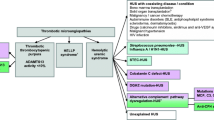
Abstract
Background
Atypical hemolytic uremic syndrome (aHUS) is characterized by platelet consumption, hemolysis, and renal injury. Eculizumab, a humanized antibody that blocks complement activity, has been successfully used in aHUS, but the best treatment schedule has not yet been clearly defined.
Methods
Herein we report our experience with eculizumab maintenance treatment, in which the interval between subsequent doses was adjusted based on classical complement pathway (CCP) activity, targeted to < 30% for the prevention of relapses. Trough circulating levels of free eculizumab were determined by an immunoenzymatic method. Genetic and serologic characteristics of the patients were also assessed.
Results
We report on 38 patients with aHUS with a median age of 25.0 years (range 0.5–60.0 years) treated with eculizumab. Once stable disease remission was obtained, the interval between eculizumab doses was extended based on target CCP activity. With this approach, presently, 22 patients regularly receive eculizumab infusion every 28 days and 16 receive it every 21. During a median observation period of 32.3 months (range 4.0–92.4 months) and a cumulative period of 1295 months, no patient relapsed. An inverse correlation between CCP activity and eculizumab circulating levels was present (r = − 0.690, p = 0.0001), with CCP activity being inhibited as long as free eculizumab was measurable in serum.
Conclusions
In patients with aHUS on eculizumab maintenance treatment, complement activity measurement can be used as a proxy for circulating levels of the drug. Monitoring complement activity allows for safe tailoring of the frequency of eculizumab administration, thus avoiding excessive drug exposure while keeping the disease in remission.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a severe, systemic thrombotic microangiopathy (TMA) often related to mutations in genes encoding complement regulatory proteins [1, 2] or to the presence of autoantibodies directed against the complement inhibitory protein factor H (CFH) [3, 4]. Since 2009, eculizumab, a humanized recombinant monoclonal IgG antibody that blocks complement component 5 (C5), has been successfully used in the treatment of patients with aHUS [5, 6]. The standard maintenance treatment suggests eculizumab administration every 2 weeks life-long, but the best treatment schedule has not been defined yet. Recent investigations have explored the possibility that determining global complement activity might be useful for better individualizing eculizumab treatment, with the aim of increasing the interval between subsequent doses thus minimizing the heavy costs of the treatment while improving the patient’s quality of life [7,8,9,10]. In addition to this pharmacodynamic approach, a pharmacokinetic one has been proposed, in which eculizumab treatment is tailored by maintaining trough concentrations of the drug within a fixed interval, known to inhibit the complement system [11, 12]. Finally, an algorithm that combines pharmacodynamics and pharmacokinetics has been proposed by Jodele et al. by measuring CH50, plasma levels of sC5b-9 and serum levels of eculizumab [13]. The safety and efficacy of eculizumab maintenance treatment tailored to global complement activity has been proven by the lack of relapses in large series [7,8,9,10], but whether global complement activity is a real proxy for eculizumab concentration to be used to optimize drug exposure without impacting on efficacy has not been established. With this as background, we analyzed the relationship between eculizumab trough concentration (pharmacokinetics) and the effect of the drug on complement activity (pharmacodynamics) in our (case list of) patients with aHUS on eculizumab maintenance treatment, to whom the drug was administered according to serum complement activity.
Patients and methods
Patients
We studied 38 patients (21 females and 17 males; median age 25 years [range 0.5–60 years]) with aHUS on maintenance eculizumab treatment for the prevention of relapse. Thirteen patients were treated to prevent the disease in kidney graft. Patients with aHUS in stable clinical remission during eculizumab treatment at our Center had the opportunity to increase the interval between subsequent doses. This was done on a routine basis with all patients. In detail, patients were addressed to a progressive extension of the interval between eculizumab doses from the standard 2 weeks to 3 or 4 weeks based on a global complement functional test for the classical complement pathway (CCP) activity. Complement activity was routinely determined before each eculizumab administration and the interval between doses was adjusted with the aim of maintaining a level of CCP activity < 30%. Strict monitoring of disease reactivation indicators (haptoglobin, lactate dehydrogenase, platelet count, serum creatinine, proteinuria, microalbuminuria and hematuria/hemoglobinuria) was regularly performed at each eculizumab administration. Relapses were defined as the concomitant detection of platelet consumption, hemolysis and signs of renal damage or worsening of renal function if already impaired (increase in serum creatinine or proteinuria). As long as target complement activity was completely suppressed (CCP activity < 10%) and the markers of disease activity documented TMA remission, the interval between administrations was extended by 1 week; in contrast, if CCP activity was > 30%, showing insufficient complement suppression, the interval between doses was shortened. If CCP activity was 10–30% the current interval was confirmed for the subsequent eculizumab administration. Additional methodological details are extensively provided in the original paper and in its update published elsewhere [7, 9].
The present retrospective study was approved by the local review board and was conducted according to the ethical principles contained in the 2013 revision of the Declaration of Helsinki and the code of Good Clinical Practice. All adult patients or parents of pediatric patients gave informed consent for the use of their data in an anonymous form for research purposes.
Methods
Genetic studies
Genomic DNA was extracted from whole peripheral blood on an automated QIAsymphonySP platform (Qiagen GmbH, Hilden, Germany). Detection of nucleotide variations was assessed by next generation sequencing (NGS) on the MiSeq platform (Illumina) by using the “targeted sequencing” technique (HaloPlex Kit; Agilent Technologies) on a multiple gene custom panel comprising CFH (NM_000186.3), MCP/CD46 (NM_002389.4), CFI (NM_000204.4), C3 (NM_000064.3), CFB (NM_001710.5), THBD (NM_000361.2), DGKE (NM_003647.2), CFHR1 (NM_002113.2), CFHR3 (NM_021023.5), and CFHR5 (NM_030787.3) at 100X coverage. Bioinformatic analysis of NGS data with filtering to identify putative causative variants was performed with the SureCall application. All variants identified by NGS analysis were then confirmed by the standard Sanger sequencing method. More details are reported elsewhere [4].
Functional immuno-enzymatic assay for the classical complement pathway
The functional test for the classical complement pathway was performed using the Wieslab Coplement System kit (Euro-Diagnostica, Malmö, Sweden), following the manufacturer’s instructions. The wells of the microtiter strips were coated with a specific activator of the classical complement pathway, and serum samples were diluted in a buffer containing specific blockers of the other two complement pathways in order to ensure that only the classical pathway was activated during incubation. The wells were then washed, and C5b-9 was detected with a specific alkaline phosphatase-labeled antibody against the neoantigen expressed on C9 during C5b-9 formation. After a further washing step, the specific antibodies were detected by means of incubation with the alkaline phosphatase substrate solution. As the amount of complement activation correlates with color intensity and is measured in terms of absorbance, results are expressed as percentages of the activity of a standard sample (i.e., normal pooled serum fixed at 100%). This method allows evaluation of complement activity detected in serum through classical pathway activation using the terminal complement complex C5b-9 as the detection system [7].
Measurement of eculizumab serum levels
Eculizumab concentration was measured in serum by an ELISA that used complement component 5 (C5) for the capture of eculizumab and anti-human IgG for its detection. Purified human C5 (Quidel, San Diego, CA, USA) at a concentration of 5 μg/mL in phosphate-buffered saline, pH 7.4 was coated overnight onto microtitration plates and, after washing, the wells were over-coated with bovine serum albumin to avoid non-specific binding. After further washes, a 1:20 dilution of serum samples was added and incubated at room temperature for 45 min. After washing, C5-bound eculizumab was identified by means of a peroxidase-conjugated goat polyclonal anti-human IgG (Sigma Aldrich, St Louis, MO, USA), which was revealed with orthophenylenediamine. Absorbance was read at 490 nm. The results were expressed as μg/mL, referred to an eculizumab standard curve.
Statistical analyses
Continuous variables are reported as median and range (min–max), while categorical variables are reported as number and percentage. The Spearman correlation coefficient was calculated to assess relationships between complement activity and eculizumab serum levels. The data were analyzed using the SPSS PC statistical package, version 26 (IBM SPSS Inc., Chicago, IL).
Results
Demographic characteristics of patients along with HUS etiology and time on eculizumab treatment are reported in table S1. Thirty-three of 38 patients (86.8%) had an identified complement regulatory gene abnormality: 23 had CFH and related large genomic rearrangement (3 with anti-factor H autoantibodies), 3 had CFI, 2 had C3, 2 had MCP, and 3 involving multiple genes. Presently, following the algorithm described in the Methods section, 22 patients regularly receive eculizumab infusion every 28 days, 16 every 21 days and none every 14 days. Patients were observed on tailored eculizumab maintenance treatment for a median of 32.3 months (range 4.0–92.4 months) with a cumulative observation period of 1295 months, during which no patient relapsed.
Out of 787 determinations throughout the entire cumulative observation period on tailored eculizumab maintenance treatment, 586 (74.5%) showed complete suppression of CCP activity with a value of ≤ 10%, and 59 (6.7%) showed partial suppression with values between 11 and 30%. The remaining determinations were distributed as follows: 79 (10.0%) had CCP activity between 31 and 70%, and 66 (8.4%) were within the normal range with CCP activity > 70% (see Fig. 1). In the 787 samples, obtained before the subsequent drug administration, eculizumab concentration was also determined (trough level) and the results are shown in Fig. 2. Ninety-eight eculizumab determinations (12.4%) were < 10 μg/ml, 295 (37.5%) were between 10 and 50 μg/ml, 284 (36.1%) were between 50 and 100 μg/ml and 110 (14.0%) were > 100 μg/ml.
The relationship between eculizumab circulating levels and complement inhibition is shown in Fig. 3. An inverse correlation was present between eculizumab levels and CCP activity (r = − 0.690, p = 0.0001), and CCP activity was inhibited as long as free eculizumab was measurable in serum and was completely suppressed even for eculizumab levels as low as 5 μg/ml. Very similar correlations between eculizumab levels and CCP activity were observed both in adult and pediatric patients (r = − 0.679 and r = − 0.713, respectively).
Given that eculizumab trough levels depend on administered dose, body weight and interval (days) since last infusion, in Fig. 4 we explored the effect of all these variables on CCP, by expressing eculizumab dose as mg of eculizumab per kg of body weight per days of interval. The median eculizumab dose per kg of body weight per days of interval was 0.75 mg/kg/days of interval (range 0.40–2.69). The lowest dose of eculizumab that minimizes both the amount of drug used and the observations with uninhibited complement activity was thus identified at 0.62 mg/kg/days of interval.
Discussion
Treatment of aHUS with the C5 inhibitor eculizumab has led to an impressive improvement in disease outcome. However, being this treatment relatively new, its optimal schedule has not yet been clearly defined. At least 30% of patients relapse if eculizumab is discontinued, thus these patients may require life-long treatment [14, 15]. The cost of maintenance treatment is as impressive as the drug efficacy: 0.4 million Euro per patient per year. As such, it represents a heavy financial burden that many countries cannot afford and others might not be able to sustain over time, given the increasing prevalence of treated patients.
Back in 2014, we proposed a treatment schedule for maintenance treatment with eculizumab being tailored to global complement activity (namely CCP activity) that, besides halving the costs, improves the patient’s quality of life by minimizing the need for drug administration [7,8,9]. Initially, we used CCP activity <10% as the threshold of complement suppression. Later on, we realized that many patients remained in stable remission even without complete suppression of complement activity and then raised the threshold to up to 30%. The lack of relapses in our case list on the tailored regimen over a long observation period (1295 months) supports the efficacy and safety of our approach. At that time, we did not explore whether complement activity could be an effective proxy for eculizumab concentration. This would allow its use to minimize drug exposure without impacting on efficacy. In addition, it would simplify clinical monitoring as not all hospitals are equipped with sophisticated methods for therapeutic drug monitoring, but can easily measure CCP activity [16].
The present study provides evidence of the correlation between eculizumab downstream levels and complement activity to the extent that the measurement of complement activity by a simple and inexpensive method can be used as a reliable proxy to monitor the adequacy of C5 inhibition. Notably, CCP activity was completely suppressed even for eculizumab levels as low as 5 mg/ml. This can be explained by considering that our method can only detect free eculizumab that is captured by means of immobilized C5; therefore, the possibly considerable amount of the drug bound to C5 in the circulating blood goes undetected. It can be argued that children may have different pharmacokinetics compared with adults. However, the correlation between eculizumab levels and CCP activity (pharmacodynamics) was almost the same in adults and children, thus the two groups can be combined.
Our data show that, even in a setting of minimal effective dose, 14% of patients were clearly over-treated, with circulating levels of eculizumab higher than 100 μg/ml (therapeutic range 10–50 μg/ml).
The relationship between CCP activity and eculizumab dose (expressed in mg per kg of body weight per days of interval) was constant to the point that an empirical formula can be derived to anticipate the interval of efficacy of a given amount of drug: eculizumab administered in mg/body weight in kg/0.62. Only 5.5% of the observations are not covered by the algorithm.
The derived formula leads to the best compromise between the desired complement inhibition and the minimal drug dose. The limitation in using the identified constant to calculate the interval in days is that it will be reliable in no more than 94.5% of cases; therefore, the theoretical interval needs to be double checked by measuring CCP activity. The flexibility of the between-dose interval is particularly relevant given that eculizumab vials contain 300 mg, therefore the administered dose can only be a multiple of that amount.
To the best of our knowledge, our study is the first to analyze the correlation between in vivo pharmacokinetics and pharmacodynamics of eculizumab in aHUS patients during maintenance treatment, albeit other investigators [10,11,12,13], including our own group [7,8,9], previously analyzed some of these aspects. Our group and Volokhina et al. analyzed pharmacodynamics [7,8,9,10], Jodele et al. mainly focused on induction treatment [13], Passot et al. considered pharmacokinetics [12] while Gatault et al. performed a pharmacokinetic study in vivo but pharmakodynamics was only evaluated in vitro [11].
In conclusion, eculizumab maintenance treatment in patients with aHUS can be monitored by the measurement of complement activity inhibition used as a proxy for drug circulating levels. This approach, which needs to be confirmed by prospective randomized studies, may allow for safe tailoring of the frequency of eculizumab administration, avoiding excessive drug exposure while keeping the disease in remission. The same strategy may also be applied to the new C5 inhibitors, including long-acting agents.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Noris M, Remuzzi G (2009) Atypical hemolytic-uremic syndrome. N Engl J Med 361:1676–87. https://doi.org/10.1056/NEJMra0902814
Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, Nester CM, Noris M, Pickering MC, Rodríguez de Córdoba S, Roumenina LT, Sethi S, Smith RJ (2017) Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 91:539–551. https://doi.org/10.1016/j.kint.2016.10.005
Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Frémeaux-Bacchi V (2005) Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 16:555–563. https://doi.org/10.1681/ASN.2004050380
Cugno M, Berra S, Depetri F, Tedeschi S, Griffini S, Grovetti E, Caccia S, Cresseri D, Messa P, Testa S, Giglio F, Peyvandi F, Ardissino G (2021) IgM autoantibodies to complement factor H in atypical hemolytic uremic syndrome. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2020081224 (Online ahead of print)
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C (2013) Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368:2169–2181. https://doi.org/10.1056/NEJMoa1208981
Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C (2016) Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89:701–711. https://doi.org/10.1016/j.kint.2015.11.026
Cugno M, Gualtierotti R, Possenti I, Testa S, Tel F, Griffini S, Grovetti E, Tedeschi S, Salardi S, Cresseri D, Messa P, Ardissino G (2014) Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost 12:1440–1448. https://doi.org/10.1111/jth.12615
Cugno M, Tedeschi S, Ardissino G (2015) Tailored eculizumab regimen for patients with atypical hemolytic uremic syndrome: requirement for comprehensive complement analysis: comment. J Thromb Haemost 13:485–486. https://doi.org/10.1111/jth.12764
Ardissino G, Tel F, Sgarbanti M, Cresseri D, Giussani A, Griffini S, Grovetto E, Possenti I, Perrone M, Testa S, Paglialonga F, Messa P, Cugno M (2018) Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome: an update. Pediatr Nephrol 33:457–461. https://doi.org/10.1007/s00467-017-3813-2
Volokhina EB, van de Kar NC, Bergseth G, van der Velden TJ, Westra D, Wetzels JF, van den Heuvel LP, Mollnes TE (2016) Sensitive, reliable and easy-performed laboratory monitoring of eculizumab therapy in atypical hemolytic uremic syndrome. Clin Immunol 160:237–243. https://doi.org/10.1016/j.clim.2015.05.018
Gatault P, Brachet G, Ternant D, Degenne D, Récipon G, Barbet C, Gyan E, Gouilleux-Gruart V, Bordes C, Farrell A, Halimi JM, Watier H (2015) Therapeutic drug monitoring of eculizumab: rationale for an individualized dosing schedule. MAbs 7:1205–11. https://doi.org/10.1080/19420862.2015.1086049
Passot C, Sberro-Soussan R, Bertrand D, Caillard S, Schvartz B, Domenger C, Contin-Bordes C, Paintaud G, Halimi JM, Ternant D, Gatault P (2021) Feasibility and safety of tailored dosing schedule for eculizumab based on therapeutic drug monitoring: lessons from a prospective multicentric study. Br J Clin Pharmacol 87:2236–46. https://doi.org/10.1111/bcp.14627
Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J, Dixon BP, Chima RS, Hirsch R, Teusink A, Lazear D, Lane A, Myers KC, Dandoy CE, Davies SM (2016) Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 22:307–315. https://doi.org/10.1016/j.bbmt.2015.10.002
Ardissino G, Testa S, Possenti I, Tel F, Paglialonga F, Salardi S, Tedeschi S, Belingheri M, Cugno M (2014) Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 64:633–637. https://doi.org/10.1053/j.ajkd.2014.01.434
Ardissino G, Possenti I, Tel F, Testa S, Salardi S, Ladisa V (2015) Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis 66:172–173. https://doi.org/10.1053/j.ajkd.2015.04.010
Galbusera M, Noris M, Gastoldi S, Bresin E, Mele C, Breno M, Cuccarolo P, Alberti M, Valoti E, Piras R, Donadelli R, Vivarelli M, Murer L, Pecoraro C, Ferrari E, Perna A, Benigni A, Portalupi V, Remuzzi G (2019) An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis 74:56–72. https://doi.org/10.1053/j.ajkd.2018.11.012
Acknowledgements
The authors are grateful to “Progetto Alice Onlus, Associazione per la lotta alla Sindrome Emolitico Uremica” for the valuable support provided to perform this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Designed the study MC and GA. Collected clinical data MC, VC and GA. Performed biological analyses SG, EG, GP, and LP. Designed and performed statistical analyses MC, EC and GA. Drafted the initial version of the manuscript MC and GA. Revised the manuscript critically for intellectual content MC, VC, SG, EG, GP, LP, EC and GA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
G. Ardissino reports consultancy agreements with Alexion and Alnylam; scientific advisory board membership with Alexion Inc. All remaining authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was approved by the local review board and was conducted according to the ethical principles contained in the 2013 revision of the Declaration of Helsinki and the code of Good Clinical Practice.
Consent to participate
Written informed consent was obtained from all individual adult participants and from the parents of pediatric patients included in the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cugno, M., Capone, V., Griffini, S. et al. Eculizumab treatment in atypical hemolytic uremic syndrome: correlation between functional complement tests and drug levels. J Nephrol 35, 1205–1211 (2022). https://doi.org/10.1007/s40620-021-01187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01187-8