Abstract
Background
To investigate the long-term neurodevelopmental outcome in children after hemolytic uremic syndrome (HUS) and to compare outcome dependent on central nervous system (CNS) involvement during HUS.
Methods
A single-center retrospective cohort of 47 children was examined at a median age of 10.6 (range 6–16.9) years and a median follow-up of 7.8 (range 0.4–15.3) years after having had HUS. Intellectual performance was assessed with the German version of the Wechsler Intelligence Scale 4th version and neuromotor performance with the Zurich Neuromotor Assessment (ZNA). The occurrence of neurological symptoms during the acute phase of HUS was evaluated retrospectively.
Results
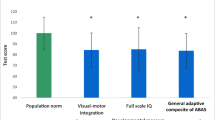
Mean IQ of the whole study population fell within the normal range (median full scale IQ 104, range 54–127). Neuromotor performance was significantly poorer in the domains “adaptive fine,” “gross motor,” “static balance” (all p < 0.05) and “associated movements” (p < 0.001); only the “pure motor” domain was within the normal reference range. Neurological findings occurred in 16/47 patients (34 %) during acute HUS. Neurodevelopmental outcome was not significantly different between children with or without CNS involvement.
Conclusions
Our follow-up of children after HUS showed a favorable cognitive outcome. However, neuromotor outcome was impaired in all study participants. Neurological impairment during acute HUS was not predictive of outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemolytic uremic syndrome (HUS) is a multi-organ and life-threatening disease characterized by hemolytic anemia, thrombocytopenia and acute renal injury. HUS is also one of the most frequent causes of acute renal failure in childhood [1] and may result in long-term renal and extrarenal sequelae [2–5].
About 90 % of HUS cases in childhood are infection-induced, i.e. they are typical HUS forms, mainly mediated by infections caused by Shiga toxin-producing bacteria, usually enterohemorrhagic Escherichia coli (STEC-HUS) but in some regions Shigella dysenteriae type 1. In addition, infections with Streptococcus pneumoniae (P-HUS) and other bacterial and viral agents can trigger HUS [6, 7]. Only 5–10 % of cases are defined as atypical HUS (aHUS) based on various hereditary and/or acquired disorders of the alternative complement pathway regulation [6–8]. Renal replacement therapy at disease onset is required in up to 65 % of STEC-HUS patients [9], 84 % of those with P-HUS [10] and 59 % of aHUS patients [5].
Extrarenal manifestations are frequent in all HUS forms, including STEC-HUS [11, 12], P-HUS [13, 14] and aHUS [5, 15], and may affect the central nervous system (CNS), gastrointestinal tract, heart, eyes, lungs, parotid glands and skin. CNS involvement represents a major complication that is associated with increased mortality [2, 11] and risk for neurological sequelae [16].
Studies reporting on neurodevelopmental outcome in children after HUS are scarce, and the results suggest a normal neurocognitive outcome [17–19]. However, a trend towards impaired full-scale and verbal comprehension IQ in these children has also been described [17]. Data on neuromotor outcome are limited to information on impaired fine motor skills in children with a history of HUS and severe CNS involvement [18].
In the study reported here, we focused on the long-term intellectual and neuromotor performance in a single-center cohort of children after HUS, including typical and atypical HUS forms. The hypothesis was that all children with HUS may have a higher risk for adverse neurodevelopmental outcome. Furthermore, the study was performed to determine the influence of CNS involvement during acute HUS disease on the long-term neurodevelopmental outcome.
Methods
Patients
The study cohort consisted of 47 children (22 males, 25 females; median age 10.6 years, age range 6–16.9 years) with a history of both typical infection-induced HUS and atypical HUS. The neurodevelopmental testing was part of a comprehensive single-center study on long-term renal outcome, psychological adjustment and quality of life in HUS patients. The study was approved by the Cantonal Ethics Committee Zurich and registered at ClinicalTrials.gov (NCT 01666548). Written informed consent was obtained by the parents and by the adolescents themselves if they were ≥15 years. Inclusion criteria for neurocognitive and neuromotor assessment were: (1) previous diagnosis of HUS and (2) age between 6 years and 16 years 11 months during the study period between February 2012 and February 2013.
HUS was defined as non-immunological hemolytic anemia (hemoglobin <100 g/l), thrombocytopenia (thrombocytes <150.000/μl) and features of acute renal injury (plasma creatinine elevation above the age-related norm range; proteinuria, hematuria or renal ultrasound abnormalities). Two of the enrolled patients—one with STEC-HUS requiring dialysis and one with recurrent aHUS due to complement factor H mutation—did not meet the criteria for thrombocytopenia. The diagnosis of HUS in all patients was confirmed by pediatric nephrologists. Based on the different approaches used in published studies to classify HUS [20–22] we categorized the disease as (1) typical, infection-induced HUS, including STEC-HUS and P-HUS, and (2) aHUS based on currently proposed HUS nomenclature [21].
The age criterion of 6–16 years was used to study long-term neurocognitive outcome using one intellectual test, namely, the German version of the Wechsler Intelligence Scale 4th version [23].
Participants were recruited from a sample of 129 patients treated for HUS at the Pediatric Nephrology Unit of Zurich University Children’s Hospital between April 1995 and February 2013. Seven patients died during an acute episode of HUS, five patients were lost to follow-up and 42 patients did not fulfil the age criterion (26 were aged <6 years and 16 were aged ≥17 years). Thus, 75 patients were eligible for the study. Twenty-six parents or children refused to participate; two additional patients were excluded due to a pre-existing neurodevelopmental impairment resulting from trisomy 21 in one and an unclassified syndrome in another. The final study cohort included 47 (63 %) of the children originally eligible for entry. Demographic and clinical characteristics did not differ significantly between enrolled patients and those not enrolled in terms of sex, HUS form, socioeconomic status, age at diagnosis of HUS, frequency of neurological complications during the acute phase of HUS, occurrence of anuria, need for dialysis during the acute phase of HUS, length of hospital stay, estimated glomerular filtration rate (eGFR) at time of discharge, need of dialysis at time of discharge and development of end-stage renal disease (ESRD).
The clinical and demographic data needed to evaluate potential risk factors were extracted from patients’ records and analyzed retrospectively. Values for the following parameters were obtained from the medical records: sex, age at disease onset, renal function, anuria defined as urine output <0.2 ml/kg per hour, requirement of dialysis and CNS involvement during the acute episode of HUS. CNS involvement was defined as presence of neurological findings including seizures, altered consciousness, ataxia, muscle tone abnormalities, hemiplegic symptoms, dysarthria, visual disorders, movement disorders and vestibular symptoms. Since conditions such as anemia or dehydration may affect mental status, CNS involvement was only considered if the clinical symptoms were severe and not attributable to an underlying non-cerebral medical condition. None of the studied children had a neurological disease prior to HUS.
Other comorbidities and ESRD with renal replacement therapy at follow-up were also recorded. Renal function was evaluated by eGFR, expressed in millimeters per minute per 1.73 m2, according to the Schwartz formula using the local factor k of 40 for all children and by the plasma creatinine concentration (in μmol/l) [24]. Information on additional potential neurological risk factors and interventions performed since HUS was retrieved from parental interviews at the time of neurodevelopmental assessment.
Neurodevelopmental outcome assessment
The neurodevelopmental outcome assessment included as assessment of intellectual and neuromotor performance and a standardized neurological examination [25]; both were performed at the Child Development Center of Zurich University Children’s Hospital by one experienced developmental pediatrician. Socioeconomic status was estimated based on maternal education level and paternal occupation using an education scale ranging from 2 to 12, with 2 being the lowest and 12 the highest education score [26].
Intellectual performance
Of the 47 participants, 46 were assessed using with the German version of the Wechsler Intelligence Scale 4th version [23]. This test provides IQ subscales for verbal comprehension, perceptual reasoning, working memory and processing speed, which together form the full-scale IQ. One 9-year-old patient with P-HUS associated with meningitis and serious neurological complications was not able to perform the Wechsler Intelligence Scale 4th version and was examined using the German version of the Wechsler Preschool and Primary Scale of Intelligence 3rd version [27].
Neuromotor performance
Neuromotor performance was examined with the Zurich Neuromotor Assessment (ZNA), a standardized, videotaped test for children aged 5 to 18 years which is used to investigate specific motor skills based on timed performances and movement quality [28, 29]. The ZNA contains five block components including: (1) pure motor domain, (2) adaptive fine motor domain, (3) adaptive gross motor domain, (4) static balance and (5) associated movements. The results are expressed as z-scores, i.e. the standard score of the reference population based on age and sex.
Statistical analysis
Statistical analysis was performed with SPSS for Windows version 20.0 and 22.0 (IBM Corp,, New York, NY). Differences between participants’ data and normative data were calculated using the univariate t test, and differences between subgroups were assessed using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Multivariate linear regression was conducted to evaluate the association between risk factors and full-scale IQ scores. Variables included in the regression model were socioeconomic status, duration of hospital stay, CNS involvement and eGFR at time of discharge. Two children with very low full-scale IQ scores (54 and 62) were excluded for the multiple regression analysis in order to comply with the requirements of a normal distribution in the study sample. A p value of <0.05 was considered to be statistically significant.
Results
Sample description
Forty-seven patients (22 boys and 25 girls; median age 10.6 years, range 6–16.9 years) with a history of STEC–HUS (n = 38), P-HUS (n = 6) and aHUS (n = 3) and a median follow-up after HUS of 7.8 (range 0.4–15.3) years participated in this study (for detailed information on each participant, see Table 1).
Of the 38 STEC-HUS patients, 24 tested positive for Shiga toxin. Genetic analysis of the three aHUS patients revealed one or more mutations of complement-related factors. The median age at onset of HUS was 1.8 (range 0.3–14.4) years. Thirty-three children (70 %) required acute renal replacement therapy combined with either peritoneal dialysis (n = 24 patients), hemofiltration or hemodialysis (n = 6) or a combination of both treatment modalities (n = 3). At time of discharge the median eGFR was 54 (range 13–178) ml/min per 1.73 m2. Forty-one (87 %) patients had an impaired eGFR defined as <90 ml/min per 1.73 m2. One patient was on dialysis when discharged and remained on dialysis for 127 days, subsequently progressing to ESRD. Five patients developed ESRD, of whom four underwent renal transplantation (RTPL) (Table 1).
The median eGFR at neurodevelopmental testing—excluding the four children who underwent RTPL—was 113 (range 12–178) ml/min per 1.73 m2; ten of these children had impaired renal function with an eGFR of <90 (range 12–88) ml/min per 1.73 m2. Two of the four patients undergoing RTPL had good renal graft function defined as an eGFR of >60 (respectively 92 and 163) ml/min per 1.73 m2, while the remaining two children showed impaired graft function (47 and 54 ml/min per 1.73 m2, respectively). The children with CNS involvement has a significantly lower median eGFR at both discharge and follow-up (46 and 89 ml/min per 1.73 m2, respectively; p = 0.014) than the children without CNS involvement (83 and 126 ml/min per 1.73 m2, respectively; p = 0.004) (Table 2).
CNS involvement during acute episode of HUS
Sixteen children (34 %) presented with CNS involvement during the acute episode of HUS with a broad spectrum of neurological symptoms, consisting predominantly of seizures (12/16) or altered consciousness (7/16) (Table 3).
In the STEC-HUS group neurological symptoms were observed in 12 of 38 patients. Two children received treatment with plasmapheresis due to severe neurological complications (Table 1). Four of the six P-HUS patients presented with neurological symptoms, including two with pneumococcal meningitis. None of the patients with aHUS manifested CNS involvement, but one was resuscitated due to respiratory failure following RTPL.
Neuroimaging studies were performed in 12 of 16 patients with CNS involvement (cerebral computed tomography in 7, cerebral magnetic resonance imaging in 5 and both investigations in 5 children), revealing cerebral abnormalities in five patients: two with cerebral infarctions (both STEC-HUS), two with meningitis-associated complications and one child with leukoencephalopathy (P-HUS) (Table 1).
Comparison of clinical and demographic characteristics between participants without and with CNS involvement during the acute episode of HUS showed that anuria (p = 0.006), longer duration of hospital stay (p = 0.03) and impaired eGFR, both at discharge (p = 0.01) and at time of neurodevelopmental testing (p = 0.004), were significantly more common in patients with CNS impairment (Table 2).
Additional comorbidities, not HUS related and potentially leading to neurodevelopmental impairment, were present in four children, with one child each having status after viral meningitis and resuscitation for pulmonary edema after RTPL, resuscitation associated with an anesthetic accident, prematurity and attention deficit hyperactivity disorder treated with methylphenidate, respectively. Two additional children with P-HUS developed severe cerebral complications (Table 1): subdural empyema and hydrocephalus, respectively.
Intellectual performance
The median full-scale IQ of the study cohort was normal with a value of 104 {54–127 points; comparison to norm of 100 [±15 = 1 standard deviation (SD)]: p = 0.39}. All subscales were in the normal range: verbal comprehension [102 (range 69–130); p–0.57], working memory [102 (54–144); p = 0.52], processing speed [100 (65–129); p = 0.69] and perceptual reasoning [108 (61–129); p–0.03].
Six children (13 %) showed a full-scale IQ of <85 (−1 SD). Two of these two patients had a full-scale IQ of <70 (−2 SD)—one with P-HUS and pneumococcal meningitis and multiple complications requiring ventriculoperitoneal shunt and cochlear implant and the second with a past history of resuscitation episode, aHUS and ESRD in infancy (Table 1).
Children with a history of ESRD showed a poorer neurocognitive outcome than children without ESRD in terms of verbal comprehension [88 (range 69–95) vs. 105 (79–130); p = 0.004], working memory [87 (54–102) vs. 102 (74–144); p = 0.008] and full-scale IQ [84 (54–103) vs. 105 (62–127); p = 0.010].
There were no significant differences between the 16 individuals with and the 31 individuals without CNS involvement during the acute phase of HUS (Table 4). Socioeconomic status did not differ between these two groups (Table 2). Furthermore, the exclusion of patients with neurodevelopmental comorbidities (n = 6) and those with development of ESRD (n = 5) did not significantly alter the results of the intellectual outcome.
Neuromotor performance
Forty-seven children (22 boys, 25 girls) performed the ZNA. Except for the pure motor domain, all other domains of the neuromotor performance were significantly impaired compared to the normal controls (Table 5). Between 15 and 38 % of the children performed poorer than the 10th percentile within the five ZNA domains (Table 5).
When participants with additional neurodevelopmental comorbidities were excluded, the neurodevelopmental outcome compared to normal controls was still impaired except for the pure motor and the adaptive fine motor domain (p > 0.07). Participants with a history of ESRD (n = 5) had significantly poorer results in the domain static balance than those without ESRD [−1.9 (range −3.0 to −0.7) vs. −0.2 (−3.0 to 1.7); p = 0.003].
Motor therapies (including psychomotor, physical and ergotherapy) were reported for nine children (19 %). There were no significant differences between children with and without CNS involvement in terms of frequency of motor therapies (6/31 vs. 3/16, respectively; p = 0.64).
Neurodevelopmental outcome in children with STEC-HUS
Table 6 presents the developmental outcome for children with only STEC-HUS—which was the commonest HUS form present in the study cohort (n = 38). Compared to normal controls, children with STEC-HUS showed a favorable intellectual outcome. In contrast, neuromotor outcome was impaired in the ZNA domains “adaptive gross motor” and “associated movements”. In these domains, 34 % and 39 % respectively performed poorer than the 10th percentile (Table 6).
Prognostic factors
Potential risk factors for poorer IQ were evaluated in a multivariate linear regression analysis . Socioeconomic status (ß = 0.474, p = 0.001) was the only factor associated with the full-scale IQ whereas CNS involvement (ß = −0.074, p = 0.62), duration of hospital stay (ß = −0.257, p = 0.08) and eGFR at time of discharge (ß = −0.117, p = 0.41) were not.
Discussion
The majority of follow-up studies of children with HUS have focused on renal outcome after HUS episode [2–5, 9, 15]. Data on neurodevelopmental outcome, however, are scarce, with only few published studies of various designs and case series available [18, 30], and little information on long-term outcome. We report here our results from a single-center cross-sectional investigation assessing neurocognitive and neuromotor long-term outcome of pediatric patients after STEC-HUS, P-HUS or aHUS. In our study we also examined the role of CNS involvement during the acute episode of HUS on subsequent neurodevelopment. In contrast to previous studies focusing on STEC-HUS [17–19], we expressly included patients with different HUS forms (STEC-HUS, P-HUS and aHUS) even though apart from thrombotic microangiopathy the underlying pathomechanisms of these HUS forms do differ.
Our patient series showed an overall favorable neurodevelopmental outcome after a history of HUS, with a normal full-scale IQ. Furthermore, the intellectual performance of our patients was not affected by CNS impairment during the acute HUS episode. Only socioeconomic status was positively correlated with full-scale IQ, which is consistent with findings in healthy controls [26]. Socioeconomic status is also a strong predictor of intellectual outcome in other populations at risk, such as preterm born children [31] or children with congenital heart defects [32].
One-third of our study patients presented with neurological symptoms during the acute episode of HUS, particularly in the form of seizures and altered consciousness, including four of the six patients with P-HUS, but none of those with aHUS. These findings are consistent with those of previous studies on neurological involvement in patients with STEC-HUS (19–30 % showing neurological symptoms) [2, 9, 33] and P-HUS patients (16–56 % with neurological symptoms) [14, 34, 35]. There are no evidence-based guidelines on the treatment of CNS complications in HUS. Nathanson et al. [16] suggested that plasmapheresis might have some benefit in children with severe CNS complications. In our series, only two children with STEC-HUS underwent plasmapheresis for severe neurological complications with full neurological recovery.
Our findings are consistent with the results of three previous studies. Schlieper et al. [17] demonstrated a favorable neurocognitive outcome in children at a mean age of 8.6 years (±3.1 SD) and mean duration of 4.1 years (±2.4 SD) after the diagnosis of HUS, with normal full-scale and subscale IQ values in 91 children after HUS (without specification of HUS type), including nine children with seizures or coma during the acute episode of HUS. However, these authors did observed mild deficits in language domains in patients with severe acute HUS [17]. Qamar et al. [18] described a normal intellectual outcome in all seven patients studied despite severe neurological complications during the acute disease. Bauer et al. [19] also reported a favorable neurocognitive outcome in 25 children affected by the STEC-HUS outbreak in 2011 in Germany due to E. coli O104:H4. However, these authors observed a slightly lower full-scale IQ in children with CNS involvement vs. those without CNS involvement during HUS. Other studies focusing on neurological involvement in adult patients with STEC-HUS due to E. coli O104:H4 also suggested a good neurological outcome [36].
In our series, only six children (12 %), including four with ESRD, had a full-scale IQ of <85, indicating an unfavorable intellectual outcome. Moreover, two of these six children had a history of severe cerebral complications after P-HUS.
Patients with a history of ESRD showed a significant poorer neurocognitive outcome after HUS compared to patients without ESRD after HUS. The development of ESRD, particularly in infancy, is a known risk factor for impaired neurocognitive outcome [37].
In our study, neuromotor performance was less favorable than intellectual outcome, with a poorer outcome particularly in fine and gross motor functioning, static balance and movement quality. Normal performance observed in the domain of pure motor functioning. This is an interesting finding. In this domain, simple motor tasks, such as repetitive or sequential finger, hand or foot movements, are performed. It is conceivable that impairments only become apparent when more complex motor functions, such as adaptive motor performances, are required. Motor performance did not differ between children with and without CNS impairment during acute HUS episode. Qamar et al. [18] also studied neuromotor outcome after HUS, reporting impaired fine motor and clumsiness in four of seven patients with severe neurological complications during the acute HUS episode. In our study, a significant proportion of patients (15–38 %) had motor performance below the 10th percentile. This poorer motor performance is clinically significant as children who perform below the 10th percentile often have difficulties participating in activities of daily life and demonstrate poorer hand writing skills and slower speed. However, we did not assess the impact of motor difficulties on daily life. Of note, motor therapies were reported in 19 % of our patients independently of CNS involvement during HUS.
The pathophysiological mechanisms leading to impaired neuromotor outcome after HUS remain to be clarified. In addition to cerebral thrombotic microangiopathy, factors such as electrolyte imbalances (e.g. severe hyponatremia), hypo-osmolality, azotemia, arterial hypertension and the direct toxic effects of Shiga toxin in STEC-HUS may be involved in pathogenetic mechanisms of neurological impairment [19, 38]. Impaired neuromotor outcome can also be found in other cohorts of pediatric patients with various diseases, such as congenital diaphragmatic hernia [39] or congenital heart disease [40]. Impaired neuromotor findings also suggest disease-unrelated factors leading to an adverse neuromotor performance, such as factors attributable to long hospital stay secondary to more severe course of a disease or more parental protection and less experience.
This study has several limitations. Due to its retrospective and cross-sectional study design, information on neurological complications during the acute illness phase was obtained retrospectively by chart review and parental interviews. Apart from the parents’ medical report, no formalized data on the neurodevelopmental status prior to HUS were available. Furthermore, the number of patients with P-HUS and aHUS was too small to analyze outcome in relation to HUS type.
In conclusion, the results of this study show that children and adolescents with HUS have a normal intellectual outcome, but a significant impairment in motor outcome. Neurological complications during the acute episode of HUS were not associated with a poorer neurodevelopmental outcome. Therefore, long-term observation of children after HUS is advisable for the early detection of neurodevelopmental deficits.
References
Andreoli SP (2009) Acute kidney injury in children. Pediatr Nephrol 24:253–263
Schifferli A, von Vigier RO, Fontana M, Spartà G, Schmid H, Bianchetti MG, Rudin C, (SPSU) TSPSU (2010) Hemolytic-uremic syndrome in Switzerland: a nationwide surveillance 1997–2003. Eur J Pediatr 169:591–598
Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF (2003) Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 290:1360–1370
Copelovitch L, Kaplan BS (2008) Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr Nephrol 23:1951–1956
Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaime F, Dragon-Durey M-A, Ngo S, Moulin B, Servais A, Provot F, Rostaing L, Burtey S, Niaudet P, Deschenes G, Lebranchu Y, Zuber J, Loirat C (2013) Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 8:554–562
Noris M, Remuzzi G (2009) Atypical hemolytic-uremic syndrome. N Engl J Med 361:1676–1687
Loirat C, Saland J, Bitzan M (2012) Management of hemolytic uremic syndrome. Presse Med 41:e115–e135
Scheiring J, Rosales A, Zimmerhackl LB (2010) Clinical practice. Today’s understanding of the haemolytic uraemic syndrome. Eur J Pediatr 169:7–13
Rosales A, Hofer J, Zimmerhackl L-B, Jungraithmayr TC, Riedl M, Giner T, Strasak A, Orth-Höller D, Würzner R, Karch H, Group G-AHS (2012) Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis 54:1413–1421
Waters AM, Kerecuk L, Luk D, Haq MR, Fitzpatrick MM, Gilbert RD, Inward C, Jones C, Pichon B, Reid C, Slack MPE, Van’t Hoff W, Dillon MJ, Taylor CM, Tullus K (2007) Hemolytic uremic syndrome associated with invasive pneumococcal disease: the United kingdom experience. J Pediatr 151:140–144
Siegler RL (1994) Spectrum of extrarenal involvement in postdiarrheal hemolytic-uremic syndrome. J Pediatr 125:511–518
Scheiring J, Andreoli SP, Zimmerhackl LB (2008) Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome (HUS). Pediatr Nephrol 23:1749–1760
Constantinescu AR, Bitzan M, Weiss LS, Christen E, Kaplan BS, Cnaan A, Trachtman H (2004) Non-enteropathic hemolytic uremic syndrome: causes and short-term course. Am J Kidney Dis 43:976–982
Brandt J, Wong C, Mihm S, Roberts J, Smith J, Brewer E, Thiagarajan R, Warady B (2002) Invasive pneumococcal disease and hemolytic uremic syndrome. Pediatrics 110:371–376
Fitzpatrick MM, Walters MDS, Trompeter RS, Dillon MJ, Barratt TM (1993) Atypical (non-diarrhea-associated) hemolytic-uremic syndrome in childhood. J Pediatr 122:532–537
Nathanson S, Kwon T, Elmaleh M, Charbit M, Launay EA, Harambat J, Brun M, Ranchin B, Bandin F, Cloarec S, Bourdat-Michel G, Pietrement C, Champion G, Ulinski T, Deschenes G (2010) Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol 5:1218–1228
Schlieper A, Orrbine E, Wells GA, Clulow M, McLaine PN, Rowe PC (1999) Neuropsychological sequelae of haemolytic uraemic syndrome. Investigators of the HUS Cognitive Study. Arch Dis Child 80:214–220
Qamar IU, Ohali M, MacGregor DL, Wasson C, Krekewich K, Marcovitch S, Arbus GS (1996) Long-term neurological sequelae of hemolytic-uremic syndrome: a preliminary report. Pediatr Nephrol 10:504–506
Bauer A, Loos S, Wehrmann C, Horstmann D, Donnerstag F, Lemke J, Hillebrand G, Lobel U, Pape L, Haffner D, Bindt C, Ahlenstiel T, Melk A, Lehnhardt A, Kemper MJ, Oh J, Hartmann H (2014) Neurological involvement in children with E. coli O104:H4-induced hemolytic uremic syndrome. Pediatr Nephrol 29:1607–1615
Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Van de Kar N, Zimmerhackl LB, HUS EPRGf (2006) A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 70:423–431
Zipfel PF, Wolf G, John U, Kentouche K, Skerka C (2011) Novel developments in thrombotic microangiopathies: is there a common link between hemolytic uremic syndrome and thrombotic thrombocytic purpura? Pediatr Nephrol 26:1947–1956
Barbour T, Johnson S, Cohney S, Hughes P (2012) Thrombotic microangiopathy and associated renal disorders. Nephrol Dial Transplant 27:2673–2685
Wechsler D (2003) Manual for the Wechsler Intelligence Scale for Children. The Psychological Corporation, New York
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM (1999) Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol 21:788–793
Largo RH, Pfister D, Molinari L, Kundu S, Lipp A, Duc G (1989) Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev Med Child Neurol 31:440–456
Wechsler D (2002) Wechsler Preschool and Primary Scale of Intelligence. The Psychological Corporation, San Antonio
Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L, Sheehy A, Gasser T (2001) Neuromotor development from 5 to 18 years. Part 1: timed performance. Dev Med Child Neurol 43:436–443
Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L (2001) Neuromotor development from 5 to 18 years. Part 2: associated movements. Dev Med Child Neurol 43:444–453
Steinborn M, Leiz S, Rüdisser K, Griebel M, Harder T, Hahn H (2004) CT and MRI in haemolytic uraemic syndrome with central nervous system involvement: distribution of lesions and prognostic value of imaging findings. Pediatr Radiol 34:805–810
Seitz J, Jenni OG, Molinari L, Caflisch J, Largo RH, Latal Hajnal B (2006) Correlations between motor performance and cognitive functions in children born <1250 g at school age. Neuropediatrics 37:6–12
von Rhein M, Dimitropoulos A, Valsangiacomo Buechel ER, Landolt MA, Latal B (2012) Risk factors for neurodevelopmental impairments in school-age children after cardiac surgery with full-flow cardiopulmonary bypass. J Thorac Cardiovasc Surg 144:577–583
Cimolai N, Morrison BJ, Carter JE (1992) Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome. Pediatrics 90:616–621
Banerjee R, Hersh AL, Newland J, Beekmann SE, Polgreen PM, Bender J, Shaw J, Copelovitch L, Kaplan BS, Shah SS (2011) Streptococcus pneumoniae-associated hemolytic uremic syndrome among children in North America. Pediatr Infect Dis J 30:736–739
Chen S-Y, Wu C-Y, Tsai I-J, Tsau Y-K, Su Y-T (2011) Nonenteropathic hemolytic uremic syndrome: the experience of a medical center. Pediatr Neonatol 52:73–77
Magnus T, Rother J, Simova O, Meier-Cillien M, Repenthin J, Moller F, Gbadamosi J, Panzer U, Wengenroth M, Hagel C, Kluge S, Stahl RK, Wegscheider K, Urban P, Eckert B, Glatzel M, Fiehler J, Gerloff C (2012) The neurological syndrome in adults during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain 135:1850–1859
Johnson RJ, Warady BA (2013) Long-term neurocognitive outcomes of patients with end-stage renal disease during infancy. Pediatr Nephrol 28:1283–1291
Donnerstag F, Ding X, Pape L, Bültmann E, Lücke T, Zajaczek J, Hoy L, Das AM, Lanfermann H, Ehrich J, Hartmann H (2012) Patterns in early diffusion-weighted MRI in children with haemolytic uraemic syndrome and CNS involvement. Eur Radiol 22:506–513
Tureczek I, Caflisch J, Moehrlen U, Natalucci G, Bernet V, Latal B (2012) Long-term motor and cognitive outcome in children with congenital diaphragmatic hernia. Acta Paediatr 101:507–512
Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, Landolt MA, Latal B (2013) Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev Med Child Neurol 55(12):1143–1149
Acknowledgments
We thank all the children, adolescents and their parents who participated in this study. We also thank Luciano Molinari, PhD, and Burkhardt Seifert, PhD, for support with the statistical analysis and Christina Schaefer, MD, University Children’s Hospital Zurich, for performing the neurodevelopmental assessment. The honorarium of Kathrin Buder was supported by the Swiss Society of Nephrology and by the foundation “Kinder für Kinder”.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buder, K., Latal, B., Nef, S. et al. Neurodevelopmental long-term outcome in children after hemolytic uremic syndrome. Pediatr Nephrol 30, 503–513 (2015). https://doi.org/10.1007/s00467-014-2950-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-2950-0