Abstract
The prevalence of non-communicable disease (NCDs) is rising globally, with a large burden recorded in sub-Saharan countries and populations of black race/ethnicity. Accelerated vascular deterioration, otherwise known as early vascular aging (EVA), is the underlying factor for highly prevalent NCDs such as hypertension. The etiology of EVA is multifactorial with a central component being arterial stiffness with subsequent development of hypertension and cardiovascular complications. Although arterial stiffness develops with increasing age, many children and adolescents are subjected to the premature development of arterial stiffness, due to genetic or epigenetic predispositions, lifestyle and behavioral risk factors, and early life programming. Race/ethnic differences in pediatric populations have also been reported with higher aortic stiffness in black (African American) compared with age-matched white (European American) counterparts independent of blood pressure, body mass index, or socioeconomic status. With known evidence of race/ethnic differences in EVA, the pathophysiological mechanisms underlying graded differences in the programming of EVA are still sparse and rarely explored. This educational review aims to address the early life determinants of EVA in children and adolescents with a particular focus on racial or ethnic differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is not only prevalent in adults and the elderly. Recent evidence highlighted the increasing trends of childhood and adolescent hypertension as well as related CVDs as future health challenges on a global scale with major economic implications [1,2,3]. Global evidence indicates that adult non-communicable diseases (NCDs), including CVD and type 2 diabetes, originate from early life or biological programming due to the interactions of environmental and genetic factors during a critical period of development, namely the first thousand days of life [4,5,6]. More importantly, even earlier changes in the biomechanical makeup of blood vessels may be compromised during fetal (intrauterine) development due to maternal nutrition [7], maternal smoking [8], maternal health (i.e., eclampsia, gestational diabetes) [9], access to health care (low socioeconomic status, lack of medical insurance) [10], and prematurity/low birthweight [11]. These early life determinants of CVD may predispose children and adolescents to accelerated biological aging, termed early vascular aging (EVA).
Aside from predispositions to genetic and environmental risk factors of certain phenotypes, there are also socioeconomic, sociocultural, and metabolic determinants to define an individual’s health status. Alongside these determining risk factors for NCDs and especially CVD is the complexity of ethnic or racial differences that are regularly reported in CVD morbidity and mortality, with an increased burden among especially black (African and African-American) and Hispanic populations [12, 13] compared with white or Asian populations. Until now, genetics studies have not succeeded in explaining genetic predispositions to CVD among various racial or ethnic groups [14, 15]. In addition, clear biological definitions of ethnicity and race are still lacking. In an analysis from the 1998 South Africa Demographic Health Survey, authors highlighted unambiguous racial and income disparities among black African and white (European descent) South Africans. Black South Africans had less access to healthcare services and mostly fell in the lower socioeconomic classes compared with white South Africans [16]. Yet, 20 years later, this disproportion is still markedly unchanged in especially the sub-Saharan Africa context. Racial inequalities should remain a global social concern, since marked differences among race groups have been reported in health-related studies with no clear pathophysiological explanations. Evidence in this regard is lacking even more in pediatric and adolescent populations.
This review will focus on the evidence of EVA in children and adolescents, its characteristics, and multifactorial determinants with specific reference to race.
Definition and diagnosis of early vascular aging
The concept of premature vascular aging was described by Nilsson (1996) as the result of adverse psychosocial and environmental factors hindering the health-preserving mechanisms of human physiology [17]. Since then, the concept of EVA has been well demonstrated, but with limited focus in children, adolescents and young adults.
Definitions
With “normal” or chronological aging, biological changes manifest and may develop to disease states when an individual reaches the end stages of life. Chronological age is the number of years, months, or days lived since the day of birth and an obvious risk factor for CVD morbidity and mortality in the presence of established risk factors [18]. Biological aging is different from chronological aging as it reflects a number of factors contributing to a gradual decline in physiological and biochemical functionality of individuals across the lifespan. Some of these factors include genetics, lifestyle behaviors, and disease states such as hypertension, atherosclerosis, and diabetes mellitus. However, the aging process can be accelerated at a biological level, rendering a mismatch between the chronological and biological age. This dissociation between chronologic and biologic aging is termed EVA. EVA is therefore a state of accelerated adverse changes in the biochemical and cellular components of the vascular tree contributing to augmented forward pulse wave reflections at younger ages [19]. These early changes have particular detrimental effects on target organs such as the heart, brain, and kidneys.
Diagnosis
EVA can be diagnosed by the presence of abnormally high arterial stiffness (arteriosclerosis) for a specific age and sex [20]. Arterial stiffness is central to the development of EVA, whereby individuals with reduced arterial elasticity can be identified by non-invasive measurement of pulse wave velocity (PWV) [21]. Carotid-femoral PWV or aortic PWV is perhaps the most commonly used measure of arterial stiffness and is defined as the velocity of the arterial pulse wave travelling between two sites (distance × 0.8) along the arterial wall (transit time) [22]. In general, mostly in adult populations, higher (> 10 m/s) PWV indicates arterial stiffness and an increased risk for future cardiovascular events [23].
Clinical importance of early vascular aging
With increasing age, pulse pressure widens and systolic hypertension develops as long-term manifestations of arterial stiffening [24]. PWV is a sensitive technique to measure arterial stiffness and can detect early adverse changes in pulse wave reflections. By measuring PWV, EVA can be assessed to identify individuals (at younger ages) with accelerated aging or adverse medial layer morphological changes due to inherent features as well as interactions with environmental exposures [25]. The impact of these vascular changes and subsequent increased arterial (aortic) stiffness on cardiac remodeling is inevitable. Aortic stiffening increases left ventricular load as well as myocardial perfusion pressure, but limits the delivery of blood to the capillary beds during diastole. Evidently, increased arterial stiffness may contribute to myocardial ischemia [26] and cardiac failure [27]. Aside from the direct relationship between aortic stiffness and cardiac dysfunction and hypertrophy is also the downstream effects of arterial stiffening on the microvasculature within vital organs including the brain [28, 29] and kidneys [30, 31]. Therefore, the non-invasive measurement of PWV in a clinical setting may assist in the risk stratification of individuals (even in young asymptomatic individuals) and the possibility for primordial prevention. Carotid-femoral PWV by applanation tonometry has been established as a highly sensitive biomarker in identifying individuals with various phenotypes and can aid in therapeutic guidance [32, 33].
Not only does PWV assess arterial stiffness, but it also provides valuable predictive information for cardiovascular outcomes as an intermediate end-point and an independent predictor of cardiovascular events and all-cause mortality [34, 35]. The predictive power of PWV for CVD was first described in high-risk groups with chronic kidney disease (CKD), hypertension, or diabetes [36,37,38]. Later, in the general population [39], one standard deviation increase of PWV predicted a 30% increased risk for future cardiovascular events [34]. However, standardization of PWV measurements with various devices and across different populations still requires attention.
Etiology and prevalence of early vascular aging over the life course
In recent consortia and research, evidence led to the appreciation of the way in which early life (including pre-conception and intrauterine development) exposures to risk factors determine the origin and trajectories of disease across the life course [16]. The life course in this context refers to the reciprocal influences of especially environmental exposures on the biological determinants of health during the different stages of life including pre-pregnancy, gestation, infancy, adolescence, and adulthood [40].
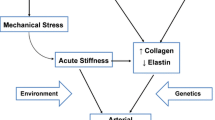
In a statement by the Lancet Commission on hypertension, the concept of EVA was used to develop a life course strategy of CVD prevention and treatment, aiming to reduce cardiovascular risk factors, target organ damage and cardiovascular events throughout the different stages of life including childhood [6]. The authors highlighted that genetic susceptibility and epigenetic imprinting during fetal life can alter the life course trajectories and underlined the importance thereof in the management of raised blood pressure (BP) [6]. In an effort to further elucidate this concept, we adapted the life course trajectory approach (Fig. 1) to indicate that the presence of intrauterine risk factors (early life programming) already predisposes the fetus to increased future cardiovascular risk and the early onset of cardiovascular abnormalities in early childhood.
An adapted life course model in the setting of early vascular aging and the consequences of early-life programming prior to birth. SES socioeconomic status, *Amended from Olsen et al., with permission from The Lancet [6]. Copyright ©2016, Elsevier
In Fig. 1, points A, B, and C indicate the relative risk of an individual on the health disease continuum based on the determinants and phenotype of fetal programming. The ideal life course is illustrated with a green line, and at point A, the risk of accelerated biological aging or EVA is much lower compared with an individual at points B and C. Such individuals (at point A) are theoretically defined as healthy or undergo supernormal vascular aging [20], with the least adverse cardiovascular disease manifestations throughout the life course. The majority of individuals intercepts the health disease continuum at point B, following an average life course in which hypertension and arterial stiffness manifests in late adulthood and middle age, with subsequent cardiovascular morbidity and mortality in advanced age. However, some individuals intercept the health-disease continuum at point C with accelerated biological aging and vascular compromise at very young ages, even in infancy, childhood, and adolescence. These individuals are believed to be subjected to maternal risk factors involved in the early life programming mismatch contributing to the increased relative risk of EVA, as discussed below.
Etiology
Evidence from BP tracking studies suggests that the development of blood vessel structure and function could be influenced by early somatic growth [41,42,43,44]. This may be the reason increased arterial wall stiffness (arteriosclerosis) and thickness (atherosclerosis) of large arteries are proposed to be present in children and adolescents in the early phases of elevated BP [45]. As with atherosclerosis, arterial stiffness is believed to start in early life on the basis of fetal programming of the medial elastin content of the arteries, as well as of other vasculature and capillaries [46, 47]. Therefore, EVA may start in utero as a result of adverse intrauterine environmental exposures for survival, which may contribute to an increased risk of vascular deterioration in early postnatal life [19].
Genetic-environmental interactions and ethnocultural influences may increase individual susceptibility to early-life programming of the vasculature [48,49,50,51]. For instance, fetal undernutrition is accompanied by suboptimal vascular growth and development with reduced elasticity, arterial compliance, and overall high peripheral resistance in the offspring [52]. Maternal nutrition and/or underlying disease states such as obesity and gestational diabetes can affect fetal nutrition and contribute to potential genetic changes during fetal development [53]. A population-based analysis indicated a 29% increased overall rate of early onset of CVD in offspring, if the mothers had diabetes during pregnancy [9]. In addition, reports have indicated higher risk of gestational diabetes among women from race groups other that non-Hispanic white race [54, 55].
Although the etiology of racial differences in early life programming is scant and perhaps only studied as biological fragments to understand the complexity of early life exposures on cardiovascular health and its future implications, evidence suggest that black children with lower birth weight compared with their white counterparts are at higher risk of having impaired vascular structure and function [56]. However, this may also be true for other race groups, as studies indicated that not only African-American babies (born at term) but also Indian, Pakistani, Bangladeshi, and black Caribbean offspring have lower birth weights than those from European ancestry [57, 58]. A study on prematurity also indicated that children born extremely preterm (compared with controls) have an increased future cardiovascular risk due to altered arterial hemodynamics of especially the smaller resistance blood vessels [59]. Systolic hypertension, elevated glucose levels, and hypercholesterolemia were also more prominent (compared with controls) as measured almost 6 years later in a group of preterm infants [60]. It appears that altered maternal lipid metabolism (higher triglycerides and total cholesterol) contributes to adverse prenatal programming of the hypothalamic-pituitary-adrenal axis by increasing a child’s stress response as evidenced by greater cortisol reactivity [61]. This could be in part the mechanism to explain the link between the early life origin of CVD and the emotional functioning or psychological stress of a child [62]. In addition, researches have shown a clear association between multiple adverse childhood experiences and increased arterial stiffness as measured by PWV [63].
Prevalence of early vascular aging
The prevalence of arterial stiffness or rather EVA remains to be clearly defined, since evidence regarding the prevalence of EVA is limited in both adult and pediatric populations. Studies have indicated higher PWV values in low cardiovascular risk populations (under 30 years of age) and estimated the prevalence of EVA at 12.5% in Portugal (n = 2542; age 18–96 years) [64] and 37.3% in Austria (n = 10 973; age 20–94 years) [65]. It is also believed that the prevalence of EVA is proportional to the prevalence of hypertension and related co-morbidities such as obesity and type 2 diabetes mellitus, due to the latter being late manifestations of increased arterial stiffness [24]. From the USA, approximately 11% of children and adolescents have high BP [1], whereas in South Africa, the prevalence of childhood hypertension ranges between 7.5% and 22.3%, dependent on location, region, and culture [2]. Whether the prevalence of EVA relates to the prevalence of hypertension remains to be determined.
Although cardiovascular morbidity and mortality are traditionally attributed to numerous modifiable risk factors including unhealthy dietary and sedentary behaviors, low physical activity, psychosocial stress, hypertension, tobacco use, abnormal lipids, glucose intolerance, and obesity [66], there are other adverse risk factors often overlooked. Among these are low socioeconomic class, psychosocial stress, infectious diseases, lack of healthcare, and poor lifestyle choices as observed in low to middle-income countries have cumulative harmful effects on cardiovascular health, especially in populations with an exceptional proportion of ethnic and/or race variation [67, 68].
Race or ethnic differences in early vascular aging among children and adolescents
The etiology of racial differences in EVA is complex, but there is an appreciation of biological and socioeconomic factors that are at least partly involved [69]. Although many determinants of EVA could explain potential racial differences, there is limited evidence to unravel the origin of such differences in the early onset of CVD. In this section, we provide a brief overview of studies comparing components of EVA (BP, PWV, carotid intima media thickness, and left ventricular mass) from studies that included two or more race/ethnic groups in children and/or adolescents.
Analyses from the Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth (SHIP-AHOY) [70] has emphasized the importance of BP measurement in children, along with the most recent sets of guidelines for BP in pediatric populations [71, 72]. Moreover, target organ damage has already been observed in adolescents with BP levels below the current clinical definition for hypertension [73]. Differences in BP based on race/ethnicity are regularly reported in adults, yet limited, evidence of these differences exists in childhood and adolescents. These race/ethnic differences in BP have been described by potential differences in intrauterine growth, based on birth weight, followed by the effects of early weight gain and growth in body height and current stature [74]. We summarized key studies that reported on BP differences by race (Table 1). The majority of studies comparing BP between different race groups are from the USA. These studies have reported mostly higher BP in black (African-American or African Caribbean) compared with children or adolescents from white or other (Asian, South Asian, Indian, Pakistan, Bangladesh) race groups. Many cross-sectional and longitudinal studies from the USA [56, 74,75,76, 78, 79, 81, 83, 84] and South Africa [82, 85] showed consistently higher BP (although inconsistent on whether the highest is systolic BP or diastolic BP) in the black compared with white population groups. The majority of these studies only compared BP without correcting for age, adiposity, stature, or other important confounders. In addition, a study from England [86] included large numbers of black, white, Asian, and other race groups and reported that black (African-Caribbean) children had similar mean systolic BP to white Europeans, but higher mean diastolic BP after correcting for age and sex. The same study also indicated that mean systolic BP tended to be slightly higher among black Caribbean, but lower among black Africans (p = 0.004); however, there was no heterogeneity for diastolic BP. Longitudinal studies mostly provide descriptive comparisons of the baseline BP profiles, and although these studies reported similar trends of higher BP in especially black children and adolescents, there are also inconsistencies. A multicenter study [77] performed an adjusted comparison between the black and white children that were born preterm and reported no race/ethnic differences, but after adjusting for neighborhood socioeconomic status, racial differences emerged over time. Similarly, studies from the USA [84, 87] and another from Brazil [88] indicated no differences in BP between black and white/non-black children and adolescents.
In a recent brief review, it became clear that studies investigating racial differences of arterial stiffness were mostly from the USA and some from Brazil and South Africa [89]. These studies reported that PWV was highest in especially black (African American, Brazilian, and South African) compared with the white groups. However, many of these studies were (i) inconsistent in reporting adjusted means of PWV, (ii) were mostly of cross-sectional design, or (iii) some studies only included one racial group. Additionally (Table 2), we provide an overview of comparative studies that measured PWV in children from different race/ethnicity.
Since there are no universal cutoffs for PWV in children and adolescents, comparative studies have proven useful in determining racial/ethnic differences in arterial stiffness and potentially EVA. Socioeconomic and psychosocial factors are becoming essential contributors to consider when investigating early manifestations of adverse vascular alterations in black populations. In a longitudinal study [87], Thurston et al. investigated association between race and socioeconomic (SES) with arterial stiffness in adolescents (age 14–16 years). The study found that PWV was higher in the black (African American) group as compared with the white group. A larger proportion in the black participants were from families with low household income, low levels of parental education, and had lower scores on the neighborhood SES assessment. Lower or medium family income and lower neighborhood SES were positively associated with PWV, even after adjustments for covariates. Despite a small sample size (n = 107, divided into black and white groups) (age 9–12 years), Lefferts et al. observed high PWV in black (African American) children as compared with white children after adjustments for covariates including age, sex, BMI, mean arterial pressure, and SES [81]. The study also noted a lower SES in the black children as compared their white counterparts.
Certain disease states may serve as facilitators for accelerated vascular aging in certain race/ethnic groups. In a population (age 11–26 years) with type 1 diabetes, a higher PWV was associated with non-Hispanic white race/ethnicity and higher in type 1 diabetes patients as compared with the controls; however, no comparative data was shown [90]. The regression analyses were adjusted for modifiable and non-modifiable risk factors. Furthermore, black (African American) adolescents with type 2 diabetes presented with a higher PWV compared with whites [91]. In the same study, multiple regression analyses further demonstrated that age, lipids, BP, and duration of diabetes were differently associated with arterial stiffness in individual race/ethnicity groups. A study in Brazil including both black and non-black children and adolescents (age 6–18 years) showed that in puberty and post-pubertal stages, black individuals had higher PWV as compared with the non-black group, even after adjustments for multiple confounders [88]. In black South African boys (age 6–8 years), there was a consistently higher PWV when measured across various segments of the vascular tree (Fig. 2) as compared with the white boys [82].
Ethnic differences in pulse wave velocity between black and white boys (ages 6–8 years) from South Africa. Adapted from Mokwatsi et al. [82]
The contribution of heritability on racial/ethnic differences in arterial stiffness remains questionable. A twin study (age 11.9–30.0) reported that heritability traits did not display any differences between blacks and whites, despite black participants presenting with a higher PWV as compared with their white counterparts [78]. It is therefore clear that exposure to unfavorable environmental factors play a significant role in the prominence of higher PWV in black children and adolescents as compared with their white peers.
Carotid intima media thickness (CIMT), as a surrogate for the detection of atherosclerosis and early development of endothelial dysfunction [92], has also been reported as a determinant of EVA [93]. Only a few studies (mostly cross-sectional) have reported differences in CIMT among children and adolescents of different race/ethnicity (Table 3). The majority among these studies included comparisons between black (American, African, Caribbean) and white or Hispanic populations, with little comparative studies that included other racial or ethnic populations. Importantly, many of these studies reported on the unadjusted means of CIMT and did not necessarily consider any adjustment for BP, body composition, age, or sex. All studies from the USA reported significantly higher CIMT in black children [81, 95, 96] and/or adolescents [94], whereas one study reported borderline higher CIMT in the black compared with white group [87]. A UK study population, with multiple race/ethnic groups, reported higher CIMT in the black (African and Caribbean) compared with white, South Asian, Asian, and other (Indian, Pakistani, or Bangladeshi) race/ethnic groups, after adjustments for multiple covariates including age and sex [97]. From South Africa, one study reported higher CIMT in black compared with white boys, after adjustment for mean arterial pressure [82]. From the community of Bogalusa, USA, children (age 4–17 years) were cross-sectionally surveyed between 1973 and 2002 (not in Table 3) and were examined with CIMT as an additional measurement (not performed at baseline). The comparative analysis showed higher CIMT in black men (0.880 mm vs. 0.839 mm) and women (0.790 mm vs. 0.762 mm) compared with their white counterparts (p < 0.00) [98].
A recent systematic review reported on studies that investigated echocardiography in children and adolescents from various parts of the world and different race groups [99]. However, most of those studies did not perform any racial comparisons, making the current literature on the racial differences in these measurements highly limited, especially from countries other than the USA. Studies claim that children with left ventricular hypertrophy (LVH) are more likely to be of non-white race and have a higher BMI z score [100]. In Table 4, we listed studies in children and adolescents that compared left ventricular mass (LVM) between two or more race groups. Multiple studies from the USA reported higher unadjusted LVM in black compared with white children and adolescents [76, 79, 102, 108]. The higher LVM was more evident in black girls compared with boys for baseline and follow-up [105]. LVM adjusted for body surface area, stature, or body height to the power 2.7, yielded similar differences with higher LVM in black than white groups [79], along with the higher LVM in black girls and boys compared with their white counterparts [76, 105, 107]. Comparative studies have also reported no differences in LVM between black and white children or adolescents [106]. A longitudinal study with a very small sample size (black: n = 25 and white: n = 36), reported higher LVM (unadjusted and adjusted for body surface area) in white girls and black boys and after an approximate 6-year follow-up. LVM was higher in white boys and girls (unadjusted) and in white boys after considering indexing for body surface area compared with the black groups [101]. A retrospective study performed a racial/ethnic comparison of data from three different sites in the USA, and reported the highest LVM (indexed by body surface area) in the Hispanic group (n = 20) compared with the black and white groups [104]. Detail on age per race/ethnic group as well as secondary causes of hypertension per race/ethnic group were not reported in this analysis. One study from an Italian group also confirmed higher LVM in black (n = 30) compared with white (n = 60) adolescents, although this study was performed in athletes visiting Italy from Central or West Africa [103]. Based on the limited and inconsistent evidence, it remains uncertain whether LVM is universally higher in certain race/ethnic populations, especially in the context of EVA in children and adolescents.
Pathological determinants of early vascular aging
Similar to the multifactorial etiology of hypertension, EVA develops in the presence of cumulative risk factors in vulnerable populations such as children and adolescents, especially those with significant differences noted by race or ethnicity. It is becoming increasingly evident that early stages of CVD, such as hypertension and LVH, start to manifest in childhood and adolescence, with black children manifesting these risk factors earlier than white children [13, 82]. Therefore, race or ethnicity is considered risk factors of EVA and subsequent CVD. In low and middle-income countries, obesity and other NCDs are consequences of a combination of poverty, living environments, the availability of fast foods, and especially the consumption of energy-dense, but micronutrient-poor diets. However, there is substantial evidence to suggest that early life nutrition and intrauterine risk factors play a pivotal role in the progression towards adult NCDs [109].
Tracking studies have shown that children and adolescents with elevated BP have a higher risk of developing hypertension in early adulthood [45, 72, 110] and this transition to adult hypertension was mostly determined by modifiable cardiovascular risk factors including poor dietary habits (high dietary salt intake, fructose, processed and fast foods), poor sleep patterns, stress, and a lack of sufficient physical activity [45]. Furthermore, childhood nutrition is a major driver of child mortality and morbidity in countries with large ethnic inequities, with a substantial burden of under-and overnutrition, and a rapid growth of obesity, driven by the excessive consumption of sugar containing drinks, ultra-processed foods, and extensive sedentary lifestyle [16]. Underweight trends among children and adolescents are still alarmingly high especially in African and Southeast Asia compared with obesity. With obesity in children and adolescents reaching a plateau in high-income countries, the prevalence is still rising in low-income and middle-income countries. This has important consequences, including the short-term developments of psychiatric, psychological, and psychosocial disorders in childhood and the increased long-term risk of developing NCDs later in life [111].
In light of the abovementioned modifiable and non-modifiable risk factors contributing to EVA, EVA is typically felt to be manifest as the long-term development of endothelial dysfunction, arterial stiffness, LVH, and early kidney damage.
The vasculature
Arterial stiffness is defined as reduced arterial distensibility or compliance (in the tunica media) as a result of continuous adaptations in the molecular and biomechanical makeup of blood vessels [112, 113] and endothelial dysfunction. Endothelial dysfunction is defined as the state of impaired vasodilation due to proinflammation and prothrombic properties of the blood vessel walls [114]. Endothelial dysfunction is also associated with several CVDs in adults, including hypertension, coronary artery disease, chronic heart failure, peripheral artery disease, diabetes mellitus, and CKD [114]. However, several mechanisms involved in reduced vasodilatory responses of the endothelium are also evident in pediatric populations including reduced nitric oxide bioavailability, oxidative stress, and the activation of vasoactive peptides known to promote vasoconstriction. A study in black and white boys (mean age of 7.29 years) reported lower urinary nitrate-to-nitrite molar ratio, as a measure of nitric oxide bioavailability, in black boys compared with the white boys, suggesting a lower reabsorption rate of nitrite or lower nitric oxide generation and underlying sub-clinical endothelial dysfunction [115]. This result may further indicate genetic differences in renal carbonic anhydrase isoforms and anion transporters among children of black African ancestry. Furthermore, markers of oxidative stress (thiobarbituric acid-reactive substances and 8-hydroxy-2-deoxy guanosine) related to increased arterial stiffness and diastolic BP in boys with linked maternal lifestyle and cardiovascular risk factors, suggesting potential family-related early onset of increased cardiovascular risk [116]. Studies in children and adolescents reported higher arterial stiffness in black and Hispanic populations at ages as early as 6 years [80, 82, 84, 87] compared with white, non-Hispanic, and Asian children. Aside from studies that reported the higher BP, PWV, and CIMT in black and Hispanic populations, there are numerous intermediate determinants of EVA that contribute to the premature development of endothelial dysfunction and subsequent arterial stiffness. Increased aortic wall thickness and impaired vasomotor function was described as functions of increased arterial stiffness [117], as observed in preterm infants with systemic hypertension [118]. In addition, low birth weight and other complications such as bronchopulmonary dysplasia also contribute to the early onset of arterial stiffening [118].
Other determinants of endothelial dysfunction and arterial stiffness include metabolic factors (impaired glucose and lipid metabolism and insulin resistance), oxidative stress [119,120,121] and inflammation, as well as the increased deposition of matrix substances, all of which contribute to altered hemodynamics and subsequent hypertension [122,123,124]. In addition, increased carotid artery intima-media thickness and early atherosclerosis, capillary rarefaction and dysfunctional vascular regulation along with microvascular and macrovascular injury have been reported in children [125, 126], but ethnic-specific comparison studies are still limited. Metabolomics analyses also confirmed race/ethnic disparities, where PWV associated adversely with β-alanine, 1-methylhistidine, and L-proline in black South African children, which may suggest potential early compromise in cardioprotective metabolic pathways in children of African ancestry [127].
The heart and the kidneys
The vascular compromise in EVA has a direct impact on the heart and other target organs. Perhaps one of the most dynamic measures of increased cardiovascular risk is LVM. Increased LVM, also defined as LVH, is a prominent independent predictor of cardiovascular morbidity and mortality in adults [128, 129] and a sensitive marker of risk in children [130]. With exposure to various environmental factors (pollution, violence, poverty, availability to drugs, access to alcohol and cigarettes), aging, and lifestyle behaviors (exercise and dietary intake of healthy and unhealthy food), the wall of the left ventricle has the ability to remodel in response. LVM may be one of the earliest markers of hypertension mediated target organ damage or manifestations of EVA. From the Bogalusa Heart Study in Louisiana, adolescence was described as a critical age period for the development of LVH in later life due to the impact of BP trajectories in childhood on adult LVH and geometric patterns [131]. This study included a population of 65% white and 35% black children, adolescents, and adults (ages 4–51 years) and reported higher BP and LVM in the black participants, highlighting the reality of ethnic specific risks in the setting of EVA.
Underweight or overweight/obesity during childhood is an additional risk factor for the development of LVH as these markers of suboptimal nutrition are associated with accelerated CV deterioration and early vascular compromise. This has been confirmed by reports indicating LVH and left ventricular diastolic dysfunction in 9–19-year olds with obesity, prior to the development of sustained hypertension [132]. LVH is also a sensitive marker of target organ damage in children with high BP and CKD [130]. In children with CKD, LVH develops early and becomes more prevalent as renal function decreases; however, this may be dependent on BP as a study reported that a reduction in BP might predict a decline in LVH in children with CKD [133].
The degree of renal function is important—children with end-stage renal disease (ESRD) on dialysis had worse measures of arterial stiffness than those with a functional kidney transplant and healthy age-sex-matched controls [134, 135]. Litwin et al. even suggested partial reversal in CKD-associated arterial wall remodeling as patients displayed attenuation of arterial pathology after kidney transplantation than patients on dialysis irrespective of exposure to similar dialysis vintage [134]. In another study, PWV was not significantly different between children with mild CKD and healthy children, while in children with mild-to-moderate CKD, PWV was independently associated with increasing age, mean arterial pressure and black ethnicity [136].
Chronic kidney disease can also promote tissue growth and adversely impact left ventricular function via non-hemodynamic pathways such as chronic inflammation, vitamin D deficiency, and higher levels of parathyroid hormone [137, 138]. Vitamin D and its interactions with the renin-angiotensin-aldosterone system (RAAS) have been implicated in arterial stiffness. Vitamin D supplementation was shown to alleviate local arterial stiffness and improved flow-mediated dilatation in children with CKD [106]. Fibroblast growth hormone-23 (FGF23), a hormone that is released from bone and works on the kidney has been positively associated with LVH in children aged 1–21 years [139, 140]. FGF23 increases activity of the RAAS by decreasing active vitamin D [141]. On the other hand, FGF23 may promote sodium retention and subsequently volume expansion independent of RAAS [142]. In the Framingham Heart Study (including the Offspring cohort and the Omni cohort), FGF23 was positively associated with African-American and Asian ethnicity [143]. However, in the CARDIA study, FGF23 was associated with an increase in BP over time and an increased incident of hypertension, with no racial/ethnic differences in hypertension [144].
Ethnic differences regarding the activity of RAAS are also well established with populations of African ancestry presenting with a suppressed RAAS across all ages [145,146,147]. The suppressed RAAS phenotype is not unique to individuals of African descent, but it is also common in Asians and elderly populations of other ethnicities [148, 149]. The low RAAS activity is due to, among others, retention of sodium and water, which increases the load against which the heart must work [146, 150]. In black boys with a mean age of 16 years, an increase in aldosterone was associated with decreased sodium excretion and increased BP and LVM [107]. Another study showed a stronger association between aldosterone and BP with aging from adolescence (mean age 10.6 years) to adulthood in black participants [147]. Early kidney damage and dysregulation in the RAAS may also stem from fetal conditions. Young adults born preterm present with smaller kidneys and higher angiotensin I, BP, and albumin-to-creatinine ratio compared with full-term controls [151], predisposing preterm babies to early vascular alterations and CVD development at young ages. A recent study developed a nomogram to predict aldosterone in children, which may improve assessment of RAAS dysfunction and treatment of pediatric hypertension to delay EVA [152]. Further studies in children and young adults from different ethnicities are needed to confirm if race-specific normal ranges are essential.
An increase is arterial stiffness was also observed in cases of children with acute post-streptococcal glomerulonephritis that progressed into CKD. Post-infectious glomerulonephritis (PIGN) is usually a result of group A streptococcal infections, and it is characterized by acute kidney injury, increased BP, glomerular hematuria, mild proteinuria, and edema [153]. Yu et al. demonstrated an association between arterial stiffness and PIGN in children [153]. The mechanisms are not yet clear, but may be due to the renal inflammatory response in PIGN [154, 155], and also suggest that glomerular changes may reflect vascular changes outside the kidney in response to infection. Rural and overcrowded communities are particularly vulnerable to epidemic clusters and outbreaks of PIGN [153]. PIGN used to be the most prevalent kidney disease in black South African children; however, focal glomerulosclerosis and rapidly progressive glomerulonephritis (mostly due to streptococcal infection) later became the first and second causes of renal failure requiring kidney transplantation [156].
Of importance, other factors contributing to the burden of kidney diseases in black South African children emanate from the quadruple burden of disease, which may not be unique to the South African context. Tuberculosis has been linked to focal glomerulosclerosis and Takayasu arteritis (inflammatory vasculitis of the aorta and its main branches), while HIV and its associated opportunistic infections, as well toxicity from antiretroviral drugs, have been implicated in kidney injury in children living with HIV [156,157,158]. Aboriginal children from Australia and New Zealand diagnosed with severe PIGN showed an increased risk to progress to advanced stages of renal damage and even ESRD as compared with the non-Aboriginal population [159,160,161]. It is not yet established if the ethnic-specific manifestations of acute kidney disease may accelerate vascular aging in children and young adults of certain race groups.
Future directions of early vascular aging and race/ethnicity in children and adolescents
While genetic studies have failed to distinguish ethnic or race-specific determinants of CVD risk [162], race/ethnicity remains one of the risk factors regularly reported in multiethnic population studies in relation to hypertension and cardiovascular disease states. Whether race/ethnicity by itself is a risk factor for EVA, or whether it is the convergence of multiple risk factors on the backdrop of race/ethnicity, remains to be confirmed.
In countries such as the USA, race/ethnic variations in health have been regularly reported, with differences in socioeconomic status as a major contributor to racial disparities in health [163]. However, in the recent 2018 World Bank report, South Africa was identified as the most unequal country in the world, with black South Africans reported to have the highest level of poverty, with less access to proper education, are most unemployed, have more female headed households, and have large families and many children per household [164]. With multiple discrepancies in environmental and sociocultural determinants that may adversely influence biological aging, research should be directed to larger, prospective, and standardized protocols to address racial differences in EVA, especially in low to middle-income countries with large ethnic diversity.
Finally, alongside the clinical treatment of the consequences of biological aging, focus should be shifted to the development of primordial prevention and educational programs to promote health from the beginning of life (starting with pregnant mothers, parents, pre-primary school children and teachers). The overall health and economic burden of treating NCDs can be improved—especially in countries with large educational and sociocultural disparities and racial variation.
Conclusion
Although this is not an exhaustive review, the main features of EVA point to important racial differences in risk factors when evaluating the early life determinants of accelerated biological aging. The manifestations of EVA are of particular global and economic interest and should be targeted for primary prevention to curb the current escalating burden of cardiovascular disease, especially in children and adolescents at increased risk by race.
Key summary points
-
Early life programming is an essential determinant of EVA in children and adolescents
-
Children of black or Hispanic race are especially vulnerable to develop EVA due to predisposed risk in hemodynamic and end organ damage
-
Ethnicity by itself can be considered a risk factor for EVA, but may be dependent on converging risk factors in early life
Multiple-choice questions
-
1.
Early vascular aging can be defined as:
-
a)
Premature CVD manifestation
-
b)
Age-related biological deterioration
-
c)
Age-related increases in cardiac function
-
d)
Vascular degeneration
-
e)
Premature deterioration of the vasculature
-
2.
Which one of the following is the gold standard measure of EVA?
-
a)
Carotid intima thickness
-
b)
Blood pressure
-
c)
Pulse wave velocity
-
d)
Nitric oxide
-
e)
Oxidative stress
-
3.
The following are key characteristics of EVA except:
-
a)
Increased pulse wave velocity
-
b)
Oxidative stress
-
c)
Increased elasticity of the arterial walls
-
d)
Microvascular rarefaction
-
e)
Increased vasoconstriction
-
4.
Ethnic differences in intrauterine programming of early vascular aging may be linked to
-
a)
Oxidative stress
-
b)
Endothelial dysfunction
-
c)
Epigenetic modifications
-
d)
A and B only
-
e)
All of the above
-
5.
EVA and its associated cardiovascular risk can be mitigated by ______.
-
a)
Intensifying treatment of hypertension, diabetes and renal diseases in resource poor settings
-
b)
Timeous diagnosis and treatment of infectious diseases
-
c)
Improving maternal and child health and nutrition
-
d)
Both A and B
-
e)
Options A, B, C
References
Flynn JT (2018) High blood pressure in the young: why should we care? Acta Paediatr 107(1):14–19
Kagura J, Ong KK, Adair LS, Pettifor JM, Norris SA (2018) Paediatric hypertension in South Africa: an underestimated problem calling for action. S Afr Med J 108(9):708–709. https://doi.org/10.7196/SAMJ.2018.v108i9.13317
Shatat IF, Brady TM (2018) Editorial: Pediatric Hypertension: Update. Front Pediatr 6(209):209. https://doi.org/10.3389/fped.2018.00209
Barker D, Lampl M, Roseboom T, Winder N (2012) Resource allocation in utero and health in later life. Placenta 33:e30–e34
Calkins K, Devaskar SU (2011) Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care 41(6):158–176
Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo A-P, Hering D (2016) A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 388(10060):2665–2712
Godfrey KM, Barker DJ (2000) Fetal nutrition and adult disease. Am J Clin Nutr 71(5 Suppl):1344S–1352S. https://doi.org/10.1093/ajcn/71.5.1344s
Taal HR, de Jonge LL, van Osch-Gevers L, Steegers EA, Hofman A, Helbing WA, van der Heijden AJ, Jaddoe VW (2013) Parental smoking during pregnancy and cardiovascular structures and function in childhood: the Generation R Study. Int J Epidemiol 42(5):1371–1380. https://doi.org/10.1093/ije/dyt178
Yu Y, Arah OA, Liew Z, Cnattingius S, Olsen J, Sorensen HT, Qin G, Li J (2019) Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ 367:l6398. https://doi.org/10.1136/bmj.l6398
Silva LM, Coolman M, Steegers EA, Jaddoe VW, Moll HA, Hofman A, Mackenbach JP, Raat H (2008) Low socioeconomic status is a risk factor for preeclampsia: the Generation R Study. J Hypertens 26(6):1200–1208. https://doi.org/10.1097/HJH.0b013e3282fcc36e
Barker DJ (2004) The developmental origins of chronic adult disease. Acta Paediatr Suppl 93(446):26–33. https://doi.org/10.1111/j.1651-2227.2004.tb00236.x
Pool LR, Ning H, Lloyd-Jones DM, Allen NB (2017) Trends in racial/ethnic disparities in cardiovascular health among US adults From 1999-2012. J Am Heart Assoc 6(9). https://doi.org/10.1161/JAHA.117.006027
Schutte AE, Botha S, Fourie CMT, Gafane-Matemane LF, Kruger R, Lammertyn L, Malan L, Mels CMC, Schutte R, Smith W, van Rooyen JM, Ware LJ, Huisman HW (2017) Recent advances in understanding hypertension development in sub-Saharan Africa. J Hum Hypertens 31(8):491–500. https://doi.org/10.1038/jhh.2017.18
Ordovas JM (2007) Medicine, genetics and race: the case of cardiovascular diseases. Perinat Med 4(1):1–6. https://doi.org/10.2217/17410541.4.1.1
Race, Ethnicity, and Genetics Working Group (2005) The use of racial, ethnic, and ancestral categories in human genetics research. Am J Hum Genet 77(4):519–532
Lake L, Shung-King M, Hendricks M, Heywood M, Nannan N, Laubscher R, Bradshaw D, Mathews C, Goga A, Ramraj T, Chirinda W (2019) Prioritising child and adolescent health: a human rights imperative. Child and adolescent health, Cape Town
Nilsson P (1996) Premature ageing: the link between psychosocial risk factors and disease. Med Hypotheses 47(1):39–42
Sniderman AD, Furberg CD (2008) Age as a modifiable risk factor for cardiovascular disease. Lancet 371(9623):1547–1549
Nilsson PM (2008) Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag 4(3):547–552. https://doi.org/10.2147/vhrm.s1094
Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM (2019) Concept of extremes in vascular aging: from early vascular aging to supernormal vascular aging. Hypertension 74(2):218–228
Kotsis V, Antza C, Doundoulakis I, Stabouli S (2017) Markers of early vascular ageing. Curr Pharm Des 23(22):3200–3204. https://doi.org/10.2174/1381612823666170328142433
Zhong Q, Hu M-J, Cui Y-J, Liang L, Zhou M-M, Yang Y-W, Huang F (2018) Carotid–femoral pulse wave velocity in the prediction of cardiovascular events and mortality: an updated systematic review and meta-analysis. Angiology 69(7):617–629
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank J, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30(3):445–448
Izzo JL, Shykoff BE (2019) Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med 2(1):29–40
Cunha PG, Boutouyrie P, Nilsson PM, Laurent S (2017) Early vascular ageing (EVA): definitions and clinical applicability. Curr Hypertens Rev 13(1):8–15. https://doi.org/10.2174/1573402113666170413094319
Kingwell BA (2002) Large artery stiffness: implications for exercise capacity and cardiovascular risk. Clin Exp Pharmacol Physiol 29(3):214–217
Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC (2001) Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 38(3):796–802
Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C (2016) Effects of arterial stiffness on brain integrity in young adults from the Framingham Heart Study. Stroke 47(4):1030–1036. https://doi.org/10.1161/strokeaha.116.012949
Saji N, Toba K, Sakurai T (2015) Cerebral small vessel disease and arterial stiffness: tsunami effect in the brain. Pulse 3(3-4):182–189
Briet M (2019) Large artery remodelling and stiffening in moderate chronic kidney disease. Artery Res 5(4):137–137
Georgianos PI, Sarafidis PA, Liakopoulos V (2015) Arterial stiffness: a novel risk factor for kidney injury progression? Am J Hypertens 28(8):958–965
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A (2014) 2013 ESH/ESC Practice guidelines for the management of arterial hypertension: ESH-ESC The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 23(1):3–16
Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S (2015) The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 241(2):507–532
Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB (2014) Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63(7):636–646. https://doi.org/10.1016/j.jacc.2013.09.063
Townsend RR (2016) Arterial stiffness: recommendations and standardization. Pulse 4(Suppl. 1):3–7
Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM (1998) Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension 32(3):570–574
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A (2001) Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37(5):1236–1241
Levisianou D, Foussas S, Skopelitis E, Adamopoulou E, Xenopoulou T, Destounis A, Koukoulis G, Skoularigis I, Melidonis A, Triposkiadis F (2013) Arterial stiffness predicts risk for long-term recurrence in patients with type 2 diabetes admitted for acute coronary event. Diabetes Res Clin Pract 99(3):315–320
Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J (2006) Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113(5):664–670. https://doi.org/10.1161/circulationaha.105.579342
Lynch J, Smith GD (2005) A life course approach to chronic disease epidemiology. Annu Rev Public Health 26:1–35. https://doi.org/10.1146/annurev.publhealth.26.021304.144505
Gillman MW, Rosner B, Evans DA, Smith LA, Taylor JO, Hennekens CH, Keough ME (1991) Use of multiple visits to increase blood pressure tracking correlations in childhood. Pediatrics 87(5):708–711
Kagura J, Adair LS, Musa MG, Pettifor JM, Norris SA (2015) Blood pressure tracking in urban black South African children: birth to twenty cohort. BMC Pediatr 15(1):78. https://doi.org/10.1186/s12887-015-0402-z
Lauer RM, Clarke WR (1988) A longitudinal view of blood pressure during childhood: the Muscatine Study. Stat Med 7(1-2):47–57. https://doi.org/10.1002/sim.4780070109
Shear CL, Burke GL, Freedman DS, Berenson GS (1986) Value of childhood blood pressure measurements and family history in predicting future blood pressure status: results from 8 years of follow-up in the Bogalusa Heart Study. Pediatrics 77(6):862–869
Falkner B (2015) Recent clinical and translational advances in pediatric hypertension. Hypertension 65(5):926–931
Cattell MA, Anderson JC, Hasleton PS (1996) Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta 245(1):73–84. https://doi.org/10.1016/0009-8981(95)06174-6
Martyn CN, Greenwald SE (1997) Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet (London, England) 350(9082):953–955. https://doi.org/10.1016/s0140-6736(96)10508-0
Bansal N (2006) Maternal and early life determinants of serum lipids and vascular function in pre-school children. The University of Manchester (United Kingdom),
Islam M, Jafar TH, Bux R, Hashmi S, Chaturvedi N, Hughes AD (2014) Association of parental blood pressure with retinal microcirculatory abnormalities indicative of endothelial dysfunction in children. J Hypertens 32(3):598–605. https://doi.org/10.1097/hjh.0000000000000063
Stirrat LI, Reynolds RM (2014) Effects of maternal obesity on early and long-term outcomes for offspring. Res Rep Neonatol 4:43
van Os J, Rutten BP, Poulton R (2010) Gene–environment interactions for searchers: collaboration between epidemiology and molecular genetics. In: Advances in Schizophrenia Research 2009. Springer, pp 19-50
Clough GF (2011) Developmental conditioning of the vasculature. Compr Physiol 5(1):397–438
Kuh D, Smith GD (2004) disease: an historical perspective with particular reference to coronary. A life course approach to chronic disease epidemiology (2):15
Hedderson MM, Darbinian JA, Ferrara A (2010) Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol 24(5):441–448. https://doi.org/10.1111/j.1365-3016.2010.01140.x
Savitz DA, Janevic TM, Engel SM, Kaufman JS, Herring AH (2008) Ethnicity and gestational diabetes in New York City, 1995-2003. BJOG 115(8):969–978. https://doi.org/10.1111/j.1471-0528.2008.01763.x
Oberg S, Ge D, Cnattingius S, Svensson A, Treiber FA, Snieder H, Iliadou A (2007) Ethnic differences in the association of birth weight and blood pressure: the Georgia cardiovascular twin study. Am J Hypertens 20(12):1235–1241. https://doi.org/10.1016/j.amjhyper.2007.07.012
Alexander GR, Kogan M, Bader D, Carlo W, Allen M, Mor J (2003) US birth weight/gestational age-specific neonatal mortality: 1995-1997 rates for whites, hispanics, and blacks. Pediatrics 111(1):e61–e66. https://doi.org/10.1542/peds.111.1.e61
Harding S, Rosato M, Cruickshank J (2004) Lack of change in birthweights of infants by generational status among Indian, Pakistani, Bangladeshi, Black Caribbean, and Black African mothers in a British cohort study. Int J Epidemiol 33(6):1279–1285
McEniery CM, Bolton CE, Fawke J, Hennessy E, Stocks J, Wilkinson IB, Cockcroft JR, Marlow N (2011) Cardiovascular consequences of extreme prematurity: the EPICure study. J Hypertens 29(7):1367–1373. https://doi.org/10.1097/HJH.0b013e328347e333
Posod A, Odri Komazec I, Kager K, Pupp Peglow U, Griesmaier E, Schermer E, Wurtinger P, Baumgartner D, Kiechl-Kohlendorfer U (2016) Former very preterm infants show an unfavorable cardiovascular risk profile at a preschool age. PLoS One 11(12):e0168162. https://doi.org/10.1371/journal.pone.0168162
Mina TH, Lahti M, Drake AJ, Forbes S, Denison FC, Raikkonen K, Norman JE, Reynolds RM (2017) Maternal lipids in pregnancy are associated with increased offspring cortisol reactivity in childhood. Psychoneuroendocrinology 83:79–83. https://doi.org/10.1016/j.psyneuen.2017.04.018
Carey LP (2018) Childhood emotional functioning and arterial stiffness in young adulthood. ProQuest Dissertations Publishing, State University of New York at Albany
Klassen SA, Chirico D, O’Leary DD, Cairney J, Wade TJ (2016) Linking systemic arterial stiffness among adolescents to adverse childhood experiences. Child Abuse Negl 56:1–10. https://doi.org/10.1016/j.chiabu.2016.04.002
Cunha PG, Cotter J, Oliveira P, Vila I, Boutouyrie P, Laurent S, Nilsson PM, Scuteri A, Sousa N (2015) Pulse wave velocity distribution in a cohort study: from arterial stiffness to early vascular aging. J Hypertens 33(7):1438–1445
Danninger K, Hafez A, Binder RK, Aichberger M, Hametner B, Wassertheurer S, Weber T (2019) High prevalence of hypertension and early vascular aging: a screening program in pharmacies in Upper Austria. J Hum Hypertens:1–9
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet (London, England) 364(9438):937–952. https://doi.org/10.1016/S0140-6736(04)17018-9
Braveman P, Gottlieb L (2014) The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep 129(1_suppl2):19–31
Rosengren A, Smyth A, Rangarajan S, Ramasundarahettige C, Bangdiwala SI, AlHabib KF, Avezum A, Boström KB, Chifamba J, Gulec S (2019) Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob Health 7(6):e748–e760
Cruickshank JK, Silva MJ, Molaodi OR, Enayat ZE, Cassidy A, Karamanos A, Read UM, Faconti L, Dall P, Stansfield B, Harding S (2016) Ethnic differences in and childhood influences on early adult pulse wave velocity: the determinants of adolescent, now young adult, social wellbeing, and health longitudinal study. Hypertension 67(6):1133–1141. https://doi.org/10.1161/HYPERTENSIONAHA.115.07079
Mendizabal B, Urbina EM, Becker R, Daniels SR, Falkner BE, Hamdani G, Hanevold CD, Hooper SR, Ingelfinger JR, Lande M, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels JA, Flynn JT (2018) SHIP-AHOY (Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth). Hypertension 72(3):625–631. https://doi.org/10.1161/HYPERTENSIONAHA.118.11434
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, Subcommittee on screening and management of high blood pressure in children (2017) Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 140 (3):e20171904. doi:https://doi.org/10.1542/peds.2017-1904
Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wuhl E, Zanchetti A (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34(10):1887–1920. https://doi.org/10.1097/HJH.0000000000001039
Urbina EM, Khoury PR, Bazzano L, Burns TL, Daniels S, Dwyer T, Hu T, Jacobs DR Jr, Juonala M, Prineas R, Raitakari O, Steinberger J, Venn A, Woo JG, Sinaiko A (2019) Relation of blood pressure in childhood to self-reported hypertension in adulthood. Hypertension 73(6):1224–1230. https://doi.org/10.1161/HYPERTENSIONAHA.118.12334
Cruickshank JK, Mzayek F, Liu L, Kieltyka L, Sherwin R, Webber LS, Srinavasan SR, Berenson GS (2005) Origins of the “black/white” difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa heart study. Circulation 111(15):1932–1937. https://doi.org/10.1161/01.CIR.0000161960.78745.33
Collins RT, Somes GW, Alpert BS (2008) Differences in arterial compliance among normotensive adolescent groups: Collins arterial compliance in adolescents. Pediatr Cardiol 29(5):929–934. https://doi.org/10.1007/s00246-008-9239-7
Dekkers C, Treiber FA, Kapuku G, Van Den Oord EJ, Snieder H (2002) Growth of left ventricular mass in African American and European American youth. Hypertension 39(5):943–951. https://doi.org/10.1161/01.hyp.0000015612.73413.91
Fuller-Rowell TE, Curtis DS, Klebanov PK, Brooks-Gunn J, Evans GW (2017) Racial disparities in blood pressure trajectories of preterm children: the role of family and neighborhood socioeconomic status. Am J Epidemiol 185(10):888–897
Ge D, Young TW, Wang X, Kapuku GK, Treiber FA, Snieder H (2007) Heritability of arterial stiffness in black and white American youth and young adults. Am J Hypertens 20(10):1065–1072. https://doi.org/10.1016/j.amjhyper.2007.05.013
Heffernan KS, Lefferts WK, Atallah-Yunes NH, Glasgow AC, Gump BB (2020) Racial differences in left ventricular mass and wave reflection intensity in children. Front Pediatr 8:132. https://doi.org/10.3389/fped.2020.00132
Hlaing WM, Prineas RJ (2006) Arterial stiffness variations by gender in African-American and Caucasian children. J Natl Med Assoc 98(2):181–189
Lefferts WK, Augustine JA, Spartano NL, Atallah-Yunes NH, Heffernan KS, Gump BB (2017) Racial differences in aortic stiffness in children. J Pediatr 180:62–67. https://doi.org/10.1016/j.jpeds.2016.09.071
Mokwatsi GG, Schutte AE, Kruger R (2017) Ethnic differences regarding arterial stiffness of 6-8-year-old black and white boys. J Hypertens 35(5):960–967. https://doi.org/10.1097/HJH.0000000000001267
Philip R, Alpert BS, Schwingshackl A, Huang X, Blakely D, Rovnaghi CR, Tran QT, Arevalo A, Anand KJ (2015) Inverse relationship between cardio-ankle vascular index and body mass index in healthy children. J Pediatr 167(2):361–365. e361
Shah AS, Dolan LM, Gao Z, Kimball TR, Urbina EM (2012) Racial differences in arterial stiffness among adolescents and young adults with type 2 diabetes. Pediatr Diabetes 13(2):170–175
Steyn K, de Wet T, Richter L, Cameron N, Levitt NS, Morrell C (2000) Cardiovascular disease risk factors in 5-year-old urban South African children—the Birth to Ten Study. S Afr Med J 90(7):719–726
Thomas C, Nightingale CM, Donin AS, Rudnicka AR, Owen CG, Cook DG, Whincup PH (2012) Ethnic and socioeconomic influences on childhood blood pressure: the Child Heart and Health Study in England. J Hypertens 30(11):2090–2097
Thurston RC, Matthews KA (2009) Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med 68(5):807–813
Zaniqueli D, Alvim RO, Luiz SG, Oliosa PR, de Sa CR, Mill JG (2017) Ethnicity and arterial stiffness in children and adolescents from a Brazilian population. J Hypertens 35(11):2257–2261. https://doi.org/10.1097/HJH.0000000000001444
Schutte AE, Kruger R, Gafane-Matemane LF, Breet Y, Strauss-Kruger M, Cruickshank JK (2020) Ethnicity and arterial stiffness. Arterioscler Thromb Vasc Biol 40(5):1044–1054. https://doi.org/10.1161/ATVBAHA.120.313133
Shah AS, Wadwa RP, Dabelea D, Hamman RF, D’Agostino R Jr, Marcovina S, Daniels SR, Dolan LM, Fino NF, Urbina EM (2015) Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes 16(5):367–374. https://doi.org/10.1111/pedi.12279
Shah AS, El Ghormli L, Gidding SS, Bacha F, Nadeau KJ, Levitt Katz LE, Tryggestad JB, Leibel N, Hale DE, Urbina EM (2018) Prevalence of arterial stiffness in adolescents with type 2 diabetes in the TODAY cohort: Relationships to glycemic control and other risk factors. J Diabetes Complicat 32(8):740–745. https://doi.org/10.1016/j.jdiacomp.2018.05.013
Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, Chen W, Srinivasan SR, Daniels SR, Kahonen M, Laitinen T, Taittonen L, Berenson GS, Viikari JS, Raitakari OT (2010) Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation 122(24):2514–2520. https://doi.org/10.1161/CIRCULATIONAHA.110.966465
Litwin M, Feber J, Ruzicka M (2016) Vascular aging: lessons from pediatric hypertension. Can J Cardiol 32(5):642–649. https://doi.org/10.1016/j.cjca.2016.02.064
Breton CV, Wang X, Mack WJ, Berhane K, Lopez M, Islam TS, Feng M, Hodis HN, Kunzli N, Avol E (2011) Carotid artery intima-media thickness in college students: race/ethnicity matters. Atherosclerosis 217(2):441–446. https://doi.org/10.1016/j.atherosclerosis.2011.05.022
Chowdhury SM, Henshaw MH, Friedman B, Saul JP, Shirali GS, Carter J, Levitan BM, Hulsey T (2014) Lean body mass may explain apparent racial differences in carotid intima-media thickness in obese children. J Am Soc Echocardiogr 27(5):561–567. https://doi.org/10.1016/j.echo.2014.01.007
Toledo-Corral CM, Myers SJ, Li Y, Hodis HN, Goran MI, Weigensberg MJ (2013) Blunted nocturnal cortisol rise is associated with higher carotid artery intima-media thickness (CIMT) in overweight African American and Latino youth. Psychoneuroendocrinology 38(9):1658–1667. https://doi.org/10.1016/j.psyneuen.2013.01.011
Whincup PH, Nightingale CM, Owen CG, Rapala A, Bhowruth DJ, Prescott MH, Ellins EA, Donin AS, Masi S, Rudnicka AR (2012) Ethnic differences in carotid intima-media thickness between UK children of black African-Caribbean and white European origin. Stroke 43(7):1747–1754
Li S, Chen W, Srinivasan SR, Tang R, Bond MG, Berenson GS (2007) Race (black-white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima-media thickness in adulthood: the Bogalusa Heart Study. Atherosclerosis 194(2):421–425. https://doi.org/10.1016/j.atherosclerosis.2006.08.026
Majonga ED, Norrish G, Rehman AM, Kranzer K, Mujuru HA, Nathoo K, Odland JO, Kaski JP, Ferrand RA (2018) Racial variation in echocardiographic reference ranges for left chamber dimensions in children and adolescents: a systematic review. Pediatr Cardiol 39(5):859–868. https://doi.org/10.1007/s00246-018-1873-0
Brady TM, Fivush B, Flynn JT, Parekh R (2008) Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr 152(1):73–78 e71. https://doi.org/10.1016/j.jpeds.2007.05.053
Allen MT, Matthews KA, Sherman FS (1997) Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension 30(4):782–787. https://doi.org/10.1161/01.hyp.30.4.782
Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA (1995) Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol 76(10):699–701
Demola P, Crocamo A, Ceriello L, Botti A, Cremonini I, Pattoneri P, Corradi D, Visioli F, Goldoni M, Pela G (2019) Hemodynamic and ECG responses to stress test in early adolescent athletes explain ethnicity-related cardiac differences. Int J Cardiol 289:125–130. https://doi.org/10.1016/j.ijcard.2019.04.084
Hanevold C, Waller J, Daniels S, Portman R, Sorof J, International Pediatric Hypertension Association (2004) The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics 113(2):328–333. https://doi.org/10.1542/peds.113.2.328
Kapuku GK, Ge D, Vemulapalli S, Harshfield GA, Treiber FA, Snieder H (2008) Change of genetic determinants of left ventricular structure in adolescence: longitudinal evidence from the Georgia cardiovascular twin study. Am J Hypertens 21(7):799–805. https://doi.org/10.1038/ajh.2008.178
Lamers L, Ensing G, Pignatelli R, Goldberg C, Bezold L, Ayres N, Gajarski R (2005) A prospective assessment of myocardial contractility in young African Americans: does ethnicity impact the wall stress-heart rate-corrected velocity of circumferential fiber shortening relationship? J Am Soc Echocardiogr 18(7):743–748
Murro DG, Beavers M, Harshfield GA, Kapuku GK (2013) Aldosterone contributes to elevated left ventricular mass in black boys. Pediatr Nephrol 28(4):655–660. https://doi.org/10.1007/s00467-012-2367-6
Schieken RM, Schwartz PF, Goble MM (1998) Tracking of left ventricular mass in children: race and sex comparisons: the MCV Twin Study. Medical College of Virginia. Circulation 97(19):1901–1906. https://doi.org/10.1161/01.cir.97.19.1901
El-Heis S, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA, Godfrey KM (2018) Early-life nutrition, epigenetics and prevention of obesity. In: Epigenetics in Human Disease. Elsevier, pp 427-456
Lurbe E, Torro MI, Alvarez-Pitti J, Redon P, Redon J (2016) Central blood pressure and pulse wave amplification across the spectrum of peripheral blood pressure in overweight and obese youth. J Hypertens 34(7):1389–1395
Ahmad FS, Cai X, Kunkel K, Ricardo AC, Lash JP, Raj DS, He J, Anderson AH, Budoff MJ, Wright Nunes JA, Roy J, Wright JT Jr, Go AS, St John Sutton MG, Kusek JW, Isakova T, Wolf M, Keane MG, Investigators CRIC (2017) Racial/ethnic differences in left ventricular structure and function in chronic kidney disease: the chronic renal insufficiency cohort. Am J Hypertens 30(8):822–829. https://doi.org/10.1093/ajh/hpx058
O’Rourke MF, Hashimoto J (2007) Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50(1):1–13. https://doi.org/10.1016/j.jacc.2006.12.050
Vlachopoulos C, O’Rourke M, Nichols WW (2011) McDonald’s blood flow in arteries: theoretical, experimental and clinical principles. CRC press
Endemann DH, Schiffrin EL (2004) Endothelial dysfunction. J Am Soc Nephrol 15(8):1983–1992. https://doi.org/10.1097/01.ASN.0000132474.50966.DA
Tsikas D, Hanff E, Bollenbach A, Kruger R, Pham VV, Chobanyan-Jürgens K, Wedekind D, Arndt T, Jörns A, Berbée JF (2018) Results, meta-analysis and a first evaluation of U NOx R, the urinary nitrate-to-nitrite molar ratio, as a measure of nitrite reabsorption in experimental and clinical settings. Amino Acids 50(7):799–821
Craig A, Mels CMC, Kruger R (2018) Thiobarbituric acid-reactive substances relate to arterial stiffness and blood pressure in 6 to 8-year-old boys stratified by maternal risk. Free Radic Res 52(2):180–187. https://doi.org/10.1080/10715762.2017.1421314
Tauzin L, Rossi P, Giusano B, Gaudart J, Boussuges A, Fraisse A, Simeoni U (2006) Characteristics of arterial stiffness in very low birth weight premature infants. Pediatr Res 60(5):592–596. https://doi.org/10.1203/01.pdr.0000242264.68586.28
Sehgal A, Malikiwi A, Paul E, Tan K, Menahem S (2016) Systemic arterial stiffness in infants with bronchopulmonary dysplasia: potential cause of systemic hypertension. J Perinatol 36(7):564–569. https://doi.org/10.1038/jp.2016.10
Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, Tracy R, Jacobs DR Jr (2005) Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation 111(15):1985–1991
Śladowska-Kozłowska J, Litwin M, Niemirska A, Płudowski P, Wierzbicka A, Skorupa E, Wawer ZT, Janas R (2012) Oxidative stress in hypertensive children before and after 1 year of antihypertensive therapy. Pediatr Nephrol 27(10):1943–1951
Túri S, Friedman A, Bereczki C, Papp F, Kovàcs J, Karg E, Németh I (2003) Oxidative stress in juvenile essential hypertension. J Hypertens 21(1):145–152
Daniels SR (2019) Understanding the global prevalence of hypertension in children and adolescents. JAMA Pediatr 173(12):1133–1134
Hinton TC, Adams ZH, Baker RP, Hope KA, Paton JF, Hart EC, Nightingale AK (2020) Investigation and treatment of high blood pressure in young people: too much medicine or appropriate risk reduction? Hypertension 75(1):16–22
Mynard JP, Goldsmith G, Springall G, Eastaugh L, Lane GK, Zannino D, Smolich JJ, Avolio A, Cheung MM (2020) Central aortic blood pressure estimation in children and adolescents: results of the KidCoreBP study. J Hypertens 38(5):821–828
Köchli S, Endes K, Steiner R, Engler L, Infanger D, Schmidt-Trucksäss A, Zahner L, Hanssen H (2019) Obesity, high blood pressure, and physical activity determine vascular phenotype in young children: the EXAMIN YOUTH study. Hypertension 73(1):153–161
Laigaard PP, Larsen M, Hansen MH, Jeppesen J, Olsen EM, Skovgaard AM, Munch IC (2020) Retinal arteriolar wall-to-lumen ratios at 16–17 years in the Copenhagen Child Cohort 2000 Study. J Hypertens 38(4):731–736
Erasmus D, Mels CMC, Louw R, Lindeque JZ, Kruger R (2018) Urinary metabolites and their link with premature arterial stiffness in black boys: the ASOS study. Pulse 6(3):144–153. https://doi.org/10.1159/000492155
Benjamin EJ, Levy D (1999) Why is left ventricular hypertrophy so predictive of morbidity and mortality? Am J Med Sci 317(3):168–175
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322(22):1561–1566
Kavey RE (2013) Left ventricular hypertrophy in hypertensive children and adolescents: predictors and prevalence. Curr Hypertens Rep 15(5):453–457. https://doi.org/10.1007/s11906-013-0370-3
Zhang T, Li S, Bazzano L, He J, Whelton P, Chen W (2018) Trajectories of childhood blood pressure and adult left ventricular hypertrophy: the Bogalusa Heart Study. Hypertension 72(1):93–101. https://doi.org/10.1161/HYPERTENSIONAHA.118.10975
Dusan P, Tamara I, Goran V, Gordana ML, Amira PA (2015) Left ventricular mass and diastolic function in obese children and adolescents. Pediatr Nephrol 30(4):645–652. https://doi.org/10.1007/s00467-014-2992-3
Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M, CKiD Group (2014) BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol 25(1):167–174. https://doi.org/10.1681/ASN.2012121197
Litwin M, Wuhl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Troger J, Mehls O, Schaefer F (2005) Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16(5):1494–1500. https://doi.org/10.1681/ASN.2004110932
Tawadrous H, Kamran H, Salciccioli L, Schoeneman MJ, Lazar J (2012) Evaluation of arterial structure and function in pediatric patients with end-stage renal disease on dialysis and after renal transplantation. Pediatr Transplant 16(5):480–485. https://doi.org/10.1111/j.1399-3046.2012.01721.x
Savant JD, Betoko A, Meyers KE, Mitsnefes M, Flynn JT, Townsend RR, Greenbaum LA, Dart A, Warady B, Furth SL (2017) Vascular stiffness in children with chronic kidney disease. Hypertension 69(5):863–869. https://doi.org/10.1161/HYPERTENSIONAHA.116.07653
Amann K, Ritz E, Wiest G, Klaus G, Mall G (1994) A role of parathyroid hormone for the activation of cardiac fibroblasts in uremia. J Am Soc Nephrol 4(10):1814–1819
Pecoits-Filho R, Lindholm B, Stenvinkel P (2002) The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—the heart of the matter. Nephrol Dial Transplant 17(suppl_11):28–31
Mitsnefes MM, Betoko A, Schneider MF, Salusky IB, Wolf MS, Juppner H, Warady BA, Furth SL, Portale AA (2018) FGF23 and left ventricular hypertrophy in children with CKD. Clin J Am Soc Nephrol 13(1):45–52. https://doi.org/10.2215/CJN.02110217
Seeherunvong W, Abitbol CL, Chandar J, Rusconi P, Zilleruelo GE, Freundlich M (2012) Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol 27(11):2129–2136. https://doi.org/10.1007/s00467-012-2224-7
de Borst MH, Vervloet MG, ter Wee PM, Navis G (2011) Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 22(9):1603–1609. https://doi.org/10.1681/ASN.2010121251
Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6(6):744–759. https://doi.org/10.1002/emmm.201303716
Haring R, Enserro D, Xanthakis V, Mitchell GF, Benjamin EJ, Hamburg NM, Sullivan L, Nauck M, Wallaschofski H, Vasan RS (2016) Plasma fibroblast growth factor 23: clinical correlates and association with cardiovascular disease and mortality in the Framingham Heart Study. J Am Heart Assoc 5(7):e003486. https://doi.org/10.1161/JAHA.116.003486
Akhabue E, Montag S, Reis JP, Pool LR, Mehta R, Yancy CW, Zhao L, Wolf M, Gutierrez OM, Carnethon MR, Isakova T (2018) FGF23 (Fibroblast Growth Factor-23) and incident hypertension in young and middle-aged adults: the CARDIA study. Hypertension 72(1):70–76. https://doi.org/10.1161/HYPERTENSIONAHA.118.11060
Gafane LF, Schutte R, Van Rooyen JM, Schutte AE (2016) Plasma renin and cardiovascular responses to the cold pressor test differ in black and white populations: the SABPA study. J Hum Hypertens 30(5):346–351. https://doi.org/10.1038/jhh.2015.88
Spence JD, Rayner BL (2018) Hypertension in blacks: individualized therapy based on renin/aldosterone phenotyping. Hypertension 72(2):263–269. https://doi.org/10.1161/HYPERTENSIONAHA.118.11064
Tu W, Eckert GJ, Hannon TS, Liu H, Pratt LM, Wagner MA, Dimeglio LA, Jung J, Pratt JH (2014) Racial differences in sensitivity of blood pressure to aldosterone. Hypertension 63(6):1212–1218. https://doi.org/10.1161/HYPERTENSIONAHA.113.02989
Higashi Y, Oshima T, Ozono R, Nakano Y, Matsuura H, Kambe M, Kajiyama G (1997) Nocturnal decline in blood pressure is attenuated by NaCl loading in salt-sensitive patients with essential hypertension: noninvasive 24-hour ambulatory blood pressure monitoring. Hypertension 30(2):163–167
Sagnella GA (2001) Why is plasma renin activity lower in populations of African origin? J Hum Hypertens 15(1):17–25. https://doi.org/10.1038/sj.jhh.1001127
Stewart AD, Millasseau SC, Dawes M, Kyd PA, Chambers JB, Ritter JM, Chowienczyk PJ (2006) Aldosterone and left ventricular hypertrophy in Afro-Caribbean subjects with low renin hypertension. Am J Hypertens 19(1):19–24. https://doi.org/10.1016/j.amjhyper.2005.06.035
Paquette K, Fernandes RO, Xie LF, Cloutier A, Fallaha C, Girard-Bock C, Mian MOR, Lukaszewski M-A, Mâsse B, El-Jalbout R (2018) Kidney size, renal function, Ang (angiotensin) peptides, and blood pressure in young adults born preterm: the HAPI study. Hypertension 72(4):918–928
Martinez-Aguayo AG, Campino C, Rodriguez-Fernandez M, Poggi H, D’apremont I, Moore R, Garcia H, Solari S, Allende F, Peredo S (2020) Urinary sodium-to-potassium ratio and plasma renin and aldosterone concentrations in normotensive children: implications for the interpretation of results. J Hypertens 38(4):671–678
Yu MC, Yu MS, Yu MK, Lee F, Huang WH (2011) Acute reversible changes of brachial-ankle pulse wave velocity in children with acute poststreptococcal glomerulonephritis. Pediatr Nephrol 26(2):233–239. https://doi.org/10.1007/s00467-010-1590-2
Sie MP, Mattace-Raso FU, Uitterlinden AG, Arp PP, Hofman A, Pols HA, Hoeks AP, Reneman RS, Asmar R, van Duijn CM, Witteman JC (2008) The interleukin-6-174 G/C promoter polymorphism and arterial stiffness; the Rotterdam Study. Vasc Health Risk Manag 4(4):863–869. https://doi.org/10.2147/vhrm.s1693
Viera N, Pedreanez A, Rincon J, Mosquera J (2007) Streptococcal exotoxin B increases interleukin-6, tumor necrosis factor alpha, interleukin-8 and transforming growth factor beta-1 in leukocytes. Pediatr Nephrol 22(9):1273–1281. https://doi.org/10.1007/s00467-007-0501-7
Thomson PD (1997) Renal problems in black South African children. Pediatr Nephrol 11(4):508–512
Jindal AK, Tiewsoh K, Pilania RK (2018) A review of renal disease in children with HIV infection. Infect Dis (Lond) 50(1):1–12. https://doi.org/10.1080/23744235.2017.1371852
McCulloch MI, Ray PE (2008) Kidney disease in HIV-positive children. In: Seminars in nephrology, vol 6. Elsevier, pp 585-594
Van Buynder PG, Gaggin JA, Martin D, Pugsley D, Mathews JD (1992) Streptococcal infection and renal disease markers in Australian aboriginal children. Med J Aust 156(8):537–540
White AV, Hoy WE, McCredie DA (2001) Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. Med J Aust 174(10):492–496
Wong W, Morris MC, Zwi J (2009) Outcome of severe acute post-streptococcal glomerulonephritis in New Zealand children. Pediatr Nephrol 24(5):1021–1026. https://doi.org/10.1007/s00467-008-1086-5
Risch N, Burchard E, Ziv E, Tang H (2002) Categorization of humans in biomedical research: genes, race and disease. Genome Biol 3(7):comment2007. https://doi.org/10.1186/gb-2002-3-7-comment2007
Williams DR, Mohammed SA, Leavell J, Collins C (2010) Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci 1186:69–101. https://doi.org/10.1111/j.1749-6632.2009.05339.x
Sulla V, Zikhali P (2018) Overcoming poverty and inequality in South Africa : an assessment of drivers, constraints and opportunities, vol 1. World Bank Group, Washington, D.C.
Funding
Ruan Kruger is funded by the Department of Science and Technology as the South African Research Chair (SARChI) in the Early Detection and Prevention of Cardiovascular Disease in South Africa – hosted by the Hypertension in Africa Research Team (HART) at the North-West University. He is also a senior member of the South African Medical Research Council Extramural Unit for Hypertension and Cardiovascular Disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Declarations
None to declare.
Additional information
Answers
1. E; 2. C; 3. C; 4. E; 5. E
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kruger, R., Gafane-Matemane, L.F. & Kagura, J. Racial differences of early vascular aging in children and adolescents. Pediatr Nephrol 36, 1087–1108 (2021). https://doi.org/10.1007/s00467-020-04593-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04593-5