Abstract
Complications of chronic kidney disease-associated mineral and bone disorders (CKD-MBD) are frequently observed in pediatric kidney transplant recipients and are associated with high morbidity, including growth failure, leg deformities, bone pain, fractures, osteonecrosis, and vascular calcification. Post-transplant CKD-MBD is mainly due to preexisting renal osteodystrophy and cardiovascular changes at the time of transplantation, glucocorticoid treatment, and reduced graft function. In addition, persistent elevated levels of parathyroid hormone (PTH) and fibroblast growth factor 23 may cause hypophosphatemia, resulting in impaired bone mineralization. Patient monitoring should include assessment of growth, leg deformities, and serum levels of calcium, phosphate, magnesium, alkaline phosphatase, 25-hydroxyvitamin D, and PTH. Therapy should primarily focus on regular physical activity, preservation of transplant function, and steroid-sparing immunosuppressive protocols. In addition, adequate monitoring and treatment of vitamin D and mineral metabolism including vitamin D supplementation, oral phosphate, and/or magnesium supplementation, in case of persistent hypophosphatemia/hypomagnesemia, and treatment with active vitamin D in cases of persistent secondary hyperparathyroidism. The latter should be done using the minimum PTH-suppressive dosages aiming at the recommended CKD stage-dependent PTH target range. Finally, treatment with recombinant human growth hormone should be considered in patients lacking catch-up growth within the first year after transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mineral and bone disorders (MBD) are a major cause of morbidity in pediatric kidney transplant recipients and include growth failure, bone pain, fractures, and ectopic (vascular) calcification [1,2,3,4]. Complications in chronic kidney disease-associated MBD (CKD-MBD) are frequently observed after kidney transplantation (KTx), even with completely restored kidney function and are given in Table 1. Children suffering from end-stage CKD (ESKD) may already present with considerable complications in CKD-MBD at the time of KTx. Thus, the degree of preexisting renal osteodystrophy and cardiovascular changes are a major contributing factor to CKD-MBD after KTx. This is especially of importance in patients suffering from metabolic bone disease due to primary diseases such as nephropathic cystinosis [5]. Several other risk factors have also been identified, including immunosuppression (steroids, calcineurin inhibitors), alterations in the parathyroid hormone (PTH)—vitamin D—fibroblast growth factor 23 (FGF23) axis, changes in mineral metabolism (hypophosphatemia, hypomagnesemia), acidosis, unhealthy diet, reduced physical activity, muscle deficits, and impaired graft function [3, 4]. Kidney transplantation may correct some of the underlying risk factors for CKD-MBD, e.g., secondary hyperparathyroidism (SHPT), but may also introduce new ones, e.g., glucocorticoid-induced growth suppression. Thus, optimum management of these risk factors is crucial for children facing a lifetime with CKD. This review summarizes recent advances in the understanding of the pathophysiology, prevention, and treatment of CKD-MBD post KTx in children.
Pathophysiology
Preexisting mineral metabolism alterations
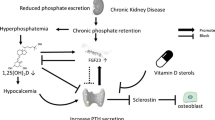
Children undergoing renal transplantation may already present with severe mineral metabolism alterations. In early CKD, high circulating FGF23 is the earliest detectable abnormality of mineral metabolism [6, 7]. FGF23 plasma concentrations start to rise as early as CKD stage 2, most likely due to an elevated phosphate load, in order to keep serum phosphate levels within the normal range by decreasing renal phosphate reabsorption and inhibiting renal synthesis of active vitamin D (calcitriol), which in turn reduces phosphate reabsorption from the gut. In addition, low levels of Klotho—the co-receptor for FGF23—may partially induce FGF23 resistance, resulting in a compensatory increase in FGF23 serum levels to maintain phosphate homeostasis. However, as renal function further declines, calcitriol deficiency results in hypocalcemia which, together with an increasing phosphate load, stimulates the synthesis of PTH by the parathyroid gland [6]. Increased PTH stimulates phosphaturia, renal 1α hydroxylase, and calcium resorption from the bone [8]. Elevated PTH levels are present in about 50% of pediatric CKD patients with an estimated glomerular filtration rate (eGFR) < 50 mL/min/1.73 m2 [7]. This allows the body to counterbalance the calcitriol deficiency-induced hypocalcemia and to keep serum phosphate levels within the normal range, despite advanced CKD, until the system decompensates and severe complications of CKD-MBD occur, i.e., renal osteodystrophy including bone pain, fractures, rickets, leg deformity, and growth failure, as well as ectopic (vascular) calcification and left ventricular hypertrophy [2, 6, 8]. Severe SHPT is associated with high bone turnover, ectopic calcification, anemia, left ventricular hypertrophy, and increased mortality in CKD patients [9,10,11,12,13,14,15]. Unfortunately, dialysis cannot reverse changes in CKD-MBD in children with ESKD and complications such as renal osteodystrophy and cardiovascular changes will progress in the majority of patients [16]. Indeed, high bone turnover, impaired bone mineralization, short stature, coronary artery calcifications, and left ventricular hypertrophy are noted in approximately 57%, 48%, 39%, 92%, and 48% of pediatric patients undergoing long-term dialysis, respectively [9, 10, 13, 17].
Changes in mineral metabolism after transplantation
The hypothetical course of circulating phosphate, PTH, and FGF23 in a patient undergoing KTx is illustrated in Fig. 1 [18]. This graph was originally based on data obtained in adult renal allograft recipients, but has also been recently confirmed in children [19]. Before KTx, the circulating levels of all three parameters increase in parallel with decreasing renal function. At the time of KTx, patients may present with excessively high levels of FGF23 and PTH. After KTx (recovery period), FGF23 and PTH may remain elevated for several months despite restored renal function. Both elevated PTH and FGF23 may contribute to the development of post-transplantation hypophosphatemia which has been noted in up to 10% of pediatric patients [19]. After the recovery period, all parameters may return to the normal range, although PTH can remain high in the case of tertiary hyperparathyroidism. In the long-term, graft function may be impaired resulting in reduced GFR and all three parameters may begin to increase again in the same order as in the pre-transplant period, i.e., starting with FGF23, followed by elevated PTH. Thus, patients with impaired graft function are prone to progressive CKD-MBD.
Graphical overview of the hypothetical course of serum phosphate, parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23) levels in patients with CKD undergoing kidney transplantation (KTx). Before KTx, the circulating levels of all three parameters increase in parallel with the renal function decline. At the time of KTx, patients may have excessively high levels of FGF23 and PTH. After KTx (recovery period), FGF23 and PTH levels can remain high for months, despite restored renal function; this effect may contribute to the development of post-transplantation hypophosphatemia. After the recovery period, all parameters may return to the normal range, although PTH can remain high in the case of tertiary hyperparathyroidism. With impaired graft function, levels of all three parameters can increase again, starting with FGF23, as observed in the pre-transplantation CKD setting. CKD, chronic kidney disease. Figure reproduced with permission from Baia et al. [18].
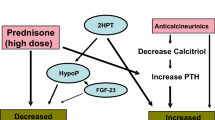
Although PTH levels usually decline in the majority of patients undergoing KTx, persistent SHPT after 12 months has been observed in 10–60% of patients [20, 21]. This was especially noted in cases of severe SHPT or tertiary hyperparathyroidism prior to KTx. Pre-transplant elevation of FGF23 is the strongest predictor of post-transplant elevation of FGF23 in children, and FGF23 levels independently predict hypophosphatemia and low 1,25-dihydroxyvitamin D levels [19]. Both may result in decreased osteoblast activity and progressive bone demineralization [3, 19]. In addition, low levels of 25-hydroxyvitamin D (25(OH)D) were noted in 50% of pediatric KTx patients and are associated with short stature and hypertension [22].
Hypomagnesemia
Hypomagnesemia has been shown to occur in approximately 40% of children after renal transplantation and is most likely due to magnesium wasting, secondary to the use of calcineurin inhibitors (CNI) [23, 24]. Magnesium deficiency may contribute to the development of osteoporosis as it is an integral component of the hydroxyapatite structure in the bone. It may also impair the magnesium-dependent hydrogen-potassium ATPase pump in bone cells, resulting in decreased pH of the extracellular matrix and, consequently, enhanced bone demineralization. In addition, magnesium deficiency is shown to impair PTH secretion and contribute to PTH resistance in target tissues in CKD patients [25]. In adult renal transplant recipients, hypomagnesemia is significantly associated with persistent SHPT 5 years after transplantation [26]. Hypomagnesemia has been associated with the development of new-onset diabetes after KTx and with decreased bone mineral content and low bone mineral density in malnourished children [27, 28]. Similarly, magnesium supplementation improved bone mineral content in healthy girls with a low dietary magnesium intake (< 220 mg/day) compared with controls [29].
Metabolic acidosis
Metabolic acidosis (serum bicarbonate < 22 mEq/L) is present in about 30% of pediatric transplant recipients and usually occurs when GFR is below 50% of norm, although nutritional intake (protein and acid load), catabolism, and alterations in electrolyte balance contribute to its development [30]. Subsequent metabolic and endocrine aberrations are triggered by metabolic acidosis and aggravate uremic growth failure. In fact, metabolic acidosis is significantly associated with decreased height gain and increased protein breakdown in children with CKD prior to and after KTx [31, 32]. Studies on metabolic acidosis in uremic animals have revealed a complex pattern of interrelated pathophysiological reactions [33]. Metabolic acidosis increases glucocorticoid production and protein degradation while concomitantly suppressing spontaneous pituitary growth hormone (GH) secretion and decreasing expression of the growth hormone (GH) receptor and insulin-like growth factor-I (IGF-I) receptor and decreasing IGF-I serum concentrations; these effects highlight the necessity for adequate control of metabolic acidosis in children with CKD [34, 35].
Immunosuppression
Glucocorticoids have a major impact on bone health. They are known to decrease bone formation, increase bone resorption, decrease calcium absorption, increase calcium wasting, decrease vitamin D, and increase PTH. In addition, they are known to impair gonadal function, and IGF-I synthesis [36,37,38,39]. A recent study in rodents showed that glucocorticoid treatment may impair bone growth via upregulation of FGF23 and FGF receptor 3 expression [40]. In line with this, pediatric KTx patients showed lower FGF23 serum levels after steroid withdrawal compared with controls kept on chronic treatment [40]. All of the aforementioned effects may contribute to impaired linear growth, osteonecrosis, fractures, and persistent deficits in cortical thickness, which are frequently noted in pediatric KTx patients on long-term glucocorticoid treatment [41].
Calcineurin inhibitors, such as cyclosporine A and tacrolimus, are known to inhibit synthesis of the vitamin D receptor and osteoprotegerin and to cause high-turnover osteoporosis [42]. In addition, they were shown to be associated with hypomagnesemia and increased PTH levels in adult KTx patients. However, their impact on bone health in pediatric renal transplant recipients remains to be clarified.
Experimental data shows evidence that treatment with mammalian target of Rapamycin (mTOR) inhibitors, including everolimus and sirolimus, negatively impacts osteoblast differentiation and growth plate structure and function [43, 44]. Treatment with sirolimus resulted in impaired linear growth and altered vascular invasion in the growth plate when given to young rats [43]. However, in case-control studies, similar growth rates were noted in transplanted children with and without mTOR inhibitor treatment [45, 46]. There is no evidence that treatment with mycophenolate mofetil or azathioprine impairs bone health.
Bone health and cardiovascular morbidity after transplantation
Bone deformities and fractures
Studies on long-term follow-up in pediatric KTx patients surviving into adulthood demonstrate a high burden of skeletal morbidity. Bartosh et al. showed a 41% prevalence of bone-joint abnormalities including genu varum an valgum, and a 23% prevalence of fractures [47]. Groothoff et al. also reported bone disease in 35% of patients, including disabling bone disorders (17.3%) and aseptic bone necrosis (11.8%) [48]. A markedly increased rate of vertebral fractures, as well as scoliosis, back pain, and disc degeneration, was noted in children after solid organ transplantation—the majority of whom received renal transplants [49, 50]. In the most recent study, a 10% prevalence of fractures was noted in children treated with concomitant glucocorticoid within the first 6 months post KTx [41]. Thus, despite a substantial improvement over the last 2 decades, transplanted children still suffer a high burden with CKD-MBD-associated complications.
Bone histomorphometry
Almost 100% of adult KTx patients show histological evidence of renal osteodystrophy [51, 52]. The most common manifestation is low bone turnover, which has been reported in up to 50% of patients. High bone turnover is associated with SHPT and observed in about 25–50% of adult KTx patients. By contrast, impaired mineralization is rarely observed (< 5%). Most studies in adults report a decline in bone formation and mineralization in the late post-transplant period [51,52,53,54,55]. A recent prospective study in adult patients undergoing bone biopsy while on dialysis and 2 years after KTx, or 2 years after baseline if KTx was not performed, showed a similar decrease in bone turnover over time in both groups [56].
Bone histology is rarely performed in pediatric KTx patients due to its invasiveness and cost. In a cross-sectional study, 10% of pediatric renal allograft recipients presented with adynamic bone disease and 23% of patients with high bone turnover [57]. The finding of persistent renal osteodystrophy in about 30% of patients, despite successful transplantation, is probably due to preexisting severe CKD-MBD related to long-term dialysis, persistent SHPT, the use of glucocorticoids, and/or vitamin D deficiency.
Bone mineral density and cortical structure
Bone mineral density (BMD) assessed by the two-dimensional technique dual-energy X-ray absorption appears to be normal in pediatric KTx patients when data is corrected for the degree of growth retardation [58]. Peripheral quantitative computed tomography (pQCT) is a three-dimensional technique which allows differentiation between trabecular and cortical bone. In addition, it measures volumetric BMD and bone dimensions [59]. In three cross-sectional studies, height-adjusted cortical thickness was found to be reduced in pediatric KTx patients when compared with controls [60,61,62]. In a prospective study, a reduced mean section modulus, which is a measure for bone strength, and a reduced muscle mass was noted in pediatric patients at the time of KTx compared with controls [41]. By contrast, trabecular BMD was significantly increased compared with controls in children aged below 13 years. Since SHPT results in the transformation of metaphyseal spongiosa, this finding is most likely due to PTH effects on the metaphysis. After KTx, cortical thickness improved significantly in this patient cohort. However, the section modulus did not improve within 12 months post-KTx, indicating persistent impaired bone strength in the patients despite marked improvement of SHPT and excellent graft function in the majority of patients. This may explain, at least partly, the high frequency of bone fractures (10% within 6 months post KTx) in this study. The persistent cortical deficits in pediatric KTx patients in the above-mentioned studies are most likely due to concomitant glucocorticoid treatment. However, pQCT data in transplanted children with complete steroid avoidance, or after steroid withdrawal, is lacking.
Growth
Although many of the metabolic and endocrine disorders contributing to uremic growth failure are resolved by renal transplantation, post-transplant catch-up growth is usually restricted to young children and occurs far from regularly [30, 47, 48, 63]. Persistent short stature is reported in about half of pediatric kidney transplant recipients. Beyond transplant function, age and extent of stunting at the time of KTx and glucocorticoid dosage is inversely associated with longitudinal growth.
Cardiovascular morbidity
Although KTx improves survival, subclinical cardiovascular organ damage is frequently noted in pediatric KTx patients, including left ventricular hypertrophy (43%), arterial stiffness (22%), atherosclerosis (58%), and endothelial dysfunction (77%) [64, 65]. Several risk factors have been shown to be associated with cardiovascular organ damage in these patients, e.g., hypertension, low eGFR, elevated body mass index, and treatment with mTOR inhibitors and glucocorticoids [64, 65]. It is important to note that the progression of vascular organ damage (aortal pulse wave velocity, carotid intima-media thickness) is significantly prevented by preemptive KTx when compared with initiating dialysis in children with ESKD [66]. By contrast, changes in left ventricular mass index were strongly associated with increased blood pressure but not with the mode of renal replacement therapy (dialysis or preemptive KTx). This highlights the need for stringent blood pressure control in KTx patients.
Coronary artery calcification is noted in 17–92% of children and young adults with childhood-onset ESKD [16]. Its presence is significantly associated with age, dialysis duration, serum phosphate, calcium, PTH, and c-reactive protein levels. Renal transplantation slows the rate of coronary artery calcification in patients with ESKD, but despite largely normalized serum calcium, phosphate, and PTH levels, they usually do not regress, at least in adults [67, 68].
Evaluation
As for every pediatric CKD patient, an anamnesis should be taken, including bone pain and walking difficulties, together with a thorough clinical assessment, including height, weight, signs of rickets or leg bowing, and calculation of annual height velocity, which should be done at regular intervals (Table 2) [69]. Young children and those who presented previously with clinical signs of CKD-MBD or impaired graft function should be seen more often. There is no evidence for performing regular X-rays in pediatric KTx patients. However, an X-ray of the left wrist should be considered in cases of persistent bone pain or SHPT to detect signs of demineralization and rickets and to establish growth potential (open epiphysis) in patients who are candidates for treatment with recombinant human GH (rhGH). In addition, calcium, phosphate, magnesium, alkaline phosphatase (ALP), PTH, and 25(OH)D levels should be regularly monitored [1, 70, 71]. The regular follow-up intervals as recommended by KDIGO are given in Table 3. These parameters should be considered together, with particular attention to trends in values [71]. Unfortunately, the above-mentioned biochemical parameters are poor predictors of bone disease, e.g., presence of impaired mineralization and high or low turnover. Therefore, KDIGO recommends assessment of bone histomorphometry, if the type of renal osteodystrophy will impact treatment decisions. However, this is rarely the case in transplanted children and may be considered in patients with unexplained fractures, especially when anticipating an underlying metabolic bone disease due to nephropathic cystinosis or primary hyperoxaluria. In adult KTx patients with an eGFR > 30 mL/min/1.73m2, KDIGO suggests using BMD to assess whether fracture risk results will alter therapy. As mentioned above, BMD values are normal in pediatric KTx patients when normalized to height and data on the predictive value of BMD measurements in assessing fracture risk in these patients is lacking. Therefore, there is currently no evidence for its clinical use in this population. The same holds true for newer techniques such as high-resolution pQCT or MRI.
Treatment options
In general, a lifestyle including a healthy diet, regular physical activity (a minimum of 30 min on most days of the week), and not smoking is recommended, as for other CKD patients. Patients should be provided with an adequate dietary calcium and phosphate intake (at least 100% of daily recommended intake in healthy children) to allow for bone mineralization and growth [1, 69]. Patients should avoid foods high in salt as high sodium intake promotes hypertension and hypercalciuria [72]. The latter may impair bone formation. In addition, a high intake of cola drinks should be avoided as this has been linked to decreased BMD and increased fracture risk in the general pediatric population and delays bone healing in rodents [73].
Correction of alterations of vitamin D and mineral metabolism
KDIGO recommends that treatment choices be influenced by the presence of CKD-MBD, as indicated by abnormal levels of calcium, phosphate, PTH, ALP, and 25(OH)D [1, 70]. It is important to note that these parameters should be considered together with particular attention to trends in values. KDIGO also recommends considering treatment with vitamin D analogs or bisphosphonates to treat bone disease in adult patients with an eGFR above 30 mL/min/1.73 m2 during the first 12 months after KTx. However, there is no evidence to recommend treatment with bisphosphonates in kidney-transplanted children.
We suggest evaluation of vitamin D deficiency as the first step, as it is present in approximately 50% of pediatric KTx patients and may promote hypophosphatemia and SHPT [22]. Oral vitamin D supplementation with cholecalciferol or ergocalciferol was recommended in vitamin D-deficient KTx patients aiming at 25(OH)D target levels of 75–12 nmol/L (30–50 ng/mL) as in CKD patients prior to transplantation [69, 74]. In the second step, treatment with active vitamin D should be considered, in the presence of PTH levels above the target range, based on the stage of CKD, if vitamin D deficiency is absent or corrected [1]. We suggest applying the minimum PTH-suppressive dosages as recommended for CKD patients prior to KTx [75]. It is important to note that there is no agreement on the optimum PTH target range and consequently recommended CKD-stage-dependent PTH target range values differ widely [69, 76,77,78,79]. However, most important is the acknowledgment that none of these recommendations have been validated in a large pediatric CKD cohort study/investigation, especially in children after renal transplantation. Parathyroidectomy should be considered in patients with persistent severe, therapy-refractory SHPT, i.e., with radiological indications and hypercalcemia [78].
In patients showing persistent post-transplant hypophosphatemia, a high phosphate diet and initiation of oral phosphate supplementation is recommended in order to reach low normal levels (for age). However, phosphate supplementation may stimulate both PTH and FGF23 levels, which may further stimulate renal phosphate wasting, causing a vicious circle. Therefore, the lowest possible phosphate dosages should be applied in these patients.
Magnesium deficiency is known to promote osteoporosis and PTH resistance, and it should be corrected with oral magnesium supplementation aiming at levels above the lower normal limit.
Correction of acidosis
Metabolic acidosis should be corrected by oral bicarbonate, aiming for bicarbonate levels above 22 mEq/L, as recommended in other CKD patients [69]. However, this may not be possible in all patients because high doses of sodium bicarbonate may promote hypertension.
Steroid avoidance
A meta-analysis of 5 randomized clinical trials (RCTs) on growth outcome using steroid minimization protocols in pediatric KTx patients showed a significant improvement in height z scores in the steroid-avoidance group, particularly within the first year after steroid withdrawal and in prepubertal patients [80]. Therefore, it is recommended to minimize or completely avoid glucocorticoid use in children who have growth potential, if possible.
Growth hormone treatment
Several RCTs have shown the benefit of rhGH therapy in short pediatric KTx patients. A meta-analysis of 5 RCTs demonstrated that patients receiving rhGH therapy had a significantly higher height velocity 1 year after initiation of therapy than the control group, with a mean difference in height z score of 0.68 (95% CI 0.25–1.11) [81]. In addition, treatment with rhGH resulted in increased osteoblast activity, bone formation, and turnover in short pediatric KTx patients [82]. Consequently, a recent European guideline recommends initiating rhGH therapy 1 year after transplantation if spontaneous catch-up growth does not occur—defined as a height below the third percentile for age and sex and a growth velocity below the twenty-fifth percentile—and steroid-free immunosuppression is not a feasible option. The latter may be the case in patients with a high immunological risk, particularly in children with suboptimal graft function (eGFR < 50 mL/min/1.73 m2) [83]. Growth hormone should be given at a dose of 0.045–0.05 mg/kg body weight per day by subcutaneous injection in the evening and parents and physicians may encourage children from about 8–10 years of age to do the rhGH injections on their own, if adequate training and adherence is ensured. Clinical visits every 3–6 months are recommended to monitor height, growth velocity, pubertal development, skeletal maturation on wrist radiography, renal function, thyroid hormone levels, and serum glucose levels. If growth velocity in the first year of rhGH treatment is less than 2 cm per year over baseline, then assessment of patient adherence to rhGH therapy, including measurement of serum IGF-I levels and weight-adjusted rhGH dosage, is recommended. Finally, rhGH should be stopped when epiphyseal closure is confirmed [83].
Key summary points
-
Monitoring of CKD-MBD in pediatric kidney transplant recipients should primarily focus on assessment of growth, leg deformities, and serum levels of calcium, phosphate, magnesium, bicarbonate, alkaline phosphatase, 25(OH)D, and PTH.
-
Regular physical activity, healthy diet, and preservation of transplant function are recommended.
-
Steroid-sparing immunosuppressive protocols and adequate treatment of alterations in vitamin D, phosphate, alkaline phosphatase, calcium, and PTH as well as correction of metabolic acidosis are recommended.
-
Treatment with active vitamin D is recommended in case of persistent secondary hyperparathyroidism, using the minimum PTH-suppressive dosages and aiming for the recommended CKD stage-dependent PTH target range.
-
Treatment with recombinant human growth hormone should be considered in patients lacking catch-up growth within the first year after renal transplantation.
Multiple choice questions
-
1.
What is a typical clinical feature of post-transplant CKD-MBD?
-
a.
Bone pain
-
b.
Delayed sexual maturation
-
c.
Increased bone mineral density
-
d.
Low bone turnover
-
a.
-
2.
Post-transplant CKD-MBD is often due to
-
a.
Mycophenolate mofetil treatment
-
b.
Decreased FGF23 levels
-
c.
Glucocorticoid treatment
-
d.
Preemptive renal transplantation
-
a.
-
3.
Management of post-transplant CKD-MBD does focus on
-
a.
Maintenance of regular physical activity
-
b.
High sodium intake
-
c.
High dose treatment with active vitamin D
-
d.
Treatment with bisphosphonates
-
a.
-
4.
Treatment with recombinant human growth hormone should be considered
-
a.
Within 12 months post transplantation
-
b.
If height velocity is below the 30th percentile for age and gender
-
c.
If eGFR is above 50 mL/min/1.73 m2
-
d.
If height is below the 3rd percentile for age and gender
-
e.
In case of concomitant glucocorticoid therapy
-
a.
-
5.
Which statement regarding control of PTH levels is right?
-
a.
Correction of vitamin D deficiency should be done before starting active vitamin D
-
b.
Active vitamin D should be started within the first three months after transplantation
-
c.
Hypercalcemia stimulates PTH levels
-
d.
PTH levels should be above 2 times the upper limit of normal
-
a.
References
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group (2017) KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 7:1–59
Wesseling-Perry K, Bacchetta J (2011) CKD-MBD after kidney transplantation. Pediatr Nephrol 26:2143–2151
Sgambat K, Moudgil A (2014) Optimization of bone health in children before and after renal transplantation: current perspectives and future directions. Front Pediatr 2:13
Haffner D, Schuler U (2014) Metabolic bone disease after renal transplantation. Curr Opin Pediatr 26:198–206
Hohenfellner K, Rauch F, Ariceta G, Awan A, Bacchetta J, Bergmann C, Bechtold S, Cassidy N, Deschenes G, Elenberg E, Gahl WA, Greil O, Harms E, Herzig N, Hoppe B, Koeppl C, Lewis MA, Levtchenko E, Nesterova G, Santos F, Schlingmann KP, Servais A, Soliman NA, Steidle G, Sweeney C, Treikauskas U, Topaloglu R, Tsygin A, Veys K, Vigier VR, Zustin J, Haffner D (2019) Management of bone disease in cystinosis: statement from an international conference. J Inherit Metab Dis 42:1019–1029
Hu MC, Shiizaki K, Kuro-o M, Moe OW (2013) Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75:503–533
Portale AA, Wolf M, Juppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB (2014) Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9:344–353
Hruska KA, Seifert M, Sugatani T (2015) Pathophysiology of the chronic kidney disease-mineral bone disorder. Curr Opin Nephrol Hypertens 24:303–309
Bakkaloglu SA, Borzych D, Soo Ha I, Serdaroglu E, Buscher R, Salas P, Patel H, Drozdz D, Vondrak K, Watanabe A, Villagra J, Yavascan O, Valenzuela M, Gipson D, Ng KH, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network (2011) Cardiac geometry in children receiving chronic peritoneal dialysis: findings from the international pediatric peritoneal dialysis network (IPPN) registry. Clin J Am Soc Nephrol 6:1926–1933
Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, Zambrano P, Ahlenstiel T, Bakkaloglu SA, Spizzirri AP, Lopez L, Ozaltin F, Printza N, Hari P, Klaus G, Bak M, Vogel A, Ariceta G, Yap HK, Warady BA, Schaefer F, International Pediatric PD Network (IPPN) (2010) The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int 78:1295–1304
Salusky IB, Ramirez JA, Oppenheim W, Gales B, Segre GV, Goodman WG (1994) Biochemical markers of renal osteodystrophy in pediatric patients undergoing CAPD/CCPD. Kidney Int 45:253–258
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342:1478–1483
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106:100–105
Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD (2006) Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70:771–780
Denburg MR, Kumar J, Jemielita T, Brooks ER, Skversky A, Portale AA, Salusky IB, Warady BA, Furth SL, Leonard MB (2016) Fracture burden and risk factors in childhood CKD: results from the CKiD cohort study. J Am Soc Nephrol 27:543–550
Querfeld U, Schaefer F (2018) Cardiovascular risk factors in children on dialysis: an update. Pediatr Nephrol. https://doi.org/10.1007/s00467-018-4125-x
Bakkaloglu SA, Wesseling-Perry K, Pereira RC, Gales B, Wang HJ, Elashoff RM, Salusky IB (2010) Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol 5:1860–1866
Baia LC, Heilberg IP, Navis G, de Borst MH, NIGRAM investigators (2015) Phosphate and FGF-23 homeostasis after kidney transplantation. Nat Rev Nephrol 11:656–666
Wesseling-Perry K, Pereira RC, Tsai E, Ettenger R, Juppner H, Salusky IB (2013) FGF23 and mineral metabolism in the early post-renal transplantation period. Pediatr Nephrol 28:2207–2215
Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH (2000) High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol 11:1093–1099
Weisinger JR, Carlini RG, Rojas E, Bellorin-Font E (2006) Bone disease after renal transplantation. Clin J Am Soc Nephrol 1:1300–1313
Shroff R, Knott C, Gullett A, Wells D, Marks SD, Rees L (2011) Vitamin D deficiency is associated with short stature and may influence blood pressure control in paediatric renal transplant recipients. Pediatr Nephrol 26:2227–2233
Uslu Gokceoglu A, Comak E, Dogan CS, Koyun M, Akbas H, Akman S (2014) Magnesium excretion and hypomagnesemia in pediatric renal transplant recipients. Ren Fail 36:1056–1059
Mazzola BL, Vannini SD, Truttmann AC, von Vigier RO, Wermuth B, Ferrari P, Bianchetti MG (2003) Long-term calcineurin inhibition and magnesium balance after renal transplantation. Transpl Int 16:76–81
Castiglioni S, Cazzaniga A, Albisetti W, Maier JA (2013) Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients 5:3022–3033
Van de Cauter J, Sennesael J, Haentjens P (2011) Long-term evolution of the mineral metabolism after renal transplantation: a prospective, single-center cohort study. Transplant Proc 43:3470–3475
Ozturk CF, Karakelleoglu C, Orbak Z, Yildiz L (2012) The effect of serum magnesium levels and serum endothelin-1 levels on bone mineral density in protein energy malnutrition. West Indian Med J 61:213–218
Hayes W, Boyle S, Carroll A, Bockenhauer D, Marks SD (2017) Hypomagnesemia and increased risk of new-onset diabetes mellitus after transplantation in pediatric renal transplant recipients. Pediatr Nephrol 32:879–884
Carpenter TO, DeLucia MC, Zhang JH, Bejnerowicz G, Tartamella L, Dziura J, Petersen KF, Befroy D, Cohen D (2006) A randomized controlled study of effects of dietary magnesium oxide supplementation on bone mineral content in healthy girls. J Clin Endocrinol Metab 91:4866–4872
Franke D, Thomas L, Steffens R, Pavicic L, Gellermann J, Froede K, Querfeld U, Haffner D, Zivicnjak M (2015) Patterns of growth after kidney transplantation among children with ESRD. Clin J Am Soc Nephrol 10:127–134
Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE (1996) The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 97:1447–1453
Boirie Y, Broyer M, Gagnadoux MF, Niaudet P, Bresson JL (2000) Alterations of protein metabolism by metabolic acidosis in children with chronic renal failure. Kidney Int 58:236–241
Brungger M, Hulter HN, Krapf R (1997) Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: new cause of growth hormone insensitivity in humans. Kidney Int 51:216–221
Challa A, Chan W, Krieg RJ, Thabet MA, Liu F, Hintz RL, Chan JC (1993) Effect of metabolic acidosis on the expression of insulin-like growth factor and growth hormone receptor. Kidney Int 44:1224–1227
Challa A, Krieg RJ, Thabet MA, Veldhuis JD, Chan JC (1993) Metabolic acidosis inhibits growth hormone secretion in rats: mechanism of growth retardation. Am J Physiol 265:E547–E553
Suzuki Y, Ichikawa Y, Saito E, Homma M (1983) Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metabolism 32:151–156
O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841
Brandenburg VM, Ketteler M, Heussen N, Politt D, Frank RD, Westenfeld R, Ittel TH, Floege J (2005) Lumbar bone mineral density in very long-term renal transplant recipients: impact of circulating sex hormones. Osteoporos Int 16:1611–1620
Klaus G, Jux C, Leiber K, Hugel U, Mehls O (1996) Interaction between insulin-like growth factor I, growth hormone, parathyroid hormone, 1 alpha,25-dihydroxyvitamin D3 and steroids on epiphyseal chondrocytes. Acta Paediatr Suppl 417:69–71
Delucchi A, Toro L, Alzamora R, Barrientos V, Gonzalez M, Andaur R, Leon P, Villanueva F, Galindo M, Heras FL, Montecino M, Moena D, Lazcano A, Pinto V, Salas P, Reyes ML, Mericq V, Michea L (2019) Glucocorticoids decrease longitudinal bone growth in paediatric kidney transplant recipients by stimulating the FGF23/FGFR3 signalling pathway. J Bone Miner Res. https://doi.org/10.1002/jbmr.3761
Terpstra AM, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Foster BJ, Strife CF, Foerster DL, Leonard MB (2012) Bone density and cortical structure after pediatric renal transplantation. J Am Soc Nephrol 23:715–726
Epstein S (1996) Post-transplantation bone disease: the role of immunosuppressive agents and the skeleton. J Bone Miner Res 11:1–7
Alvarez-Garcia O, Carbajo-Perez E, Garcia E, Gil H, Molinos I, Rodriguez J, Ordonez FA, Santos F (2007) Rapamycin retards growth and causes marked alterations in the growth plate of young rats. Pediatr Nephrol 22:954–961
Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G (2008) Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem 103:434–446
Billing H, Burmeister G, Plotnicki L, Ahlenstiel T, Fichtner A, Sander A, Hocker B, Tonshoff B, Pape L (2013) Longitudinal growth on an everolimus- versus an MMF-based steroid-free immunosuppressive regimen in paediatric renal transplant recipients. Transpl Int 26:903–909
Forster J, Ahlenstiel-Grunow T, Zapf A, Mynarek M, Pape L (2016) Pubertal development in pediatric kidney transplant patients receiving mammalian target of rapamycin inhibitors or conventional immunosuppression. Transplantation 100:2461–2470
Bartosh SM, Leverson G, Robillard D, Sollinger HW (2003) Long-term outcomes in pediatric renal transplant recipients who survive into adulthood. Transplantation 76:1195–1200
Groothoff JW, Cransberg K, Offringa M, van de Kar NJ, Lilien MR, Davin JC, Heymans HS, Dutch cohort study (2004) Long-term follow-up of renal transplantation in children: a dutch cohort study. Transplantation 78:453–460
Helenius I, Remes V, Salminen S, Valta H, Makitie O, Holmberg C, Palmu P, Tervahartiala P, Sarna S, Helenius M, Peltonen J, Jalanko H (2006) Incidence and predictors of fractures in children after solid organ transplantation: a 5-year prospective, population-based study. J Bone Miner Res 21:380–387
Helenius I, Remes V, Tervahartiala P, Salminen S, Sairanen H, Holmberg C, Palmu P, Helenius M, Peltonen J, Jalanko H (2006) Spine after solid organ transplantation in childhood: a clinical, radiographic, and magnetic resonance imaging analysis of 40 patients. Spine (Phila Pa 1976) 31:2130–2136
Lehmann G, Ott U, Stein G, Steiner T, Wolf G (2007) Renal osteodystrophy after successful renal transplantation: a histomorphometric analysis in 57 patients. Transplant Proc 39:3153–3158
Rojas E, Carlini RG, Clesca P, Arminio A, Suniaga O, De Elguezabal K, Weisinger JR, Hruska KA, Bellorin-Font E (2003) The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int 63:1915–1923
Cruz EA, Lugon JR, Jorgetti V, Draibe SA, Carvalho AB (2004) Histologic evolution of bone disease 6 months after successful kidney transplantation. Am J Kidney Dis 44:747–756
Borchhardt K, Sulzbacher I, Benesch T, Fodinger M, Sunder-Plassmann G, Haas M (2007) Low-turnover bone disease in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Transplant 7:2515–2521
Cueto-Manzano AM, Konel S, Crowley V, France MW, Freemont AJ, Adams JE, Mawer B, Gokal R, Hutchison AJ (2003) Bone histopathology and densitometry comparison between cyclosporine a monotherapy and prednisolone plus azathioprine dual immunosuppression in renal transplant patients. Transplantation 75:2053–2058
Keronen S, Martola L, Finne P, Burton IS, Kroger H, Honkanen E (2019) Changes in bone histomorphometry after kidney transplantation. Clin J Am Soc Nephrol 14:894–903
Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, Goodman WG (1998) Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int 53:1358–1364
Leonard MB (2005) Assessment of bone mass following renal transplantation in children. Pediatr Nephrol 20:360–367
Leonard MB (2007) A structural approach to the assessment of fracture risk in children and adolescents with chronic kidney disease. Pediatr Nephrol 22:1815–1824
Reusz GS, Szabo AJ, Peter F, Kenesei E, Sallay P, Latta K, Szabo A, Szabo A, Tulassay T (2000) Bone metabolism and mineral density following renal transplantation. Arch Dis Child 83:146–151
Behnke B, Altrogge H, Delling G, Kruse HP, Muller-Wiefel DE (1996) Bone mineral density in pediatric patients after renal transplantation. Clin Nephrol 46:24–29
Ruth EM, Weber LT, Schoenau E, Wunsch R, Seibel MJ, Feneberg R, Mehls O, Tonshoff B (2004) Analysis of the functional muscle-bone unit of the forearm in pediatric renal transplant recipients. Kidney Int 66:1694–1706
Klare B, Montoya CR, Fischer DC, Stangl MJ, Haffner D (2012) Normal adult height after steroid-withdrawal within 6 months of pediatric kidney transplantation: a 20 years single center experience. Transpl Int 25:276–282
Ruben S, Kreuzer M, Buscher A, Buscher R, Thumfart J, Querfeld U, Staude H, Ahlenstiel-Grunow T, Melk A, Fischer DC, Leifheit-Nestler M, Pape L, Haffner D (2018) Impaired microcirculation in children after kidney transplantation: everolimus versus mycophenolate based immunosuppression regimen. Kidney Blood Press Res 43:793–806
Borchert-Morlins B, Thurn D, Schmidt BMW, Buscher AK, Oh J, Kier T, Bauer E, Baig S, Kanzelmeyer N, Kemper MJ, Buscher R, Melk A (2017) Factors associated with cardiovascular target organ damage in children after renal transplantation. Pediatr Nephrol 32:2143–2154
Schmidt BMW, Sugianto RI, Thurn D, Azukaitis K, Bayazit AK, Canpolat N, Eroglu AG, Caliskan S, Doyon A, Duzova A, Karagoz T, Anarat A, Deveci M, Mir S, Ranchin B, Shroff R, Baskin E, Litwin M, Ozcakar ZB, Buscher R, Soylemezoglu O, Dusek J, Kemper MJ, Matteucci MC, Habbig S, Laube G, Wuhl E, Querfeld U, Sander A, Schaefer F, Melk A, 4C Study Consortium (2018) Early effects of renal replacement therapy on cardiovascular comorbidity in children with end-stage kidney disease: findings from the 4C-T study. Transplantation 102:484–492
Hristova M, van Beek C, Schurgers LJ, Lanske B, Danziger J (2010) Rapidly progressive severe vascular calcification sparing the kidney allograft following warfarin initiation. Am J Kidney Dis 56:1158–1162
Moe SM, O'Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA (2004) Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 19:2387–2393
KDOQI Work Group (2009) KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. executive summary. Am J Kidney Dis 53:S11–S104
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9(Suppl 3):S1–S155
Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB (2017) Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int 92:26–36
Ahmed MA, Abd El Samad AA (2013) Benefits of omega-3 fatty acid against bone changes in salt-loaded rats: possible role of kidney. Physiol Rep 1:e00106
Teofilo JM, Leonel DV, Lamano T (2010) Cola beverage consumption delays alveolar bone healing: a histometric study in rats. Braz Oral Res 24:177–181
Shroff R, Wan M, Nagler EV, Bakkaloglu S, Fischer DC, Bishop N, Cozzolino M, Bacchetta J, Edefonti A, Stefanidis CJ, Vande Walle J, Haffner D, Klaus G, Schmitt CP, European Society for Paediatric Nephrology Chronic Kidney Disease Mineral and Bone Disorders and Dialysis Working Groups (2017) Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease stages 2-5 and on dialysis. Nephrol Dial Transplant 32:1098–1113
Shroff R, Wan M, Nagler EV, Bakkaloglu S, Cozzolino M, Bacchetta J, Edefonti A, Stefanidis CJ, Vande Walle J, Ariceta G, Klaus G, Haffner D, Schmitt CP; European Society for Paediatric Nephrology Chronic Kidney Disease Mineral and Bone Disorders and Dialysis Working Groups (2017) Clinical practice recommendations for treatment with active vitamin D analogues in children with chronic kidney disease stages 2-5 and on dialysis. Nephrol Dial Transplant 32:1114-1127
Rees L (2008) What parathyroid hormone levels should we aim for in children with stage 5 chronic kidney disease; what is the evidence? Pediatr Nephrol 23:179–184
Klaus G, Watson A, Edefonti A, Fischbach M, Ronnholm K, Schaefer F, Simkova E, Stefanidis CJ, Strazdins V, Vande Walle J, Schroder C, Zurowska A, Ekim M, European Pediatric Dialysis Working Group (EPDWG) (2006) Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr Nephrol 21:151–159
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 113:S1–S130
Haffner D, Schaefer F (2013) Searching the optimal PTH target range in children undergoing peritoneal dialysis: new insights from international cohort studies. Pediatr Nephrol 28:537–545
Zhang H, Zheng Y, Liu L, Fu Q, Li J, Huang Q, Liu H, Deng R, Wang C (2016) Steroid avoidance or withdrawal regimens in paediatric kidney transplantation: a meta-analysis of randomised controlled trials. PLoS One 11:e0146523
Wu Y, Cheng W, Yang XD, Xiang B (2013) Growth hormone improves growth in pediatric renal transplant recipients--a systemic review and meta-analysis of randomized controlled trials. Pediatr Nephrol 28:129–133
Sanchez CP, Kuizon BD, Goodman WG, Gales B, Ettenger RB, Boechat MI, Wang Y, Elashoff R, Salusky IB (2002) Growth hormone and the skeleton in pediatric renal allograft recipients. Pediatr Nephrol 17:322–328
Drube J, Wan M, Bonthuis M, Wuhl E, Bacchetta J, Santos F, Grenda R, Edefonti A, Harambat J, Shroff R, Tonshoff B, Haffner D, European Society for Paediatric Nephrology Chronic Kidney Disease Mineral and Bone Disorders, Dialysis (2019) Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat Rev Nephrol 15:577–589
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D.H. has received research grants from Sandoz, Kyowa Kirin, Horizon, and Amgen and has received speaker and/or consultant fees from Amgen, Sandoz, Kyowa Kirn, Pfizer, Merck Serono, Horizon, and Chiesi. M.L.N. received travel grants from Amgen.
Additional information
Answers to multiple choice questions:
1. a; 2. c; 3. a; 4. d; 5. a
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haffner, D., Leifheit-Nestler, M. CKD-MBD post kidney transplantation. Pediatr Nephrol 36, 41–50 (2021). https://doi.org/10.1007/s00467-019-04421-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04421-5