capacity. Plant Cell Environ 26:547–560
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Franco AC, Bustamante M, Caldas LS, Goldstein G, Meinzer FC, Kozovits AR, Rundel P, Coradin VTR (2005) Leaf functional traits of neotropical savanna trees in relation to seasonal water deficit. Trees 19:326–335
Gamon JA, Surfus JS (1999) Assessing leaf pigment content and activity with a reflectometer. New Phytol 143:105–117
Gamon JA, Serrano L, Surfus JS (1997) The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 112:492–501
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gilmore AM (1997) Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant 99:197–209
Gould KS, McKelvie J, Markham KR (2002) Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ 25:1261–1269
Gulías J, Flexas J, Abadía A, Medrano H (2002) Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species. Tree Physiol 22:687–697
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Jin ZZ, Ou XK (2000) Vegetations in the hot and dry valleys along the Yuanjiang, Nujiang, Jinshajiang, and Lanchangjiang Rivers. Yunnan University Press, Kunming
Knight CA, Ackerly DD (2003a) Evolution and plasticity of photosynthetic thermal tolerance, specific leaf area and leaf size: congeneric species from desert and coastal environments. New Phytol 160:337–347
Knight CA, Ackerly DD (2003b) Small heat shock protein responses of a closely related pair of desert and coastal Encelia. Int J Plant Sci 164:53–60
Kondoh S, Yahata H, Nakashizuka T, Kondoh M (2006) Interspecific variation in vessel size, growth and drought tolerance of broad-leaved trees in semi-arid regions of Kenya. Tree Physiol 26:899–904
Lauer MJ, Boyer JS (1992) Internal CO2 measured directly in leaves. Abscisic acid and low leaf water potential cause opposing effects. Plant Physiol 98:1310–1316
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Ann Rev Plant Physiol Plant Mol Biol 45:633–662
Maroco JP, Rodrigues ML, Lopes C, Chaves MM (2002) Limitations to leaf photosynthesis in field-grown grapevine under drought-metabolic and modeling approaches. Funct Plant Biol 29:451–459
Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31:203–216
Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T (2005) Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ 28:1552–1566
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35:14–44
Prado CHBA, De Moraes JAPV (1997) Photosynthetic capacity and specific leaf mass in twenty woody species of Cerrado vegetation under field conditions. Photosynthetica 33:103–112
Prior LD, Eamus D (2000) Seasonal changes in hydraulic conductance, xylem embolism and leaf area in Eucalyptus tetrodonta and Eucalyptus miniata saplings in a north Australian savanna. Plant Cell Environ 23:955–965
Prior LD, Eamus D, Duff GA (1997) Seasonal and diurnal patterns of carbon assimilation, stomatal conductance and leaf water potential in Eucalyptus tetrodonta saplings in a wet-dry savanna in northern Australia. Aust J Bot 45:241–258
Prior LD, Bowman DMJS, Eamus D (2004) Seasonal differences in leaf attributes in Australian tropical tree species: family and habitat comparisons. Funct Ecol 18:707–718
Qu C-M, Han X-G, Su B, Huang J-H, Jiang G-M (2001) The characteristics of foliar δ13C values of plants and plant water use efficiency indicated by δ13C values in two fragmented rainforests in Xishuangbanna, Yunnan. Acta Bot Sin 43:186–192
Rice KJ, Matzner SL, Byer W, Brown JR (2004) Patterns of tree dieback in Queensland, Australia: the importance of drought stress and the role of resistance to cavitation. Oecologia 139:190–198
Richardson AD, Duigan SP, Berlyn GP (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194
Rivera G, Elliott S, Caldas LS, Nicolossi G, Coradin VTR, Borchert R (2002) Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Trees 16:445–456
Shackleton CM (1999) Rainfall and topo-edaphic influences on woody community phenology in South Africa savannas. Global Ecol Biogeogr 8:125–136
Song FQ, Cao KF (2005) Anatomical and nutrient features of plant leaves in Yuanjiang savanna valley. Chin J Appl Ecol 16:33–38
Sternberg LDSL, Bucci S, Franco A, Goldstein G, Hoffman W, Meinzer FC, Moreira MZ, Scholz F (2004) Long range lateral root activity by neo-tropical savanna trees. Plant Soil 270:169–178
Steudle E (2000) Water uptake by roots: effects of water deficit. J Exp Bot 51:1531–1542
Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G (2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 163:547–561
Terashima I, Miyazawa SI, Hanba YT (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114:93–105
Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Tyree MT, Vargas G, Engelbrecht BMJ, Kursar TA (2002) Drought until death do us part: a case study of the desiccation-tolerance of a tropical moist forest seedling-tree, Licania platypus (Hemsl.) Fritsch. J Exp Bot 53:2239–2247
Valladares F, Pearcy RW (1997) Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant Cell Environ 20:25–36
Walsh RPD, Lawler DM (1981) Rainfall seasonality: description, spatial patterns and change through time. Weather 36:201–208
Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D (1997) Leaf phenology of woody species in a north Australian tropical savanna. Ecology 78:2542–2558
Wu ZY (1995) The vegetation of China. Science Press, Beijing, pp 578–582
Yeh DM, Lin HF (2003) Thermostability of cell membranes as a measure of heat tolerance and relationship to flowering delay in chrysanthemum. J Am Soc Hortic Sci 128:656–660
Acknowledgments
We thank Mr. Guoda Tao for identification of the study plants, Mr. Fuqiang Song for assistance with some of the field measurements, and the three anonymous reviewers for their helpful comments on our manuscript. The Yuanjiang Meteorological Station provided the climatic data. This study was co-funded by the National Science Foundation of China and the Chinese Academy of Sciences through two research projects (No. 90302013 and No. KSCX2-SW-116) led by KFC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Bilger.
Rights and permissions
About this article
Cite this article
Zhang, JL., Zhu, JJ. & Cao, KF. Seasonal variation in photosynthesis in six woody species with different leaf phenology in a valley savanna in southwestern China. Trees 21, 631–643 (2007). https://doi.org/10.1007/s00468-007-0156-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-007-0156-9