Abstract
Background
Atypical hemolytic uremic syndrome (aHUS) is a thrombotic microangiopathy (TMA) characterized by platelet consumption, hemolysis, and organ damage. Eculizumab (ECU), a humanized antibody that blocks complement activity, has been successfully used in aHUS, but the best treatment schedule is not yet clear.
Methods
Here, we report our experience with ECU maintenance treatment and the interval between subsequent doses being extended based on global classical complement pathway (CCP) activity aimed at <30% for maintaining aHUS into remission.
Results
We report on 38 patients with aHUS, 13 children, 21 female, with a median age of 25.0 years (range 0.5–60) at disease onset treated with ECU standard schedule for a median of 2.6 months (range 0.4–24.6). Once stable TMA remission was obtained, the interval between ECU doses was extended based on complement function, with a target CCP activity of <30%. With this approach, 22 patients regularly receive ECU infusion every 28 days and 16 every 21. During a median observation period on ECU, an extended interval of 26.9 months (range 0.8–80.9), with a cumulative observation period of 1,208 months, none of the patients relapsed.
Conclusion
Monitoring complement activity allows a safe reduction in the frequency of ECU administration in aHUS while keeping the disease in remission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a severe, systemic thrombotic microangiopathy (TMA) often related to mutations in genes encoding complement regulatory proteins. Since 2009, Eculizumab (ECU), a humanized recombinant monoclonal IgG antibody that blocks complement component 5 (C5), has been successfully used in the treatment of patients with aHUS [1, 2]. The standard maintenance treatment suggests ECU administration every 2 weeks, life-long, but the best treatment schedule is not yet defined. Recently, KDIGO has promoted an international consensus conference that has suggested that global complement activity might be useful for monitoring ECU treatment with a target of classical complement pathway (CCP) activity <10% [3]. Here, we update our previously published experience [4, 5] on ECU maintenance treatment tailored to global complement activity, as later confirmed by independent groups [6], to prevent relapses with the rationale of improving the patient’s quality of life, reducing the risk of adverse reactions, and minimizing the heavy costs of the treatment.
Materials and methods
Once stable clinical remission had been obtained, patients undergoing ECU treatment for aHUS at our Center were offered the opportunity to increase the interval between subsequent ECU doses. This was done on a routine basis with all patients being treated at our Center. In practice, patients were introduced to a progressive extension of the interval between ECU doses from the standard 2 weeks to 3 or 4 weeks based on global complement functional tests (Wieslab for the classical, alternative, and mannose-binding lectin complement pathways). Complement activity was routinely determined before each ECU administration and the interval between doses was adjusted with the target of maintaining a level of CCP activity <30%. Strict monitoring of indicators of disease reactivation (haptoglobin, lactic dehydrogenase, platelet count, serum creatinine, proteinuria, microalbuminuria, and hematuria) were regularly performed at every ECU administration. Relapses were defined as the concomitant detection of platelet consumption, hemolysis, and signs of renal damage or worsening of already impaired renal function (increase in serum creatinine or proteinuria). As long as target complement activity was completely suppressed (CCP activity <10%) and the markers of disease activity documented TMA remission, the interval between administrations was increased. In contrast, if complement activity was >30%, showing insufficient complement suppression, the interval between doses was shortened. If CCP activity was 10–30%, the current interval was confirmed for the subsequent ECU administration. For the present analysis, CCP activity was defined as “blocked” if <10%, “impaired” if 10–70%, and “normal” if >70%. Additional methodological details are extensively provided in the original paper published previously [3]. The present retrospective study was approved by our institutional Ethical Committee.
Results
Out of 47 patients who were successfully treated with ECU, 17 were able to discontinue dialysis (the remaining 30 had not been dialyzed during the acute phase). Nine patients (19.1%) discontinued ECU treatment after fewer than 6 doses of ECU (early discontinuation) [7,8,9]; therefore, they did not continue maintenance treatment. The interval between doses was extended in the remaining 38 patients (including 13 children; median age at the time of aHUS diagnosis of 25 years with range 0.5–60 years), 25 with native kidney and 13 with kidney graft. Six of them had been switched to ECU from plasma therapy and 8 had previously discontinued ECU treatment; however, since their relapse, treatment had been reinitiated and they continued on the maintenance regimen. The general characteristics of the patients, their complement function, and identified gene mutations are summarized in Table 1. The patients had received ECU treatment for a median of 2.6 months (range 0.4–24.6) before the interval between doses started to be extended. With complement function targeted as described in the materials and methods section, presently 22 patients regularly receive ECU infusion every 28 days and 16 every 21. Only 1 of the patients, who had persistent nephrotic-range proteinuria required a prolonged period (months) of intensive ECU administration (1,200 mg every 2 weeks) to maintain complement activity within the target level. When proteinuria decreased to <1 g/day, his complement activity was completely suppressed and the treatment schedule was changed accordingly (every 21 days).
Regarding the dose of ECU to maintain the target CCP activity; in children of <20 kg body weight, the single dose is currently 300 mg, in those between 20 and 30 kg it is 600 mg, whereas for patients weighing 30–60 kg, it is 900 mg, and in those >60 kg, it is 1,200 mg. The median ECU dose per kilogram of body weight per day of the interval was 0.75 mg/kg/day (interquartile range: 0.67–0.95). Patients were observed on tailored ECU maintenance treatment for a median of 26.9 months (range 0.8–80.9), and during a cumulative observation period of 1,208 months on tailored ECU treatment, no renal or extrarenal relapse was detected. No serious adverse events were observed during the period of extended ECU treatment.
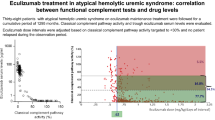
With a total of 709 determinations of global complement activity performed throughout the entire cumulative observation period on tailored ECU maintenance treatment, in 528 (74.5%) complement activity was completely suppressed (CCP ≤ 10%), in 122 (17.1%) it was impaired (CCP activity 10–70%), and in 59 (8.4%), it was within the normal range (CCP > 70%; Fig. 1). No differences in the mean laboratory parameters of TMA activity were observed in patients with impaired activity in comparison with those with blocked activity, particularly with regard to the most sensitive parameters: haptoglobin, proteinuria, and microalbuminuria.
Discussion
Data reported in the present update strengthen the usefulness of complement functional tests for monitoring the frequency of ECU administration for the prevention of relapses, which we previously demonstrated, but with a shorter follow-up period and fewer patients [4, 5]. All patients showed suppression of complement activity after ECU and, although the interval between subsequent drug dosing was progressively increased from the standard 14 days to 21 days and in several cases to 28 days, none of them experienced a disease relapse during a very long cumulative observation period (as many as 100 patient-years). In addition, in many patients with a body weight between 40 and 60 kg, 900 mg of ECU proved to be enough to obtain an adequate complement inhibition for as long as 3–4 weeks. The single patient who required 1,200 mg every 14 days (later postponed to 21 days) persistently had severe proteinuria. We speculate that his increased need for ECU (also observed during pregnancy in another case) might be related to the nephrotic-range proteinuria and the consequent urinary drug loss. The management strategy presented in this present paper differs from the one recently suggested by KDIGO recommendations [3], as we do not necessarily target a blocked complement function (CCP activity <10%), but rather an impairment of its function (CCP activity <30%).
As infections are known to increase the production of C5, thus increasing the need for C5 inhibition, in the case of serious and prolonged infections (such as cytomegalovirus in kidney transplant patients), the interval between subsequent ECU doses was preventively and temporarily reduced. With this approach, again, no relapse was recorded during the entire observation period including periods characterized by incomplete complement inhibition with CCP activity well above complete blockade.
Even the observations with CCP activity within the normal range (although a minority: 8.4% of all determinations) were not associated with relapses, perhaps because patients with normalized CCP activity have remained in the state of unblocked complement activity for too short a period to allow disease reactivation before receiving the next ECU dose. Another possible interpretation is that once the disease is in remission, a relapse requires more than a few days of exposure to incomplete complement inhibition.
We believe that ECU maintenance treatment in aHUS can be individualized based on global complement activity; a tailored approach leads to an improvement in patients’ quality of life and a significant reduction (weighted mean −33%) of the costs of maintenance treatment, which, combined with a policy of treatment discontinuation whenever possible [9], make this highly effective treatment more affordable, with an average cost per patient per year of less than 150,000 Euro.
References
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C (2013) Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368:2169–2181
Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C (2016) Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89:701–711
Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, Nester CM, Noris M, Pickering MC, Rodríguez de Córdoba S, Roumenina LT, Sethi S, Smith RJ (2017) Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 91:539–551
Cugno M, Gualtierotti R, Possenti I, Testa S, Tel F, Griffini S, Grovetti E, Tedeschi S, Salardi S, Cresseri D, Messa P, Ardissino G (2014) Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost 12:1440–1448
Cugno M, Tedeschi S, Ardissino G (2015) Tailored eculizumab regimen for patients with atypical hemolytic uremic syndrome: requirement for comprehensive complement analysis: comment. J Thromb Haemost 13:485–486
Volokhina EB, van de Kar NC, Bergseth G, van der Velden TJ, Westra D, Wetzels JF, van den Heuvel LP, Mollnes TE (2016) Sensitive, reliable and easy-performed laboratory monitoring of eculizumab therapy in atypical hemolytic uremic syndrome. Clin Immunol 160:237–243
Ardissino G, Testa S, Possenti I, Tel F, Paglialonga F, Salardi S, Tedeschi S, Belingheri M, Cugno M (2014) Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 64:633–637
Ardissino G, Possenti I, Tel F (2015) In reply to ‘Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome’. Am J Kidney Dis 65:342–343
Ardissino G, Possenti I, Tel F, Testa S, Salardi S, Ladisa V (2015) Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis 66:172–173
Acknowledgements
We are grateful to the following physicians, whose collaboration in the management of patients was essential: B. Basolo (Torino), M. Belingheri (Milan), F. Bertola (Legnano), A. Castiglioni (Busto Arsizio), G. Colussi (Milan), L. Costantini (Vercelli), R. Cravero (Biella), M. D’Amico (Como), L. Del Vecchio (Lecco), F. Catalano (Reggio Calabria), P. Fabbrini (Monza), A. Inzoli (Crema), S. Marenghini (Palermo), M. martini (Arezzo), C. Milocco (Monfalcone), L. Morabito (Imperia), A. Naticchia (Rome), F. Paglialonga (Milan), A. Pani (Cagliari), L. Potenza (Modena), A. Rigotti (Rimini), S. Testa (Milan), and G. Visconti (Palermo). We are also grateful to “Progetto ALICE ONLUS. Associazione per la Lotta alla SEU” for their continuous and precious support. We thank “Progetto ALICE ONLUS. Associazione per la lotta alla SEU” for their continuous and precious support.
Funding
This study was supported by a research grant provided by “Progetto ALICE ONLUS—Associazione per la Lotta alla SEU.”
Author information
Authors and Affiliations
Contributions
Research idea and study design: GA, DC, MC; data acquisition: GA, DC, MC, FT, MP, MS, IP, SG, AG; data analysis/interpretation: GA, FT, MC, SG, MP, IP; statistical analysis: GA, MC, AG. MS; supervision or mentorship: AG, MC, DC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. GA takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Corresponding author
Ethics declarations
Conflicts of interest
D. Cresseri: national (Italy) coordinator of the Global aHUS Registry supported by Alexion Pharmaceuticals, Inc.; G. Ardissino: member of the scientific advisory board of the Global aHUS Registry supported by Alexion Pharmaceuticals, Inc. The other authors state that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ardissino, G., Tel, F., Sgarbanti, M. et al. Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome: an update. Pediatr Nephrol 33, 457–461 (2018). https://doi.org/10.1007/s00467-017-3813-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3813-2