Abstract
Canonical Wnt signaling activity contributes to physiological and adaptive bone mineralization and is an essential player in bone remodeling. Sclerostin is a prototypic soluble canonical Wnt signaling pathway inhibitor that is produced in osteocytes and blocks osteoblast differentiation and function. Therefore, sclerostin is a potent inhibitor of bone formation and mineralization. Accordingly, rodent sclerostin-deficiency models exhibit a strong bone phenotype. Moreover, blocking sclerostin represents a promising treatment perspective against osteoporosis. Beyond the bone field novel data definitely associate Wnt signaling in general and sclerostin in particular with ectopic extraosseous mineralization processes, as is evident in cardiovascular calcification or calciphylaxis. Uremia is characterized by parallel occurrence of disordered bone mineralization and accelerated cardiovascular calcification (chronic kidney disease – mineral and bone disorder, CKD-MBD), linking skeletal and cardiovascular disease—the so-called bone-vascular calcification paradox. In consequence, sclerostin may qualify as an emerging player in CKD-MBD. We present a stepwise review approach regarding the rapidly evolving field sclerostin participation in CKD-MBD. Starting from data originating in the classical bone field we look separately at three major areas of CKD-MBD: disturbed mineral metabolism, renal osteodystrophy, and uremic cardiovascular disease. Our review is intended to help the nephrologist revise the potential importance of sclerostin in CKD by focusing on how sclerostin research is gradually evolving from the classical osteoporosis niche into the area of CKD-MBD. In particular, we integrate the limited amount of available data in the context of pediatric nephrology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Step 1: What is Wnt signaling?
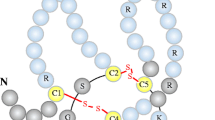
The wingless-type mouse mammary tumor virus integration site (Wnt) pathway plays a key role in a wide array of biological processes such as cell proliferation, migration, and differentiation [1]. Wnt signaling encompasses at least three different complex pathways with partial functional overlap, one of which is the canonical Wnt/beta-catenin pathway [1, 2]. The canonical Wnt pathway involves interaction of several Wnt ligands with their cognate receptors and co-receptors (Fig. 1a and b). Important substructures of this dual-receptor complex are formed by transmembrane Frizzled (Frz) receptors and the low-density lipoprotein receptor-related proteins (LRP) 5 and 6. The key function of the canonical Wnt pathway is stabilization of beta-catenin by inhibiting the activity of the beta-catenin degradation complex (Fig. 1a). Beta-catenin can be considered as a central regulatory player within the cytoplasm. The function of the beta-catenin degradation complex is to phosphorylate beta-catenin through e.g., glycogen synthase kinase (GSK)-3beta-mediated phosphorylation (Fig. 1a) [3]. Phosphorylated beta-catenin is prone to proteolysis and would not accumulate in the nucleus. Only unphosphorylated beta-catenin can translocate into the nucleus and modulate target gene transcription (Fig. 1b) [3].
a Soluble Wnt ligands bind to a receptor complex consisting of Frizzled and LDL-receptor-related protein 5 and 6 (LRP5/6). Upon extracellular Wnt ligand binding, the receptor complex interacts with the phosphoprotein Dishevelled (Dsh), which is located in the cytoplasm. Wnt ligand binding and activation of Dsh result in the relocation of Axin to the plasma membrane receptor complex resulting in disassembly of the ß-catenin degradation complex. This destruction complex consists of casein kinase 1 (CK1), Axin, adenomatosis polyposis coli (APC) and glycogen synthase kinase 3 (GSK 3) (the multimeric CK1-APC-GSK3-Axin complex). ß-catenin is consecutively released from this complex and stabilized since the absence of phosphorylation prevents degradation. In consequence, unphosphorylated ß-catenin accumulates, translocates into the nucleus and modifies gene transcription via its role as coactivator of transcription factors that belong to the TCF/lEF family. b In the presence of the soluble Wnt inhibitor sclerostin Wnt ligands are blocked from binding the LRP5/6-Frizzled receptor complex which in turn allows activation of the ß-catenin degradation complex (CK-APC-GSK3-Axin complex). Phosphorylation of ß-catenin by GSK3 and CK1 blocks the translocation of ß-catenin into the nucleus and enhances ß-catenin degradation via proteolysis
The signaling pathways of Wnt ligands vary with the cell type involved and with the receptors and co-receptors expressed on particular effector cells. Loss-of-function mutations in key players of the canonical Wnt signaling cause distinct phenotypes, as reviewed elsewhere [4]. These genetic modification models reveal the plethora of biological functions Wnt signaling participates in. For example, individuals with LRP6 mutations exhibit premature coronary artery disease as well as severe osteoporosis [5], providing the first evidence that Wnt signaling is a player in the bone-vascular axis.
Wnt signaling appears prototypic for the crosstalk within the bone-vascular axis since it encompasses vascular development and endothelial cell specification, as well as the regulation of bone modeling and remodeling [6–9]. The term bone-vascular calcification paradox describes various disease states with dysregulated calcification processes characterized by the coincidence of skeletal demineralization and vascular calcification. This calcification paradox is one of the characteristic features of chronic kidney disease – mineral and bone disorder (CKD-MBD), or vice versa, CKD-MBD is a prototypic condition for paradoxic calcification [10]. Therefore, it deserves comprehensive analyses as to what extent disturbances in Wnt signaling contribute and associate with CKD.
Step 2: What are Wnt inhibitors?
Secreted Wnt inhibitors are a group of various proteins that interfere with the extracellular binding of Wnt ligands with the transmembrane receptor complex. These inhibitors either directly bind and block Wnt ligands and thereby prevent their interactions with receptors (e.g., secreted frizzled-related protein and Wnt inhibitory factor), or bind to LRP5/6, inducing receptor internalization and/or reducing their availability to Wnt ligands (e.g., Dickkopf factors (DKK) and sclerostin (Fig. 1b)) [11]. Among the numerous Wnt inhibitors, DKK-1 and sclerostin have been most intensively studied – mainly in animal models and various adult populations [9]. When directly compared with DKK-1, sclerostin in particular gained attention in the context of the bone-vascular calcification paradox. Sclerostin is a member of the cystatin-knot family of proteins and is a product of the SOST gene [12]. This glycoprotein (22 kDa) is secreted almost exclusively by osteocytes (Fig. 2) and to a lesser extent by other cell types, including osteoclast precursors, renal and vascular cells [13]. The major sclerostin producers, the osteocytes, are terminally differentiated osteoblasts trapped within the bone matrix. The discovery of sclerostin production contributes to our understanding that osteoblasts and osteocytes are endocrine cells secreting a number of fascinating proteins, e.g., osteoprotegerin (OPG), osteocalcin, sclerostin and fibroblast growth factor 23 (FGF-23) [14]. These factors and their rather distant physiological effects (e.g., in energy metabolism or tubular function) establish the skeleton as one of the endocrine organs [9].
Step 3: What is the biological effect of these canonical Wnt inhibitors—particularly in bone?
The biological effects of Wnt inhibitors were first elucidated in bone. Secreted (extracellular) Wnt inhibitors such as sclerostin or DKK-1 are important negative feed-back regulators of Wnt-beta catenin signaling. Sclerostin activities are complex, and besides interaction with the LRP5/6 co-receptors, there are other modes of action of sclerostin on bone metabolism, e.g., interaction with bone morphogenetic proteins [15, 16]. Additionally, sclerostin binds to LRP4, promoting receptor-blocking activities at the Frz-LRP5/6 complex [17]. Sclerostin interferes with another well-known bone metabolism regulating system: the OPG-RANK-RANKL system. Wnt signaling stimulates OPG which, in turn, binds and inhibits the receptor activator of nuclear factor kB-ligand (RANKL) expressed by osteoblasts, thus preventing osteoclast precursor differentiation [18].
Wnt inhibitors allow the catenin degradation complex, consisting of APC, Axin, and GSK3 [19], to degrade cytosolic beta-catenin (Fig. 1b). By doing so, Wnt inhibitors prevent beta-catenin nuclear signaling and target gene expression. Insight into the role of the various receptors, messengers, and modulators of the Wnt signaling in skeletal health and disease has been gained from rodent and human genetic modification models, including sclerosteosis and Van Buchem’s disease [11, 20–24]. In particular, genetic modification of sclerostin or DKK-1 activity provides comprehensive insight on how soluble Wnt inhibitors affect bone metabolism. While reduced activity of sclerostin and DKK-1 in vivo contributes to increased bone mass and strength via a stimulated osteo-anabolic action [23, 25], the opposite is true in experimental models with overexpression of both sclerostin and DKK-1 [12, 26]. These models reveal how potent the anti-anabolic actions of sclerostin and DKK-1 are. Moreover, transgenic overexpression of sclerostin was associated with chondrodysplasia in lumbar vertebrae. The vertebrae were also shorter, wider and lacked the normal trabecular and inter-vertebral disc architecture [12]. Interestingly, the observed high bone formation and mineralization indices typically detected in sclerostin-null mice might, at least in part, be related to significantly increased 1,25 dihydroxyvitamin D levels and significantly reduced FGF-23 levels in these animals compared to wild-type mice [27]. With all these data in mind, it is surprising to see a positive association between serum sclerostin levels and high bone mineral density in humans, which at first sight is counter-intuitive [28]. Register and co-workers speculated that high levels of circulating sclerostin might be indicative of impaired receptor binding and therefore reduced local skeletal activity [28]. However, this assumption, as well as the potential role of parathyroid hormone (PTH) resistance therein, needs further investigation and especially confirmation in patients with CKD. Sclerostin levels are modified and influenced by, among other things, age, fracture healing, gender, estrogen usage, hyperparathyroidism, and physical activity. Although beyond the scope of the present review, it is justified to summarize that the absence or presence of such (patho)physiological stimuli or suppressors of bone metabolism influences sclerostin [29–31]. Needless to say that, regarding such interactions any conclusion of analogy between bone physiology in non-renal patients and CKD-MBD, should be drawn with substantial caution.
In summary, the osteocyte and its SOST gene product sclerostin are outstanding regulators of bone metabolism and target structures for several extraskeletal mediators. Viable osteocytes regulate the proper functionality of the skeleton which, as outlined below, in turn, has substantial effects beyond bone metabolism [9, 32, 33].
Step 4: Is targeting canonical Wnt signaling clinically meaningful?
Targeting Wnt signaling offers fascinating clinical perspectives, currently expected to be most beneficial in the bone field [11]. To date, interfering with Wnt signaling by blocking sclerostin is most advanced in postmenopausal osteoporotic patients. In a recent 12-month phase 2 trial, blocking sclerostin activity by monoclonal antibodies significantly increased bone mass in postmenopausal osteoporosis [34]. The trial compared the efficacy of various anti-osteoporotic therapies (alendronate, teriparatide) with different dosages of romosozumab, a human monoclonal sclerostin-antibody, through evaluation of lumbar and femoral bone mineral density in 419 women with low bone mass. Bone mass was assessed by densitometry at the lumbar spine and the femoral neck. Additionally, the authors investigated the development of serum levels of markers of bone metabolism over time. The primary end point was the percentage change from baseline in bone mineral density at the lumbar spine. Compared to placebo, as well as to the two other active intervention arms (teriparatide and alendronate), romosozumab treated patients exhibited a significant, dose-dependent bone mineral density increase up to 11.3 % at the lumbar spine, 4.1 % at the total hip, and 3.7 % at the femoral neck. In parallel, the study revealed a (transient) increase in bone formation markers and a decrease in bone resorption markers. The authors of this trial speculate about the assumed safety of romosozumab treatment with two major arguments: first, heterozygous human carriers of SOST mutations have a benign clinical course with high bone density but without clinical signs of bone overgrowth such as nerval compression symptoms [35]; second, the authors claim that SOST expression is limited to the skeleton and therefore blocking sclerostin activity is limited to the skeleton, too. However, this might actually be an over-simplification and step 9 of the present review will describe concerns about unpredictable extra-skeletal effects of romosozumab which recently have been announced by our group [36].
Step 5: Why is sclerostin in particular the most promising parameter to consider among the various Wnt inhibitors in the concert of CKD-MBD?
Both sclerostin and DKK-1 are prototypic soluble Wnt inhibitors [11]. The—still limited—data that are currently available regarding the potential association and diagnostic value in terms of relevant CKD-MBD parameters, is insufficient to attribute clear “scientific” or “clinical” superiority to either sclerostin or DKK-1. However, several lines of evidence support our view that sclerostin is one step ahead of DKK-1 in bone and also vascular research. This evidence is fuelled, for example, by the numbers of publications (e.g., retrieved via PubMed search for “DKK-1” versus “sclerostin” as updated in September 2014). Sclerostin research is also more advanced in terms of treatment for human osteoporosis as already pointed out in Step 4. A large-scale phase III trial is on the way to compare the efficacy of fracture prevention between romosozumab and alendronate in osteoporotic postmenopausal women (ClinicalTrials.gov Identifier: NCT01631214). It is important to note that DKK-1 and sclerostin exhibit distinct biological effects and are differently regulated [17, 37, 38]. In this context it has been hypothesized that sclerostin exerts more specific regulatory actions within the Wnt cascade, while DKK-1 is more a pan-Wnt inhibitor encompassing different Wnt pathways [11]. Accordingly, notable differences exist between the two rodent knock-out models SOST −/− and DKK-1 −/−. While SOST −/− mice present a macroscopically rather unremarkable phenotype, with the predominant characteristic finding of strong bones, DKK-1 null mutant embryos lack head structures anterior of the midbrain [39], which is associated with premature death. These animal models suggest that sclerostin activity appears to be more focused upon (although not limited to) mineralization processes. Therefore, therapeutic interventions targeting sclerostin might produce more predictable effects as compared to DKK-1. Also, the association between sclerostin and DKK-1 serum levels and cardiovascular calcification are opposite [40]. Thus, the available evidence suggests that it is not justified to simply lump these two inhibitors together. Instead, sclerostin and DKK-1 should be considered two different types of Wnt inhibitors, despite some homologies and similarities. Based on the amount and quality of data available, the following sections focus mainly on sclerostin.
Step 6: Potential role of Wnt inhibitors in nephrology
The Wnt signaling pathway plays an important role in renal (patho)physiology via its involvement in nephrogenesis [41], renal repair, and fibrosis [42]. Experimental renal failure dramatically upregulates renal beta-catenin production [42]. This goes along with an increased expression of DKK-1 and sclerostin, as recently observed in the tubular epithelium of mice subjected to renal injury mimicking stage 2 CKD [43]. In addition, “standard” nephrological therapeutics such as vitamin D [42] and ACE-inhibition [44] interfere with the Wnt signaling cascade.
Given the important role of Wnt signaling in the biology of bone and vasculature it is tempting to speculate about the potential role of Wnt-signaling in CKD-MBD. In particular, what is the contribution of sclerostin and DKK-1 to CKD-MBD [45]? CKD-MBD encompasses three major disturbances associated with reduced renal function: bone disease, cardio-vascular disorders and dysregulated mineral metabolism [10]. Sclerostin especially fulfils the prerequisites to qualify as relevant new player (biomarker) in CKD-MBD. These are: (i) CKD by itself influences the candidate biomarker in terms of concentration and/or biological activity, and (ii) there are links between the candidate biomarker and all three aspects of CKD-MBD. Indeed, CKD in humans associates with signs of disturbed Wnt signaling. Compared to adult individuals with normal renal function, circulating levels of both DKK-1 and sclerostin are increased in humans with advanced or end-stage renal disease (ESRD) [46]. Sclerostin levels gradually increase, from healthy individuals to patients with calcifying arteriosclerosis, to heavily calcified dialysis patients [46]. It has not yet been fully determined to what extent this reflects increased production, renal retention or both. As far as sclerostin is concerned, recent clinical data argue against simple renal retention, since in parallel with increasing serum concentrations, urinary sclerostin concentrations as well as fractional sclerostin excretion also increase with declining renal function (the latter from 0.45 ± 0.26 % in patients with CKD stage 1 to 26 ± 18 % (n = 15) in CKD stage 5 (n = 19) [47]. Serum levels rapidly return to the normal range following successful renal transplantation [48]. If elevated sclerostin levels are not solely consequential to renal retention, increased production comes into play. The bone compartment is one candidate for increased production. However, the exact source of sclerostin detected in the circulation is not yet known. In vivo evidence has been provided that osseous sclerostin expression is upregulated in early CKD as detected by analyzing mouse models of moderate renal failure (juvenile cystic kidney (jck) mouse, a genetic model for polycystic kidney disease) [49]. The jck mouse is characterized by biochemical changes (starting at about week 10), cardiovascular abnormalities (starting at about week 15) and skeletal changes (starting at about week 9) typically seen in CKD-MBD [49]. As previously speculated, sclerostin production at sites outside the skeleton, for example the calcified vasculature, might also occur in CKD-MBD and contribute via “spill-over” to the observed serum levels [46, 47, 50]. If sclerostin metabolism is altered in CKD (i.e., the above-mentioned requirement (i) is fulfilled) – what about the second item, (ii) – is there a causal association with CKD-MBD, or is sclerostin entirely innocent?
Step 7: Sclerostin associates with various CKD-MBD-related biomarkers
In contrast to the highly elevated PTH levels (up to factor 20, 30, or even more) and in particularly FGF-23 levels (up to factor 1,000 above the upper normal limit) in patients with ESRD, circulating sclerostin concentrations are only moderately, albeit significantly, increased by a factor 2 to 3 [46]. These ESRD-related sclerostin levels reveal significant associations with the classical CKD-MBD biomarkers such as PTH. Plasma sclerostin levels within the NECOSAD cohort (NEtherlands COoperative Study on the Adequacy of Dialysis) cohort (n = 673, age 63 ± 14 years, mean serum sclerostin (ELISA) 1.24 ± 0.57 ng/ml) were negatively correlated with PTH levels (r = −0.25, p < 0.001) [51].
Other researchers confirmed statistically relevant associations between sclerostin and classical CKD-MBD parameters in the serum of CKD patients (e.g., with FGF-23 [52]). These mathematical correlations may reflect a biological (causal) background, as described below, for the interaction between sclerostin and PTH (PTH suppresses sclerostin expression in osteocytes). Sclerostin is thought to participate in several biochemical feedback loops, as evidenced by the fact that sclerostin-deficient mice reveal alterations in a number of classical biochemical CKD-MBD-related parameters [27]. While serum calcium and PTH levels are not different between sclerostin-knockout and wild-type mice, FGF-23 levels are about 2.5 times lower in sclerostin-deficient mice compared to the wild type, and conversely 1,25-dihydroxyvitamin D and serum phosphate levels were significantly elevated. Sclerostin alters vitamin D synthesis in proximal tubular cells [27]. Hence, the above-mentioned endocrine skeletal activity of sclerostin is not limited to paracrine effects. Taken together, elevated concentrations of circulating sclerostin (plasma, serum) are a novel player participating in the biochemical concert of CKD-MBD alterations.
Step 8: Wnt signaling and inhibitors in uremic bone disease
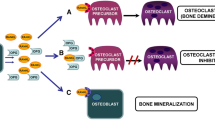
An important aspect to consider is a potentially causal role of Wnt signaling and its dysregulation, respectively, in the development of renal osteodystrophy. Data regarding how sclerostin and DKK-1 can cause or mediate renal osteodystrophy are still sparse. In rodent models, absence of sclerostin is undoubtedly associated with a high bone mass and a “healthy” bone phenotype as reviewed above. So theoretically, uremic SOST overexpression might be one contributor to disturbed bone metabolism as seen in renal osteodystrophy (low bone mass and altered metabolism).
PTH downregulates sclerostin expression in osteocytes and this interaction represents an important aspect of how PTH stimulates bone metabolism [53]. There is substantial experimental evidence available that a balanced cross-talk between PTH and sclerostin is relevant for bone physiology. PTH administration rapidly reduces sclerostin mRNA as well as protein production in osteocytes [53, 54]. In healthy conditions, PTH anabolism to a certain extent is mediated by suppressing the anti-anabolic activity of sclerostin. Patients with primary hyperparathyroidism have lower serum sclerostin. As already discussed, a negative correlation has been reported between PTH and sclerostin concentrations both in renal and non-renal individuals [51, 55]. Kramer and co-workers convincingly showed how much skeletal PTH actions rely upon sclerostin physiology [25]. They investigated the effects of intermittent PTH-administration in mouse models with sclerostin overexpression and also with sclerostin deficiency. Six month-old genetically engineered mice underwent a 2-month treatment period with 1–34 PTH. Both sclerostin-deficient and sclerostin-overexpressing mice revealed the expected skeletal phenotype, i.e., high bone mass in the former and severe osteopenia in the latter. In both of these genetically engineered mouse models, the response to intermittent PTH treatment in terms of stimulation of bone metabolism was significantly diminished. Therefore the authors came to the conclusion that suppressibility of sclerostin in osteocytes is necessary to mediate anabolic responses to PTH [25]. Another interesting aspect within this context is that PTH also exerts its bone anabolic effects via LRP6 – as already discussed, an important co-receptor within the canonical Wnt signaling [56].
Conditions associated with supra-physiological sclerostin activity thus may impede PTH-mediated bone anabolism. Adynamic bone disease (ABD) is a subtype of renal osteodystrophy characterized by substantially reduced bone formation rate, impaired remodeling activity, and reduced osteoblastic and osteoclastic activity [57] (Fig. 3). In uremia, high sclerostin levels may exacerbate PTH-resistance, which could cause and/or aggravate ABD. Bone biopsy findings support this theory. A study in which 60 adult dialysis patients underwent a bone biopsy after tetracycline labeling revealed a statistically negative correlation between serum sclerostin and PTH [58]. Most importantly, sclerostin showed strong negative associations with parameters of bone turnover, pointing towards a role of high sclerostin levels in the development of ABD [58]. So the slightly elevated PTH levels in ABD might actually resemble a state of relative hypoparathyroidism in which PTH cannot overcome skeletal actions of bone suppressive mediators contributing to uremic PTH resistance of bone. Based on our current knowledge, sclerostin qualifies as a suitable candidate to aggravate this PTH resistance. Experimental evidence indicates that the phosphorus load triggers development of renal osteodystrophy and that this causative role may at least in part be mediated via stimulation of sclerostin expression [59]. Ferreira and co-workers performed subtotal nephrectomy and total parathyroidectomy in rats, which afterwards were fed a high- or low-phosphorus diet for 8 weeks. Compared to the 0.6 % phosphorus diet, the 1.2 % phosphorus diet significantly stimulated SOST gene expression in tibial tissue and serum sclerostin levels (about eight times higher each). This in turn was associated with a lower bone volume. However, bone formation rate per bone surface, as a sensitive marker for ABD, was comparable between the two diets [59].
A very recent publication about the jck mouse model, i.e., a mouse model of moderate renal failure, contributed remarkably to our understanding about the timely evolution of CKD-MBD parameters during the course of the disease [49]. Interestingly, osteocytic sclerostin expression and consecutive suppression of beta-catenin signaling in this mouse model occurs earlier (week 5) than biochemical changes in serum PTH or FGF-23 (week 10). The increased sclerostin expression also preceded cardiovascular (starting at about week 15) and skeletal (starting at about week 9) changes typically seen in CKD-MBD [34]. The rise in sclerostin-positive osteocytes was transient and diminished with increasing hyperparathyroidism. These findings turn sclerostin alterations into a particularly interesting CKD-MBD parameter, since the time course of activation might point towards causality.
Step 9: Wnt signaling and inhibitors in (uremic) vascular disease
Wnt signaling plays a role in human atherosclerosis [60] and missense mutations in LRP6 are associated with premature coronary artery disease and osteoporosis [5]. Another study showed that rare (611C) and common (1062 V) loss-of-function variants of LRP6 display reduced activation of Wnt/beta-catenin signaling. The rare gene variant 611C was associated with hypertension, metabolic abnormalities, and early coronary artery disease. Moreover, also the more common LRP6 variant 1062 V associates strongly with the presence of coronary artery disease in hypertensive patients [61].
Vitamin K antagonist treatment such as warfarin is suspected to trigger vascular calcification [62, 63]. Beazley and co-workers showed that warfarin activates beta-catenin signaling in vascular smooth muscle cells (VSMCs) in vitro by: i) increasing the amount of total beta-catenin protein, ii) upregulating its nuclear translocation, and iii) stimulating transcription of beta-catenin target genes [64]. To the best of our knowledge, there are no data regarding the cardiovascular status in sclerosteosis, van Buchem’s disease or mice with genetically modified sclerostin expression. While participation of Wnt signaling in general atherosclerosis has already been extensively reviewed [60], the particular role of sclerostin (and also DKK-1) in uremic vascular disease is a novel, evolving field. Their role is currently incompletely understood, since previous findings are partly contradictory and important research questions have not been addressed yet. Recent research indicates that in human aortic valve tissue from hemodialysis patients with microscopic as well as macroscopic calcification, a significant local sclerostin mRNA upregulation is detectable which is absent in aortic valve tissue from hemodialysis patients without calcification [46]. So it is not uremia per se but the calcification process which is seemingly responsible for local sclerostin expression in the vascular system. Similarly, local sclerostin deposition was detectable in human atherosclerotic lesions via IHC staining (Fig. 4, courtesy of Dr. Nadine Kaesler, Aachen). This up-regulated local expression in calcified tissue is accompanied by augmented circulating levels of sclerostin which gradually increase, when measured with the same ELISA, from non-uremic individuals without calcific aortic disease, to patients with either one of the diseases, to uremic patients with calcific aortic disease in parallel [46]. It remains unclear whether elevated serum sclerostin levels originate from the vasculature or the bone or both [46, 49]. Experimental in vitro data from Zhu and co-workers confirm sclerostin expression in calcifying VSMCs [65]. In vitro, VSMCs express osteocytic markers when grown in a pro-calcific environment, indicative of an osteoblastic to osteocytic transition (terminal transdifferentiation) [65]. Accordingly, the same authors found in vivo expression of sclerostin in calcified mouse aortas. Occurrence of sclerostin in calcified aortic valve tissue is not limited to hemodialysis patients but also occurs in patients with dominant calcific aortic stenosis [66]. Moreover, ectopic sclerostin production and deposition was also detectable in skin specimens from patients with calciphylaxis (Fig. 5) [67], while no such local sclerostin was found in control skin specimens from dialysis patients without cutaneous calcification. Vascular calcification can be quantified by non-invasive means, such as X-ray or computed tomography, and several reports about the association between calcification burden and serum sclerostin levels have been published. The correlation between calcification and circulating sclerostin was described as positive in non-uremic calcific aortic stenosis [66], in uremia-associated calcific aortic stenosis [46] and in aortic artery calcification in postmenopausal women [40]. However, no such statistically significant correlation has been reported between uremia-associated coronary artery calcification and sclerostin levels [46]. Of note, even contradictory findings have been published: Register and coworkers [28] investigated vascular calcification in 450 diabetic African Americans. After full adjustment, only calcification of the carotid artery in men (not women) remained inversely and significantly associated with serum sclerostin [28]. Similarly, Claes et al. also found that lower, not higher, sclerostin levels were determined as independent predictors of aortic calcification as assessed by lumbar X-ray in a cohort of 154 CKD patients [68]. Interpretation of these heterogeneous and partly conflicting data needs to take into account the anatomical structures (aortic valve, coronary artery, large elastic arteries), heterogeneity of study populations (particularly dialysis versus non-dialysis cohorts) and different calcification detection methods, as well as technical differences between various sclerostin assays. It is important to mention that comparisons of mean Wnt inhibitor levels between cohorts should be limited to samples analyzed with the same assays [46, 66]. Our group for example previously used two different ELISA-based sclerostin assays in hemodialysis patients (Biomedica, Austria and TECOmedical, Switzerland). Using the TECOmedical assay in 673 patients the mean serum level was determined as 1.24 ± 0.57 ng/ml [51], which is different to the median level of 110 (82–151) pmol/l obtained in 100 hemodialysis patients [50]. Moreover, gender- and diabetes-related differences regarding sclerostin levels could only be obtained within the latter cohort and the Biomedica assay [50]. We are currently unable to define diagnostic superiority for one of these two assays, but such studies are on their way.
Taken together, Wnt signaling activity in general and sclerostin activity in particular are linked to ectopic, vascular calcification processes and therefore exert calcification effects beyond bone mineralization. Further research needs to determine whether sclerostin’s role in arteriosclerosis is pro- or anti-calcific and whether this role might vary dependent on background conditions, e.g., CKD.
Step 10: Determination of Wnt signaling and outcome—is there a link?
Since vascular calcification influences cardiovascular event rate and mortality in CKD patients, researchers also explored the relationship between sclerostin and future outcome in ESRD patients. This association was for example assessed in the NECOSAD cohort [51]. After adjustment for various clinical and biochemical parameters, patients in the highest sclerostin tertile 3 months after initiation of dialysis had a significantly lower risk of cardiovascular death (HR 0.29, 95 % CI 0.13–0.62) and all-cause mortality (0.39, 95 % CI 0.22–0.68) within 18 months compared to patients of the lowest tertile. These findings are not in line with a previously published smaller cohort of CKD patients, in which the authors detected a reciprocal sclerostin effect upon mortality; i.e., levels above the median were associated with worse cardiovascular outcome [52]. Another study by Viaene and co-workers dichotomized a cohort of long-term dialysis patients according to the median level of sclerostin and detected a significant survival benefit for patients above the median after adjusting for age and gender (HR 0.33, 95 % CI 0.15–0.73, p = 0.006). However, within a fully adjusted model including bone-specific alkaline phosphates, the association between survival and sclerostin lost statistical significance [50]. Again, data are not homogeneous. Similarly to the situation in the above-mentioned calcification studies, these three survival studies were also performed in cohorts with different clinical background and applying different sclerostin assays—factors that potentially influence these findings. It remains an open question as to whether sclerostin will ever qualify as a biomarker for cardiovascular health status and for survival prediction in CKD-MBD.
Step 11: Sclerostin in pediatric populations
To our knowledge, no data are available on sclerostin in CKD or ESRD children and adolescents. The role of sclerostin in the growing skeleton under uremic conditions has not yet been investigated. Since puberty is the time when 25–50 % of the adult bone mass is accrued, the growing skeleton might be particularly vulnerable to over-activity of Wnt-signaling inhibitors. Very little data has been published recently on sclerostin in pediatric populations and these are conflicting. On the one hand, it has been demonstrated that sclerostin levels increase with age in adults of both sexes with a significantly higher levels in men compared to women [69]; on the other hand, Fischer et al. showed in a cross-sectional study of 424 healthy children, adolescents and young adults (aged 0.1–21 years, 221 males) that sclerostin levels were independent of age and gender [70]. However, Kirmani et al. showed that sclerostin levels were higher in boys than in girls and this gender difference already appears during puberty, pointing towards a role of estrogen in reducing sclerostin levels [71]. Furthermore, they demonstrated that serum sclerostin levels peak early in life (at about age 10 in girls and 14 in boys), decline during the later stages of puberty towards a nadir at the end of puberty, and then increase again over the remaining adult lifetime.
Step 12: The future: research perspectives regarding sclerostin in CKD-MBD
We consider the following open questions as important issues and we think they qualify as research targets in order to better define the role of sclerostin in CKD-MBD:
-
1)
Assay reliability and comparability for sclerostin measurements need to be assessed prior to implementation of sclerostin measurement in clinical practice.
-
2)
It is unknown whether, and to what extent, nephrological standards of care, e.g., active vitamin D or calcimimetics, alter levels of soluble Wnt inhibitors.
-
3)
The potential association between sclerostin serum levels and outcome should translate into research efforts defining whether therapeutic modification of sclerostin levels associates with modified outcome.
-
4)
It is not known to what extent circulating Wnt signaling inhibitor levels in CKD patients originate from the vasculature or the bone. At least in postmenopausal women, serum sclerostin levels correlate closely with bone marrow plasma levels, suggesting that serum levels, at least to some extent, reflect status in the bone microenvironment [72]. So further research needs to define the origin of increased blood levels of sclerostin and if circulating levels in CKD-MBD patients reflect distinct tissue activities.
-
5)
Further research is needed to explore to what extent increased local and/or systemic Wnt signaling antagonistic activities contribute to renal osteodystrophy in general, and particularly with respect to ABD.
-
6)
The potential impact of disturbed Wnt signaling in children and adolescents with CKD or ESRD deserves special attention. We need to evaluate how Wnt signaling participates in the regulation of bone mass and structure during this critical period for skeletal development under uremic conditions.
-
7)
The potential contribution of Wnt signaling in uremic vascular disease, as reflected for example by local tissue activities of sclerostin, fuels speculations about a contribution of Wnt signaling in accelerated uremic arteriosclerosis. More research is needed to clarify the role of sclerostin as pro- or anti-calcific.
-
8)
While anti-sclerostin antibodies theoretically may help to overcome situations with over-suppressed bone metabolism in uremia (=ABD) it seems of outstanding importance to further investigate the consequences of therapeutic blockade of sclerostin in accelerated uremic calcification.
Conclusions
In conclusion, sclerostin has made its way down the road from the bone niche to the cardiovascular field. Due to its strong links to CKD, sclerostin qualifies as a novel mediator of CKD-MBD. Based on our current knowledge, sclerostin may be considered as one of the driving forces of the calcification paradox in the bone-vascular axis. The available data about sclerostin and CKD-MBD as summarized in the present review originate solely from studies in adults. Corresponding data form children are absent, yet it is tempting to speculate how sclerostin might be involved in adolescent CKD-MBD. These gaps in our understanding bring us directly to potential future research targets and make us speculate about what burning questions should be urgently addressed.
References
Kestler HA, Kuhl M (2008) From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci 363:1333–1347
Kim W, Kim M, Jho EH (2013) Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J 450:9–21
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ (2012) Update on Wnt signaling in bone cell biology and bone disease. Gene 492:1–18
Kubota T, Michigami T, Ozono K (2010) Wnt signaling in bone. Clin Pediatr Endocrinol 19:49–56
Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP (2007) LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315:1278–1282
Dejana E (2010) The role of Wnt signaling in physiological and pathological angiogenesis. Circ Res 107:943–952
Johnson ML, Kamel MA (2007) The Wnt signaling pathway and bone metabolism. Curr Opin Rheumatol 19:376–382
Rajamannan NM (2011) The role of Lrp5/6 in cardiac valve disease: LDL-density-pressure theory. J Cell Biochem 112:2222–2229
Vervloet MG, Massy ZA, Brandenburg VM, Mazzaferro S, Cozzolino M, Urena-Torres P, Bover J, Goldsmith D (2014) Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol 2:427–436
Cozzolino M, Urena-Torres P, Vervloet MG, Brandenburg V, Bover J, Goldsmith D, Larsson TE, Massy ZA, Mazzaferro S (2014) Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant 29:1815–1820
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33:747–783
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Capulli M, Paone R, Rucci N (2014) Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys 561:3–12
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ (2008) Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A 105:20764–20769
Baron R, Rawadi G (2007) Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep 5:73–80
Choi HY, Dieckmann M, Herz J, Niemeier A (2009) Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One 4:e7930
Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Schmitz Y, Rateitschak K, Wolkenhauer O (2013) Analysing the impact of nucleo-cytoplasmic shuttling of beta-catenin and its antagonists APC, Axin and GSK3 on Wnt/beta-catenin signalling. Cell Signal 25:2210–2221
Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346:1513–1521
Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18:960–974
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39:91–97
Morvan F, Boulukos K, Clement-Lacroix P, Roman RS, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G (2006) Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21:934–945
Robinson MK, Caminis J, Brunkow ME (2013) Sclerostin: how human mutations have helped reveal a new target for the treatment of osteoporosis. Drug Discov Today 18:637–643
Kramer I, Loots GG, Studer A, Keller H, Kneissel M (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189
Yao GQ, Wu JJ, Troiano N, Insogna K (2011) Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab 29:141–148
Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A 110:6199–6204
Register TC, Hruska KA, Divers J, Bowden DW, Palmer ND, Carr JJ, Wagenknecht LE, Hightower RC, Xu J, Smith SC, Dietzen DJ, Langefeld CD, Freedman BI (2014) Sclerostin is positively associated with bone mineral density in men and women and negatively associated with carotid calcified atherosclerotic plaque in men from the African American-Diabetes Heart Study. J Clin Endocrinol Metab 99:315–321
Dovjak P, Dorfer S, Foger-Samwald U, Kudlacek S, Marculescu R, Pietschmann P (2014) Serum levels of sclerostin and dickkopf-1: effects of age, gender and fracture status. Gerontology 60:493–501
Viapiana O, Fracassi E, Troplini S, Idolazzi L, Rossini M, Adami S, Gatti D (2013) Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 92:324–329
Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A (2012) Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab 97:148–154
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420
van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE (2011) Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res 26:2804–2811
Evenepoel P, D’Haese P, Brandenburg V (2014) Romosozumab in postmenopausal women with osteopenia. N Engl J Med 370:1664
Nakamura T, Nakamura T, Matsumoto K (2008) The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J Cell Mol Med 12:391–408
Gifre L, Ruiz-Gaspa S, Monegal A, Nomdedeu B, Filella X, Guanabens N, Peris P (2013) Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone 57:272–276
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Izpisua Belmonte JC, Westphal H (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1:423–434
Hampson G, Edwards S, Conroy S, Blake GM, Fogelman I, Frost ML (2013) The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone 56:42–47
Vivante A, Mark-Danieli M, Davidovits M, Harari-Steinberg O, Omer D, Gnatek Y, Cleper R, Landau D, Kovalski Y, Weissman I, Eisenstein I, Soudack M, Wolf HR, Issler N, Lotan D, Anikster Y, Dekel B (2013) Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol 24:550–558
He W, Kang YS, Dai C, Liu Y (2011) Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22:90–103
Fang Y, Ginsberg C, Seifert M, Agapova O, Sugatani T, Register TC, Freedman BI, Monier-Faugere MC, Malluche H, Hruska KA (2014) CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol 25:1760–1773
Floege J (2014) Antagonism of canonical Wnt/beta-catenin signaling: taking RAS blockade to the next level? J Am Soc Nephrol 26:3–5
Cannata-Andia JB, Roman-Garcia P, Hruska K (2011) The connections between vascular calcification and bone health. Nephrol Dial Transplant 26:3429–3436
Brandenburg VM, Kramann R, Koos R, Kruger T, Schurgers L, Muhlenbruch G, Hubner S, Gladziwa U, Drechsler C, Ketteler M (2013) Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol 14:219
Cejka D, Marculescu R, Kozakowski N, Plischke M, Reiter T, Gessl A, Haas M (2014) Renal elimination of sclerostin increases with declining kidney function. J Clin Endocrinol Metab 99:248–255
Bonani M, Rodriguez D, Fehr T, Mohebbi N, Brockmann J, Blum M, Graf N, Frey D, Wuthrich RP (2014) Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press Res 39:230–239
Sabbagh Y, Graciolli FG, O’Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC (2012) Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 27:1757–1772
Viaene L, Behets GJ, Claes K, Meijers B, Blocki F, Brandenburg V, Evenepoel P, D’Haese PC (2013) Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant 28:3024–3030
Drechsler C, Evenepoel P, Vervloet MG, Wanner C, Ketteler M, Marx N, Floege J, Dekker FW, Brandenburg VM (2014) High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. doi:10.1093/ndt/gfu301
Kanbay M, Siriopol D, Saglam M, Gulcan KY, Gok M, Cetinkaya H, Karaman M, Umut UH, Oguz Y, Sari S, Eyileten T, Goldsmith D, Vural A, Veisa G, Covic A, Ilker YM (2014) Serum sclerostin and adverse outcomes in non-dialyzed chronic kidney disease patients. J Clin Endocrinol Metab 99:E1854–E1861
Bellido T, Saini V, Pajevic PD (2013) Effects of PTH on osteocyte function. Bone 54:250–257
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37:148–158
Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R (2013) Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab 98:3873–3883
Li C, Xing Q, Yu B, Xie H, Wang W, Shi C, Crane JL, Cao X, Wan M (2013) Disruption of LRP6 in osteoblasts blunts the bone anabolic activity of PTH. J Bone Miner Res 28:2094–2108
Cannata-Andia JB, Rodriguez GM, Gomez AC (2013) Osteoporosis and adynamic bone in chronic kidney disease. J Nephrol 26:73–80
Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, Haas M, Malluche HH (2011) Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6:877–882
Ferreira JC, Ferrari GO, Neves KR, Cavallari RT, Dominguez WV, dos Reis LM, Graciolli FG, Oliveira EC, Liu S, Sabbagh Y, Jorgetti V, Schiavi S, Moyses RM (2013) Effects of dietary phosphate on adynamic bone disease in rats with chronic kidney disease–role of sclerostin? PLoS One 8:e79721
Marinou K, Christodoulides C, Antoniades C, Koutsilieris M (2012) Wnt signaling in cardiovascular physiology. Trends Endocrinol Metab 23:628–636
Sarzani R, Salvi F, Bordicchia M, Guerra F, Battistoni I, Pagliariccio G, Carbonari L, Dessi-Fulgheri P, Rappelli A (2011) Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor-related protein 6 gene variant. Nutr Metab Cardiovasc Dis 21:150–156
Cozzolino M, Brandenburg V (2010) Warfarin: to use or not to use in chronic kidney disease patients? J Nephrol 23:648–652
Kruger T, Oelenberg S, Kaesler N, Schurgers LJ, van de Sandt AM, Boor P, Schlieper G, Brandenburg VM, Fekete BC, Veulemans V, Ketteler M, Vermeer C, Jahnen-Dechent W, Floege J, Westenfeld R (2013) Warfarin induces cardiovascular damage in mice. Arterioscler Thromb Vasc Biol 33:2618–2624
Beazley KE, Deasey S, Lima F, Nurminskaya MV (2012) Transglutaminase 2-mediated activation of beta-catenin signaling has a critical role in warfarin-induced vascular calcification. Arterioscler Thromb Vasc Biol 32:123–130
Zhu D, Mackenzie NC, Millan JL, Farquharson C, MacRae VE (2011) The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One 6:e19595
Koos R, Brandenburg V, Mahnken AH, Schneider R, Dohmen G, Autschbach R, Marx N, Kramann R (2013) Sclerostin as a potential novel biomarker for aortic valve calcification: an in-vivo and ex-vivo study. J Heart Valve Dis 22:317–325
Kramann R, Brandenburg VM, Schurgers LJ, Ketteler M, Westphal S, Leisten I, Bovi M, Jahnen-Dechent W, Knuchel R, Floege J, Schneider RK (2013) Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant 28:856–868
Claes KJ, Viaene L, Heye S, Meijers B, D’Haese P, Evenepoel P (2013) Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab 98:3221–3228
Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ III, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379
Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D (2012) Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem 49:546–553
Kirmani S, Amin S, McCready LK, Atkinson EJ, Melton LJ III, Muller R, Khosla S (2012) Sclerostin levels during growth in children. Osteoporos Int 23:1123–1130
Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandenburg, V.M., D’Haese, P., Deck, A. et al. From skeletal to cardiovascular disease in 12 steps—the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol 31, 195–206 (2016). https://doi.org/10.1007/s00467-015-3069-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3069-7