Abstract
Declining kidney function is associated with sequential systemic changes in mineral homeostasis leading to pathologic alterations in the cardiovascular system and the skeleton. One of the earliest changes in response to renal injury is the increased osteocyte production of secreted factors including the anti-anabolic protein, sclerostin. Elevated sclerostin is associated with reduced Wnt/β-catenin signaling in bone and decreased osteoblast differentiation/activity. Agents that directly or indirectly inhibit β-catenin signaling have differential skeletal effects suggesting additional mechanisms contribute to the diversity of renal osteodystrophies. Similarly, Wnt/β-catenin activation in smooth muscle cells contributes to cardiovascular calcification yet emerging data suggests that this pathway may also be protective when elevated in neighboring tissues. The ongoing epidemiology studies examining the relationship between circulating sclerostin and cardiovascular disease, particularly those that investigate stage specific and/or patient sub-populations, will be useful in understanding the overall contributions of this pathway, its antagonist sclerostin, and the progression of CKD-MBD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease-mineral bone disorder (CKD-MBD) is a syndrome that occurs secondary to declining renal function and involves alterations in systemic hormone levels with resulting dysregulation of calcium and phosphorus metabolism [1, 2]. Multiple epidemiology studies have demonstrated that these changes are associated with decline in bone health leading to enhanced fracture rates that are coupled to pathologic changes in the cardiovascular system (including reduced vascular wall elasticity, vascular calcifications, and left ventricular hypertrophy) [3•, 4]. Given that cardiovascular disease is the major cause of death in individuals with kidney disease, numerous studies have been aimed at unraveling the pathologic mechanisms responsible for the parallel changes in the skeletal and cardiovascular systems. As a consequence, new findings over the last decade and a half have enhanced our understanding of these mechanisms but also illustrated the complexity of the systematic hormonal changes responsible for these pathologies. Alterations in the phosphaturic and calcemic hormones, fibroblast growth factor 23 (FGF23)/Klotho, 1,25 Vitamin D3, and parathyroid hormone (PTH) have been well described elsewhere [5, 6]. This review summarizes the emerging understanding of the relationship between sclerostin, the Wnt/β-catenin pathway, and the pathology of CKD-MBD.
The Role of Sclerostin in Wnt/β-Catenin Signaling in Bone Metabolism
Based on the influence of naturally occurring human mutations and the consequences of directed mouse genetics, it is now well established that under normal physiologic conditions, canonical Wnt/β-catenin signaling in bone is predominantly an anabolic pathway [7, 8••]. In humans, loss-of-function mutations that attenuate Wnt/β-catenin signaling are generally associated with decreased bone mass whereas the converse is true for gain-of-function mutations. These findings are also substantiated by mouse genetic studies and by genome-wide association studies that have identified relationships between gene polymorphisms within specific pathway components and bone mass [8••].
In general, Wnt/β-catenin signaling is associated with increased differentiation and/or function of cells within the osteoblast/osteocyte lineage. Activation of this pathway promotes differentiation of mesenchymal stem cells toward the osteoblast lineage and increases proliferation of committed osteoblast precursors. The anabolic actions of Wnt/β-catenin signaling in osteoblast/osteocytes are also enhanced by its indirect inhibition of osteoclast differentiation through up-regulation of OPG, the antagonist to the osteoclast differentiation factor, RANKL.
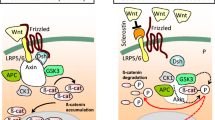
Wnt ligands bind to a cell surface complex comprised of their receptor, Frizzled, and one of the co-receptors, LRP5 or 6 thereby promoting cytoplasmic β-catenin stabilization, translocation to the nucleus, and regulated gene transcription [7, 8••]. In addition to the local expression of specialized Wnt ligands, regulation of this canonical pathway is also dependent on the relative expression of inhibitors that bind to either Wnts themselves, (Frizzled Related Proteins, FRPs), or to one of the LRP co-receptors (sclerostin or Dkk1). Thus, under normal conditions, bone homeostasis is fine tuned by the relative expression of specific Wnts versus pathway antagonists.
Although multiple Wnt antagonists have been linked to disturbances in bone homeostasis, recent attention has focused on sclerostin, in part due to the promising therapeutic potential of sclerostin antibodies as an anabolic therapy for osteoporosis. Declining bone mass is associated with aging, attenuation of Wnt/β-catenin signaling in bone, and increased sclerostin expression in osteocytes [9]. Anti-sclerostin antibodies in rats and primates promote osteoanabolic effects with increases in bone formation and bone strength [10, 11]. In clinical studies, anti-sclerostin antibodies have been shown to induce changes in serum markers of bone turnover and improvements in bone mineral density in individuals with osteopenia/osteoporosis [12]. Although skeletal complications are commonly associated with CKD, it is only until recently that evidence has accumulated for potential roles of sclerostin and Wnt/β-catenin signaling in renal osteodystrophy.
Repression of Wnt/β-Catenin Signaling in Renal Osteodystrophy
Renal osteodystrophy is a spectrum of bone disorders ranging from low to high turnover [2]. Classically, PTH elevation beginning about stage 3 induces high turnover bone disease (osteitis fibrosis) through indirect increase in osteoclast numbers. The mechanism for the predominant catabolic activity of sustained elevated PTH is consistent with studies examining the influence of therapeutic PTH administration. PTH is known to bind directly to cells of the osteoblast/osteocyte lineage and promote increased RANKL expression leading to osteoclast activation. In the case of intermittent PTH administration, the effect is transient, with RANKL actions balanced by the subsequent rising levels of its antagonist, osteoprotegerin (OPG), thus accounting for a short burst of osteoclast activity. The transient activation of osteoclast activity leads to increased osteoblast actions causing the classic bone anabolic PTH response [13]. In contrast, catabolic activity is observed when PTH concentrations are sustained, such as in the context of continuous PTH therapy or pathologic hyperparathyroidism, since RANKL expression remains high. Therapies that suppress PTH production or actions are mainstream in the treatment of mid-to-late stage CKD patients with high serum PTH levels. Mechanistically, PTH’s differential regulation of the RANKL/OPG ratio appears to occur via cross talk with the Wnt/β-catenin pathway [14, 15]. The PTH/PTHR1 receptor complex has been shown to directly bind and phosphorylate the Wnt co-receptor, LRP6 in a cAMP-dependent manner thereby promoting β-catenin stabilization in the absence of Wnt binding [16]. PTH’s effect on β-catenin explains its influence on sclerostin, as sclerostin down-regulation is a classic target of β-catenin-controlled transcription. Taken together, the mechanism of high bone turnover via a PTH-dependent influence on β-catenin pathway appears clear. However, as discussed below, this mechanism cannot account for bone changes associated with CKD.
A single cause of low turnover bone disease in CKD has not been identified although occurrences late in disease are attributed to therapeutic over-suppression of PTH [17]. Low bone turnover disease can also be found at earlier stages in individuals who have not received PTH lowering therapies or do not have obvious risk factors for pre-existing osteoporosis [18]. These observations together with studies examining bone changes in a diabetic mouse model with induced CKD [19] raised the possibility that a reduction in osteoblast differentiation/activity might be an early event in CKD. To explore this hypothesis further, a recent study used a progressive genetic model of CKD to correlate the temporal histomorphometric alterations with molecular changes in bone. An overall repression in Wnt/β-catenin signaling was suggested based on the finding of an early increase in sclerostin positive osteocytes that correlated with increased expression of inactive phosphorylated β-catenin [20••]. Attenuated signaling was confirmed by a corresponding reduction in the expression of Wnt/β-catenin target genes that are typically associated with osteoblast/osteocyte differentiation. These alterations occurred despite a paradoxical increase in osteoclast gene expression and enhanced bone formation rates that occurred before detectable PTH elevation [20••]. It is not clear whether the PTH independent changes in BFR are unique to the particular animal model, but several independent studies support a mechanistic link with decreased osteocyte β-catenin activity and osteoclast inactivation. Osteocyte-specific deletion of β-catenin causes an increase in the RANK/OPG ratio and enhanced osteoclast activity [21]. Furthermore, sclerostin has been shown to increase osteocyte expression of RANK/OPG in vitro [22]. Thus, abnormal increases in osteoclast activity may reflect sustained alterations in osteocytes’ relative RANK/OPG expression that is typically associated with a transient and/or context-dependent response.
The changes in osteocyte expression of sclerostin and β-catenin activation were also observed in clinical bone biopsies representing different types of osteodystrophy and from individuals with varying disease severity [20••, 23]. Similar to observations in animal models, clinical biopsies revealed elevated sclerostin and inactivate β-catenin positive osteocytes with the greatest effect observed in early stages. Consistent with the known effect of PTH to reduce sclerostin transcription [24, 25], expression declined somewhat in biopsies from individuals with elevated PTH but remained high relative to non-CKD. These findings raise the possibility that a common feature of renal osteodystrophy is an overall decrease in osteoblast differentiation and/or activity regardless of the relative osteoclast activity. Thus, in the context of high formation rates, while osteoblast activity may be elevated above normal, it may be insufficient to dampen the enhanced osteoclast activity.
Consequence of Sclerostin and β-Catenin Changes
Several studies have assessed the consequences of defective Wnt/β-catenin by testing the influence of β-catenin agonist therapies in animal models of CKD-MBD. A recent study by Moe and colleagues assessed the effects of anti-sclerostin in normal animals relative to a slow-progressing model of CKD-MBD with imposed low or high PTH levels [26••]. Antibody treatment had no influence on bone health in CKD animals with high PTH, elevated bone turnover, and cortical porosity, but did improve trabecular bone volume and mineralization in CKD animals with low PTH values that had bone defects associated with low bone formation. These results were correlated with a reduction in bone expression of inactive phosphorylated β-catenin, confirming its ability to restore Wnt/β-catenin signaling. In normal animals, the antibody treatment improved bone volume, cortical geometry, and biomechanical properties. The failure of the antibody to improve bone strength in the context of CKD suggests that while β-catenin contributes to bone disease, restoration of Wnt binding to the receptor may not be sufficient to overcome all bone changes associated with low bone turnover osteodystrophy and that additional mechanisms may contribute to underlying defects in CKD.
Despite the limitations, the positive benefits of the sclerostin antibody to restore Wnt binding is consistent with a previous study demonstrating that antibodies to another LRP5/6 antagonist, Dkk1 also improved bone health and cardiovascular disease in a mouse model of diabetes with injury-induced CKD progression [27]. The investigators had previously characterized the bone, vascular, and biochemical changes related to CKD-MBD in this model with pre-existing atherosclerotic lesions [28•]. Transient hyperphosphatemia was associated with the acute renal failure phase with corresponding increased serum levels of the osteocyte-expressed proteins, FGF23, and sclerostin that were consistent with CKD progression into CKD [27]. Additionally, molecular changes associated with vascular calcification, including evidence of vascular osteoblast transition, were observed when glomerular filtration rates were equivalent to human CKD stage 2. Bone changes were characterized by reduced bone formation rates greater than increased resorption reflecting a low bone turnover state similar to the model described above in the anti-sclerostin studies. Renal injury was associated with enhanced Wnt production, a well-characterized repair response, with corresponding over-production, via a negative feedback mechanism, of the pathway antagonists DKK1, sclerostin, and sFRP 1, 2, and 4. In the context of kidney disease, these authors have suggested that enhanced renal production of Dkk1 and sclerostin contribute to their increased circulating levels leading to downstream consequences in bone and vasculature. Administration of a neutralizing DKK1 antibody at the end of the renal injury phase decreased circulating sclerostin levels, prevented vascular calcification, and improved bone health without restoring renal function.
Taken together, these data from the anti-sclerostin and anti-Dkk1 studies are consistent with a model in which elevated local or systemic levels of the Wnt-binding antagonists, sclerostin, and/or Dkk1 contribute to low turnover osteodystrophy via inhibition of the canonical Wnt/β-catenin pathway. However, as described above, reduced activation of the Wnt/β-catenin pathway also appears to be associated with high turnover in the setting of CKD suggesting that additional mechanism(s) contribute to the diversity of osteodystrophy pathologies.
Further insight into potential mechanistic differences between high and low bone turnover have been obtained through studies using neutralizing antibodies to transforming growth factor beta (TGFβ) [29••]. TGFβ is a family of ubiquitous growth factors that play a prominent role in bone biology [30]. Similar to other autocrine/paracrine factors, TGFβ activities on bone are diverse and dependent on regulation by negative and positive regulators that orchestrate specific TGFβ functions in a spatial and temporal manner. In normal bone remodeling, TGFβ1 released from the bone matrix by osteoclasts attracts mesenchymal stem cells to sites of resorption thereby ensuring that new bone restores eroded older bone [31]. TGFβ also promotes osteoblast proliferation but later restricts osteoblast maturation by repressing the expression of genes involved in bone formation [32].
TGFβ is elevated in serum, bone, and other tissues in CKD [29••]. The availability of a neutralizing TGFβ antibody provided an opportunity to evaluate its potential role in the pathology of renal osteodystrophy [29••]. In contrast to the influence of anti-sclerostin and anti-Dkk1 antibodies on low or normal bone turnover, TGFβ neutralization improved bone architecture only in normal or high turnover states. TGFβ neutralization led to normalization of bone turnover markers, reduction in bone formation, improved trabecular and cortical architecture, and reduced cortical porosity in three independent models of high turnover. The influence of the antibody is consistent with efficacy on other high bone turnover conditions including Calmurati-Engelmann disease, osteitis fibrosis, and on localized lesions in osteoarthritis [31, 33, 34]. Surprisingly, TGFβ neutralization, with expected reductions in SMAD signaling, decreased SOST mRNA expression and restored β-catenin to normal levels as evidenced by enhanced expression of genes downstream of Wnt/β-catenin signaling. These alterations were independent of changes in circulating PTH levels.
The mechanistic link of TGFβ neutralization and restoration or β-catenin signaling has yet to be delineated. Like, PTH, TGFβ has been shown to stabilize β-catenin levels downstream of Wnt signaling [35]. Since TGFβ is known for temporal actions on osteoblasts that first promote and then attenuate osteoblast differentiation, it is conceivable that continuous pathologic over-expression could be associated with sustained inhibition of osteoblast maturation and perhaps attenuation of β-catenin. TGFβ has been shown to attenuate its own and PTH responses by promoting complex formation between PTHR1 and its receptor, TβRII [36]. Thus, crosstalk between TGFβ and PTH signaling could result in dampening of the anabolic responses of each hormone, consistent with an overall de-stabilization of β-catenin. Additionally, recent evidence demonstrating that inhibition of SMAD3-dependent TGFβ signaling is required for decreased sclerostin expression that occurs in response to mechanical loading raises the possibility that early activation of TGFβ could be responsible for the elevated sclerostin expression in CKD [37•]. Indeed, increased TGFβ and pSMAD was observed concomitantly with the elevated number of sclerostin positive osteocytes, and TGFβ neutralization was associated with normalization of sclerostin mRNA.
Overall, the current data provides compelling evidence that elevation of local and/or circulating levels of other antagonists such as Dkk1, leads to repression of Wnt/β-catenin signaling thereby contributing to CKD-associated skeletal defects. These studies also highlight deficits in our current understanding since demonstration that restoring signaling in CKD models can normalize bone strength is lacking. It is unclear whether TGFβ elevation plays a critical role in all forms of CKD as efficacy of neutralizing antibodies is only observed in the context of high turnover disease and clinical evidence for elevation in bone is limited. Perhaps, PTH elevations occur in the subset of individuals with high bone turnover disease as a response to overall inhibition of bone anabolism induced by TGFβ, sclerostin or other Wnt inhibitors such as Dkk1.
Dysregulated Serum Sclerostin Levels in CKD
Circulating sclerostin levels have been evaluated across several small cohorts of individuals with CKD. Cejka and colleagues were the first to show elevated serum sclerostin levels in a small cross-sectional study of patients on dialysis [38•]. This finding has been further validated by other small studies in both patients with stage 5D [39•, 40, 41•, 42•] and CKD not on dialysis [42•, 43, 44]. Studies monitoring expression across all disease stages reveal accumulation of serum levels with declining renal filtration [39•, 46].
Relative differences in the magnitude of serum changes across studies may reflect variation in patients and/or reflect assay difference. Most studies assessed sclerostin changes using one of two commercial assays. While an early comparison noted major differences between assays [46], recent improvements result in data that is relatively similar, albeit larger differences between assays are observed at the higher ranges of sclerostin concentrations [47].
Although serum sclerostin levels generally correlate with local bone expression under most conditions, it is not clear to what extent the serum levels reflect changes in expression versus accumulation in individuals with kidney disease. The local expression of sclerostin described above suggested the highest expression occurred at initial stages of the disease. However, these studies should be viewed as inherently qualitative since the number of sclerostin positive osteocytes was monitored rather than absolute protein levels. Nonetheless, these data are consistent with the premise that sclerostin accumulation is at least partially due to increased osteocyte production. Additional data suggest that aberrant sclerostin production as observed in injured mouse kidneys and at the sites of vascular calcification in mice and humans might also contribute to expression levels [27, 44]. Rapid return of serum sclerostin to the normal range post transplant suggests that decreased renal clearance may be responsible for accumulation at least in late stages [48]. Finally, the potential for circulating sclerostin fragments, similar to what is known for PTH has not been fully examined. Thus, additional investigation will be required to determine whether the mild sclerostin elevation in CKD is due to accumulation or increased production or both. Perhaps increased production plays a greater role earlier in the course of CKD and accumulation is more relevant later in CKD/dialysis.
Association of Sclerostin with Clinical Outcomes
High sclerostin levels are associated with increased fracture risk in individuals with post-menopausal osteoporosis and in patients with type 2 diabetes [49–53]. Ongoing studies have begun to examine the relationship of sclerostin levels with bone health, cardiovascular-related events, and/or mortality in CKD. In a cross-sectional study of 60 patients with stage 5 dialysis (stage 5D), serum sclerostin levels were inversely correlated with bone formation rates [38•]. This data is in contrast to several cross-sectional studies in which sclerostin values had a positive correlation with BMD [41•]. However, a subsequent prospective study of 81 stage 5D patients found that higher sclerostin serum concentrations predicted a greater loss of bone mass over a 1-year period [41•]. Overall, these data are consistent with the hypothesis that higher sclerostin levels promote low bone turnover that leads to loss in bone mass over time, as would be expected of a negative regulator of bone formation. Additional studies will be required to delineate whether differential levels of sclerostin are associated with bone disease associated with high versus low bone formation and to determine whether there is a correlation with increased sclerostin and fracture rates.
The impact of high sclerostin levels on cardiovascular disease and mortality is also relevant given the known role of canonical Wnt signaling on vascular calcification, a contributor to cardiovascular risk. Vascular calcification results from phenotypic conversion of vascular smooth muscle cells into an osteoblast-like phenotype [54] that involves induction of an osteoblast transcriptional program (including the osteocytes specific proteins FGF23 and sclerostin) via a Wnt-dependent mechanism [55]. The appearance of negative regulators of Wnt signaling might therefore be expected to attenuate aberrant osteoblast maturation and progression of cardiovascular calcification. Indeed, in a cross-sectional study of 154 CKD pre-dialysis patients, higher sclerostin levels correlated with the absence of calcification in addition to increased age, male gender, lower bone-specific alkaline phosphatase, and renal function [44]. In a short 6-week, post hoc analysis of 40 normophosphatemic stage 3–4 CKD patients, sevelamer-HCl, but not calcium acetate, treatment was associated with a significant decrease in sclerostin levels [45]. However, this study did not correlate expression with vascular calcification or other cardiac complications. Post hoc analysis of 100 prevalent hemodialysis patients monitored over a 2-year period revealed that improved survival was associated with higher sclerostin levels [40]. A prospective cohort study of 800 incident dialysis patients also found that high serum sclerostin was associated with lower cardiovascular mortality over an 18-month period, but this association was less pronounced after 4 years [42•]. Additionally, preliminary evaluation of the relationship between sclerostin levels and mortality associated with 800 patients from the CRIC study failed to reveal an independent association with outcomes [46]. It is possible that the failure to identify long-term correlations may be related to progressive increases in positive regulators of vascular calcification that swamp out any beneficially effects of sclerostin.
A potential protective effect of sclerostin on cardiovascular disease is in apparent conflict with pre-clinical studies reported above in which antibodies to the Wnt antagonist, Dkk1 ameliorated cardiovascular calcification. In this study, repressed signaling was associated with endothelial cells as opposed to smooth muscle cells, and the assessment was performed in a model representing earlier stage of disease. Thus, a full understanding of the contribution of Wnt signaling will require stage- and tissue-specific analysis. Additionally, relative contributions may be distinct across different patient sub-populations.
Conclusions
Disturbances in Wnt/β-catenin signaling with associated elevations in the Wnt antagonist, sclerostin can be added to the array of changes associated with the CKD-MBD progression. The decrease in overall osteoblast maturation genes is consistent with an overall reduction in bone anabolism that appears to be independent of osteoclast activity and overall BFRs. The differential responses to antibodies that neutralize Wnt antagonists versus TGFβ on distinct types of renal bone disease further suggest that additional mechanisms contribute to the diversity of skeletal defects in CKD. Whether activation of this pathway is sufficient to reduce fracture rates remains to be determined.
Similarly, the relative impact of circulating Wnt antagonists on the progression of cardiovascular calcification and importantly on cardiovascular mortality requires additional investigation. A potential concern is that the use of therapies to promote bone anabolism might have a negative impact on the cardiovascular disease. Conversely, improvement of bone health may reduce other risk factors that have higher impact on cardiac disease such as serum phosphate and FGF23.
The current information regarding sclerostin and its role in CKD-MBD provides yet another example of the critical need to take a systems biology approach in understanding the full weight of changes associated with disease pathogenesis. It is likely that incorporation of emerging tools such as total RNA sequencing and informatic analysis to fully assess patient samples will provide new opportunities to stratify patient populations and choose the best therapeutic options for individual sub-populations.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance • Of importance
Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2006;69:1945–53.
Moe SM. Definition and classification of renal osteodystrophy and chronic kidney disease-mineral bone disorder (CKD-MBD). In: Olgaard K, Salusky IB, Silver J, editors. The spectrum of mineral and bone disorders in chronic kidney disease. New York: Oxford University; 2010. p. 1–14.
Lu K-C, Wu C-C, Yen JF. Vascular calcification and renal bone disorders. Sci World J. 2014;637065:1–20. A comprehensive review summarizing the link between poor bone health and cardiovascular disease in CKD.
Cannata-Andia JB, Roan-Garcia P, Hruska K. The connections between vascular calcification and bone health. Nephrol Dial Transplant. 2011;26:3429–36.
Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–33.
Quarles D. A systems biology preview of the relationships between mineral and metabolic complications in chronic kidney disease. Semin Nephrol. 2013;33:130–42.
Krisnan V, Bryant HU, MacDougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature Med. 2013;19:179–92. Outstanding review article describing evidence based mechanistic understanding of canonical Wnt/β-catenin signaling and its role in bone biology.
Modder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–9.
Li X, Ominsky MS, Warminghton KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of post-menopausal osteoporosis. J Bone Miner Res. 2009;24:578–88.
Ominsky MS, Vlasseros F, Jolette J, et al. Two doses of anti-sclerostin antibody in cynomologus monkeys increases bone formation, bone mieral density, and bone strength. J Bone Miner Res. 2010;25:948–59.
Padhi D, Jang G, Stouch B, et al. Single dose placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26.
Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–46.
Poole KE, Reeve J. Parathyroid hormone: a double-edged sword for bone metabolism. Curr Opin Pharmacol. 2005;5:612–7.
Kramer I, Kellar H, Leupin O, Kniessel M. Does osteocytic SOST suppression mediate PTH anabolism? Trends in Endocr Metab. 2010;21:237–44.
Wan M, Yang C, Li J, et al. Parathyroid signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–79.
Brandenburg VM, Floege J. Adynamic bone disease—bone and beyond. Nephrol Dial Transpl. 2008;3:135–47.
Rocha LA, Higa A, Barreto FC, et al. Variant of adynamic bone disease in hemodialysis patients: fact or fiction? Am J Kidney Dis. 2006;48:430–6.
Lund RJ, Davies MR, Matthew S, Hruska K. New discoveries in the pathogenesis of renal osteodystrophy. J Bone Miner Res. 2006;24:169–71.
Sabbagh Y, Graciolli FG, O’Brien S, et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Min Res. 2012;27:1757–72. First mechanistic evidence that repression of Wnt/β-catenin signaling is associated with CKD in mouse bones and clinical biopsies.
Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3072–85.
Wijenayaka AR, Kogawa M, Lim HP. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;10:e25900.
Moyses R. Osteocyte regulation in renal osteodystrophy. Presented Am Soc Nephrology, Philadelphia, PA, 2014.
Bellido T, Ali AA, Gubrij I, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–83.
Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–58.
Moe SM, Chen NX, Newman CL, et al. Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy. J Bone Miner Res. 2014. doi:10.1002/jbmr.2372. Describes preclinical effects of anti-sclerostin antibody on low and not high bone turnover.
Fang Y, Ginsberg C, Sugatani T, et al. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Intern. 2013;85:142–50.
Fang Y, Ginsberg C, Seifert M. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-MBD. J Am Soc Nephrol. 2014;25:1760–73. Study provides evidence that injured kidneys may release Wnt antagonists into circulation and that antagonism with neutralizing antibodies can treat low bone disease and vascular calcification. Evidence that aberrant Wnt signaling in endocrine cells is associated with vascular calcification.
Liu S, Song W, Boulanger JH, et al. Role of TGF-b in a mouse model of high turnover renal osteodystrophy. J Bone Min Res. 2014;29:1141–57. Important study suggesting a role for TGFβ involvement in early pathogenesis of high turnover bone disease.
Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor β1 to the bone. Endocr Rev. 2005;26:743–74.
Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–65.
Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20:2254–72.
Grafe I, Yang T, Alexander S, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20:670–5.
Zhen G, Wen C, Jia X, Li Y, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–12.
Gu X, Wang XF. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19:71–88.
Qui T, Wu X, Zhang F, et al. TGF-β type II receptor phosphorylates PTH receptor to integrate bone remodeling signaling. Nature Cell Biol. 2010;12:224–34.
Nguyen J, Tang SY, Nguyen D, Alliston T. Load regulates bone formation and sclerostin expression through a TGFβ-dependent mechanism. PLoS One. 2013;8:1547–53. Provides mechanistic link between TGFβ and sclerostin regulation.
Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6:877–82. First report of elevated sclerostin levels in CKD demonstrating relationship with bone formation rates.
Pelletier S, Dubourg L, Carlier MC, et al. The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol. 2012;8:819–23. Provides assessment of sclerostin serum expression across all stages of CKD progression.
Viaene L, Behets GJ, Claes K, et al. Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Dephrol Dial Transplant. 2013;28:3024–30.
Malluche HH, Davenport DL, Cantor T, Monier-Faugere M-C. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin J Am Soc Nephrol. 2014;9:1254–62. Prospective study examining the relationship over time between serum sclerostin and bone mass.
Drechsler C, Evenepoel P, Vervloet MG, et al. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2014;0:1–6. Largest prospective study to date examining relationship between serum sclerostin and cardiovascular events.
Thambiah S, Roplekar R, Manghat P, et al. 2012; Circulating sclerostin and dickkopf1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int. 2012;90:473–80.
Claes KJ, Viaene L, Heye S, et al. Sclerostin: another vascular calcification inhibitor? J Clin Endocrin Metab. 2013;98:3221–8.
De Oliveira RB, Graciolli FG, dos Reis LM, et al. Disturbances of Wnt/β-catenin pathway and energy metabolism in early CKD: effect of phosphate binders. Nephrol Dial Transplant. 2013;28:2510–7.
Isakova T Circulating sclerostin as a marker of bone health and disease. Presented at the American Society of Nephrology, Philadelphia, PA, 2014.
McNulty M, Singh RJ, Li X. et al. Determination of serum and plasma sclerostin concentrations by enzyme-linked immunoassays. J Clin Endocrinol Metab. 2011;96:E11159-62.
Costa AG, Cremers S, Dworakowski E, et al. Comparison of two commercially available ELISAs for circulating sclerostin. Osteoporos Int. 2014;25:1547–54.
Bonani M, Rodriguez D, Fehr T, et al. Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press Res. 2014;39:230–9.
Arasu A, Cawthon PM, Lui LY, et al. Serum sclerostin and risk of hip fracture in older Caucasian women. J Clin Endocrinol Metab. 2012;97:2027–32.
Ardawi MS, Rouzi AA, Al-Sibiani SA. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the Center of Excellence for Osteoporosis Research Study. J Bone Miner Res. 2012;27:2592–602.
Ardawi MS1, Akhbar DH, Alshaikh A, et al., Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone. 2013;56:355–62.
Yamamoto M, Yamauchi M, Sugimoto T. Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:4030–7.
Steitz SA, Speer MY, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification. Circ Res. 2001;89:1147–54.
Shao JS, Cheng SL, Pingsterhaus JM, et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–20.
Compliance with Ethics Guidelines
Conflict of Interest
SC Schiavi declares no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by SC Schiavi involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Kidney and Bone
Rights and permissions
About this article
Cite this article
Schiavi, S.C. Sclerostin and CKD-MBD. Curr Osteoporos Rep 13, 159–165 (2015). https://doi.org/10.1007/s11914-015-0263-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-015-0263-2