Abstract
Background
There is limited evidence regarding the overall feasibility and success rates of the laparoscopic approach in major emergency surgery, despite its potential to improve outcomes. This study aims to investigate the association between patient, procedural, and surgical factors and likelihood of successful laparoscopic completion in emergency major surgery and derive a predictive model to aid clinical decision-making.
Method
All patients recorded in the NELA emergency laparotomy database 1 December 2013–31 November 2018 who underwent laparoscopically attempted surgery were included. A retrospective cohort multivariable regression analysis was conducted for the outcome of conversion to open surgery. A predictive model was developed and internally validated.
Results
Of 118,355 patients, 17,040 (7.7%) underwent attempted laparoscopic surgery, of which 7.915 (46.4%) were converted to open surgery. Procedure type was the strongest predictor of conversion (compared to washout as reference, small bowel resection OR 25.93 (95% CI 20.42–32.94), right colectomy OR 6.92 (5.5–8.71)). Diagnostic [free pus, blood, or blood OR 3.67 (3.29–4.1)] and surgeon [subspecialist surgeon OR 0.56 (0.52–0.61)] factors were also significant, whereas age, gender, and pre-operative mortality risk were not. A derived predictive model had high internal validity, C-index 0.758 (95% CI 0.748–0.768), and is available for free-use online.
Conclusion
Surgical, patient, and diagnostic variables can be used to predict likelihood of laparoscopic success with a high degree of accuracy. This information can be used to inform peri-operative decision-making and patient selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients undergoing emergency laparotomy are amongst the highest-risk patients for morbidity and mortality [1, 2]. Recent studies have suggested that a laparoscopic approach may improve patient outcomes compared to traditional laparotomy [3,4,5]. A population analysis of major emergency abdominal surgery, case-matched for patient, surgeon, and procedural variables, found that laparoscopy was associated with a significantly reduced rate of mortality (6.0% vs. 9.1%, p < 0.001) [6]. A previous cohort study of laparoscopy versus laparotomy for all emergency major abdominal procedures suggested that open surgery was amongst the strongest predictors of risk-adjusted in-hospital mortality [3].
Prospective trials of patients undergoing emergency general surgery have, to date, been limited in number and to highly selected populations. The LASSO trial randomised patients with uncomplicated band adhesional bowel obstruction to laparoscopy or open surgery, with laparoscopic patients benefitting from reduced pain, length of stay, and quicker return to bowel function [7]. The LaCeS trial is similarly randomising patients for emergent colorectal surgery and has reported positive early feasibility and safety data [8]. A meta-analysis of 38,927 patients from retrospective studies of adhesional bowel obstruction demonstrated a significant reduction in morbidity, mortality, and reoperation rate [4]. However, with perforated ulcer disease, a meta-analysis of trials of laparoscopic versus open repair demonstrated equivocal short-term outcomes [9].
Despite this growing body of evidence, the use of laparoscopy in emergency surgery remains variable. According to the most recent annual National Emergency Laparotomy Audit (NELA) data for England and Wales, approximately a fifth of all cases are attempted laparoscopically, with a laparoscopic completion rate of less than 10% [2]. Concern regarding high conversion rates [10], and the perceived risk of prolonged operative time or iatrogenic injury [11], have been variably cited as barriers to further uptake of laparoscopy in the past, but may be the relics of past attitudes and experiences. On the contrary to this, the improved short-term outcomes suggested by recent studies following emergency laparoscopy are likely to be further enhanced by the reduction of long-term complications such as incisional hernia or adhesional obstruction [12].
Given the high conversion rates seen with emergency laparoscopy, however, it is imperative to better understand how surgeons might better select patients to best benefit from an initial minimally invasive approach. The factors which influence the likelihood of successful laparoscopic completion versus conversion to open surgery remain unclear. Predictive modelling has been limited to predicting conversion in specific operations [13, 14] or elective colorectal surgery [15], with emergency general surgery cohorts not adequately studied. An improved understanding of which patients are most likely to be able to be completed laparoscopically would have significant benefits for pre-operative planning and shared decision-making. It would help post-operative resource allocation, such as by prioritising intensive care beds for those most likely to require them. Increasing success rates and improving patient selection, furthermore, could improve surgeon confidence in attempting laparoscopy, potentially improving clinical outcomes by increasing rates of minimally invasive surgery as well.
The aims of this study were firstly to investigate the association between patient, procedural, and surgical factors and likelihood of success of laparoscopic completion of emergency major abdominal surgery and secondly to derive a predictive model that reliably predicts the risk of conversion to aid in clinical decision-making.
Methods
Anonymised demographic, clinical, and outcome data were retrieved from the NELA dataset, an obligatory prospective national database of all major (non-trauma) emergency abdominal surgery in England and Wales [2], excluding those patients with a diagnosis of appendicitis, uncomplicated hernia, or biliary disease. The study period included all patients who underwent surgery between 1 December 2013 and 31 November 2018, inclusive. This study was approved by the national NELA review committee; data analysis is approved under the NHS Act 2006. Our findings are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [16].
All patients who received attempted laparoscopic surgery—i.e. laparoscopically completed or laparoscopic converted to open surgery—were included. Patients whose surgery was coded as “laparoscopically assisted” were excluded to maintain data homogeneity as there was no clear definition for this code.
Laparoscopically completed and converted to open surgery groups were compared using non-parametric tests. A p value of less than 0.05 was considered statistically significant. Multivariate regression analysis for odds of conversion to open surgery was performed using R (v 3.5.3), incorporating patient, disease, and surgeon covariables. To adjust for patient variables, we used age, gender, and P-POSSUM mortality risk. P-POSSUM [17], a well-validated mortality risk scoring system incorporating vital signs, blood results, patient details, and disease severity, was used to categorise patients as low (< 5% mortality risk), high (5 to < 10%), and very high (≥ 10%) in accordance with NELA definitions. For disease variables, we adjusted for procedure (as coded by NELA), blood loss, degree of peritoneal soiling, and presence of malignancy. Individual procedure types were adjusted for procedures with the lowest frequencies were combined as ‘other’. Finally, we considered surgeon and care process variables: whether a preoperative CT scan had been performed, predicted blood loss, whether surgery was undertaken in or out of hours (in hours defined as patient entry into the operating theatre between 08:00 and 17:00), surgeon grade, and whether a subspecialist performed the surgery.
In order to account for surgical subspecialist expertise, an initial analysis demonstrated that for colorectal procedures, colorectal surgeons were significantly less likely to convert than upper gastrointestinal (upper GI/foregut) surgeons or non-GI specialists (both of whom had similar conversion rates); similar trends were seen for upper GI surgeons performing upper GI procedures (odds ratios reported in results). Furthermore, it was noted that both types of GI specialists were significantly less likely to convert than non-specialists for other general surgical (non-colorectal or upper GI) procedures. As such, for purposes of this analysis, we defined a subspecialty surgeon as a colorectal surgeon undertaking any colonic operation, an upper gastrointestinal surgeon (upper GI/foregut) undertaking peptic ulcer repair or gastric surgery, or either type of GI surgeon performing any other procedure (complete classification list, see supplementary table 3).
A final prediction model was derived by entering all candidate variables into a multivariate logistic regression and selecting important variables by a backwards stepwise technique, with variables excluded if the multivariate p value > 0.05. Discrimination of the model was quantified using the area under curve (AUC or C-statistic) where the value represents the proportion of random pairs of cases where the predicted probability of conversion is ordered correctly. Calibration was assessed visually by comparing observed against predicted probability of conversion along with the respective slope and intercept. Validation was conducted internally, using bootstrapping with 1000 resampled datasets. For multivariate analysis, missing data were handled using multiple imputation by chained Eqs. [18] with ten imputed datasets.
Results
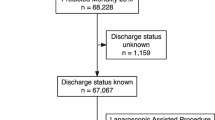
A total of 118,355 patients underwent NELA-eligible surgery during the study period. Exclusion of patients who underwent open (n = 99,880) and laparoscopically assisted (n = 1435) surgery resulted in the inclusion of 17,040 patients who underwent attempted laparoscopic surgery (7.7% of all recorded cases) in the final analysis. Of these, 9125 (53.6%) had laparoscopically completed surgery, whilst the remainder, 7915 (46.4%), were converted to open surgery (Table 1).
The most commonly undertaken procedures were adhesiolysis (n = 3088, 38.1% conversion rate) and right colectomy (n = 2283, 58.0% conversion rate). Overall, adhesiolysis was the procedure most likely to be attempted laparoscopically (21.4% overall), whilst left colectomy (2.8%) was least likely. Cases coded as small bowel resection were the mostly likely to be converted (n = 1815, 75.6% conversion rate), whereas subtotal colectomy (n = 930, 22.3% conversion rate) and gastric surgery (n = 402, 23.4% conversion rate) were most likely to be completed laparoscopically.
Demographic, diagnostic, and disease variables were significantly different between patient groups (Table 2). The overall in-hospital mortality rate was 5.4%, with a median length of stay of 8 days (IQR 5–14 days). Patients who were managed laparoscopically demonstrated improved (unadjusted) outcomes versus those converted to open, mortality 4.1% versus 7.0% (p < 0.001) and median length of stay 7 (4–12) days versus 9 (6–16) days (p < 0.001), respectively.
After adjusting for relevant factors through multivariate regression analysis (Table 3), no significant association was found for age, gender, or availability of preoperative CT scan. High-risk patients (predicted mortality risk 5–9.9%) were no more likely to be converted to open than low-risk patients. Patients with higher volumes of predicted blood loss or higher grades of peritoneal soiling were more likely to be converted to open. Surgery in the presence of localised malignancy was less likely to be converted to open, OR 0.83 (0.71–0.96), but not if nodal or distant metastases were present. Surgery was also more likely to be converted to open if performed by a non-consultant grade, OR 1.3 (95% CI 1.13–1.6), or out of hours, OR 1.31 (1.2–1.43).
Factors related to the presenting pathology were the strongest predictors of conversion. Procedure type was the most strongly associated factor, with the procedures most likely to be converted (odds of conversion compared to “washout” as reference) being small bowel resection, OR 25.93 (20.42–32.94), right colectomy, OR 6.92 (5.5–8.71), and Hartmann’s procedure, OR 6.47 (5.04–8.3). Beyond procedure type, the degree of peritoneal soiling was also strongly associated.
A gastrointestinal specialist performing either general surgery or gastrointestinal resection/repair matched to their subspecialty was less likely to convert from laparoscopy, OR 0.56 (0.52–0.61). Specialty-specific secondary analysis confirmed that when compared to non-gastrointestinal surgeons, there was a lower rate of conversion for colorectal surgeons performing colonic (OR 0.46 95% CI 0.39–0.55, p < 0.001) or ‘general’ (OR 0.72, 0.64–0.80, p < 0.001), but not foregut procedures (OR 0.92, 0.71–1.20, p = 0.548). Similarly, upper GI surgeons had a lower rate of conversion for peptic ulcer/gastric (OR 0.48, 0.37–0.63, p < 0.001) and ‘general’ (OR 0.63, 0.55–0.71, p < 0.001), but not colonic procedures (OR 1.11, 0.86–1.43, p = 0.413) when compared to non-gastrointestinal specialists.
Predictive model
Backwards stepwise selection identified seven important variables that were included in the final prediction model: gender, predicted blood loss, senior operator grade, timing of surgery, subspecialty-specific surgeon, and peritoneal soiling. At least one data point was missing in 1526 out of 17,040 cases (8.9%). All variables had less than 5% missing data. Further details are provided in Supplementary Table 1.
Final model coefficients are provided in Supplementary Table 2. Bootstrap validation demonstrated a C-index of 0.758 (95% CI 0.748–0.768), representing very good discrimination. Calibration was visually excellent (Fig. 1) with agreement between observed and predicted probability of conversion near perfect throughout a broad range of values. Similarly, the calibration slope was 1.00 with an intercept of 0.00.
At time of publication, a free-to-use online calculator for the risk of conversion is available at https://sar03.shinyapps.io/LapCon/ based on the shiny framework in R. Code used to produce the calculator is available at https://github.com/saqibrahmanUGI/LapCon.
Discussion
This study is the first to describe predictive factors for success in major emergency laparoscopic surgery. Despite surgical patient selection, almost half (46%) of cases selected for a laparoscopic approach were converted to open. Using a large national population dataset, we report the procedure types most likely to be completed laparoscopically, in addition to patient and surgeon factors which further influence the probability of conversion to open surgery. A multivariate pre-operative predictive model for conversion, using seven variables available at the start of surgery and 17,040 cases, exhibited both good discrimination and excellent calibration and can be accessed at https://sar03.shinyapps.io/LapCon/. This data can be used to inform perioperative discussions with patients during the consent and decision-making process, and with anaesthetists and other health professionals when planning intra- and post-operative care.
As part of this analysis, it was noted that patient selection for laparoscopy on part of the surgical and anaesthetic teams would appear to have been appropriate and safe. There was no significant difference in conversion rates across age and predicted mortality rate groups, as might have otherwise been expected if patients had been unable to tolerate the need for pneumoperitoneum, on-table tilting, and potentially increased operative time associated with laparoscopy. Laparoscopic success in this dataset was dictated other factors, such as underlying pathology and surgeon factors, instead.
Whilst procedures requiring major bowel resection, or involving generalised peritoneal contamination, were the most likely to be performed laparoscopically, a significant proportion of cases were still completed laparoscopically (24% of small bowel resections, 28% of Hartmann’s procedures), further reinforcing how surgeon experience and environmental factors may influence the decision to convert to laparotomy for such cases. This is supported by the observation that non-consultant grades, surgeons not sub-specialised for the procedure type, and surgery performed out of hours, for example, were both more likely to result in conversion.
The influence of hospital and surgeon-specific factors on patient outcomes has been long acknowledged. A recent analysis of over 33,000 patients from the NELA database found significantly improved rates of mortality and length of stay for patients undergoing emergency colorectal surgery when performed by a colorectal surgeon [19]. Out-of-hours operating has been theorised to impact on surgeon performance. Whilst evidence from simulation-based studies has suggested potential deteriorations in technical skill associated with sleep deprivation, [20] reviews of clinical data have found no relationship between in-hours and out-of-hours mortality, suggesting adequate compensatory mechanisms by surgeons [21, 22]. Earlier conversion to open surgery may be an example of such a coping mechanism, reducing technical difficulty, mental load, and surgical time at the expense of a larger incision.
This analysis does have limitations which should be considered. Additional factors which are likely to influence laparoscopic success, such as patient body mass index, previous surgical history (with potential adhesions), or duration of symptoms, were not available. However, it is also unclear how much these factors may have affected the patients in this retrospective dataset, given that many of these factors may influence the pre-operative decision to attempt laparoscopy in the first place (i.e. selection bias). Also, the calibration of our predictive model using the data available was excellent.
This predictive model does not purport to predict appropriateness of laparoscopy for all patients, as the analysed group includes only those surgically selected for attempted laparoscopy in the first place. Surgical patient selection remains critical. In those patients that were converted to open surgery, the reasons for conversion in each individual case were unknown; some cases may have been started laparoscopically to establish a visual diagnosis before proceeding to planned laparotomy, or converted due to iatrogenic injury rather than technical difficulty of the case, or even due to equipment failure. Future prospective work is required to quantify surgeons’ judgement on the appropriateness of patients and pathology for attempted laparoscopy, and reasons for abandonment or conversion. Finally, the exclusion of “laparoscopically assisted” cases meant that these patients could not be considered in the analysis and represents a source of bias. However, this represented less than 1% of all operations in the study period; further prospective study would be required to capture exactly the nature of cases coded as “assisted” and the implications on patient outcomes.
Despite the relatively high conversion rate seen in this cohort, it is important to highlight that conversion to open surgery should not be considered a negative outcome. Elective surgical trials have almost universally reported increased surgical times with laparoscopy despite moderately high conversion rates, which has not impacted on the ability of minimally invasive surgery to improve outcomes [23, 24]. Starting laparoscopically is unlikely to disadvantage patients, and even performing part of the procedure laparoscopically may allow a more focussed incision, to the benefit of the patient’s post-operative recovery. A recent population analysis suggested that risk-adjusted outcomes for converted cases were superior to those after laparotomy [6]. Whilst some may raise concerns regarding the resource implications of laparoscopic equipment, the cost of re-sterilising reusable laparoscopic instruments, even if in addition to ultimately an open surgical instrument set, is negligible. The potential benefit to the patient is not. Every attempt at emergency laparoscopy may be seen as an opportunity to improve outcomes, whether successful or not.
In conclusion, this study highlights factors for laparoscopic surgery which are associated with successful minimally invasive surgery or conversion to laparotomy. Whilst the underlying pathology and required procedure are the strongest determinants, surgeon and environmental remain important factors. Even in the most frequently converted procedures, almost a quarter of procedures were completed laparoscopically, confirming that no procedure can be considered an absolute indication for any given surgical approach. This data, along with our prediction model, can be used to better inform peri-operative discussions and may assist surgeons in patient selection. Appropriate patient selection and surgical experience remains key, and further research into surgeon and patient considerations for emergency laparoscopy is required.
References
Haider AH, Obirieze A, Velopulos CG et al (2015) Incremental cost of emergency versus elective surgery. Ann Surg 262(2):260–266
NELA Project Team (2019) Fifth Patient Report of the National Emergency Laparotomy Audit. RCoA London
Pucher PH, Carter NC, Knight BC et al (2018) Impact of laparoscopic approach in emergency major abdominal surgery: single-centre analysis of 748 consecutive cases. Ann R Coll Surg Engl 100(4):279–284
Quah GS, Eslick GD, Cox MR (2019) Laparoscopic versus open surgery for adhesional small bowel obstruction: a systematic review and meta-analysis of case-control studies. Surg Endosc 33(10):3209–3217
Wiggins T, Markar SR, Harris A (2015) Laparoscopic adhesiolysis for acute small bowel obstruction: systematic review and pooled analysis. Surg Endosc 29(12):3432–3442
Pucher PH, Mackenzie H, Tucker V, Mercer SJ. Laparoscopy in major emergency surgery reduces hospital stay and improves survival: national database propensity score-matched analysis. BJS 2020 [in press]
Sallinen V, Di Saverio S, Haukijarvi E et al (2019) Laparoscopic versus open adhesiolysis for adhesive small bowel obstruction (LASSO): an international, multicentre, randomised, open-label trial. Lancet Gastroenterol Hepatol 4(4):278–286
Harji DP, Marshall H, Gordon K et al (2020) Laparoscopic versus open colorectal surgery in the acute setting (LaCeS trial): a multicentre randomized feasibility trial. Br J Surg 107(12):1595–1604
Tan S, Wu G, Zhuang Q et al (2016) Laparoscopic versus open repair for perforated peptic ulcer: a meta analysis of randomized controlled trials. Int J Surg 33:124–132
Coe PO, Lee MJ, Boyd-Carson H et al (2020) Open versus laparoscopic repair of perforated peptic ulcer disease: a propensity-matched study of the national emergency laparotomy audit. Ann Surg. https://doi.org/10.1097/SLA.0000000000004332
Sajid MS, Khawaja AH, Sains P et al (2016) A systematic review comparing laparoscopic vs open adhesiolysis in patients with adhesional small bowel obstruction. Am J Surg 212(1):138–150
Bartels SA, Vlug MS, Hollmann MW et al (2014) Small bowel obstruction, incisional hernia and survival after laparoscopic and open colonic resection (LAFA study). Br J Surg 101(9):1153–1159
Kim MS, Kwon HJ, Park HW et al (2014) Preoperative prediction model for conversion of laparoscopic to open cholecystectomy in patient with acute cholecystitis: based on clinical, laboratory, and CT parameters. J Comput Assist Tomogr 38(5):727–732
Finnerty BM, Wu X, Giambrone GP et al (2017) Conversion-to-open in laparoscopic appendectomy: a cohort analysis of risk factors and outcomes. Int J Surg 40:169–175
Tekkis PP, Senagore AJ, Delaney CP (2005) Conversion rates in laparoscopic colorectal surgery: a predictive model with, 1253 patients. Surg Endosc 19(1):47–54
von Elm E, Altman DG, Egger M et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335(7624):806–808
Prytherch DR, Whiteley MS, Higgins B et al (1998) POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and operative severity score for the enumeration of mortality and morbidity. Br J Surg 85(9):1217–1220
van Buuren S, Groothuis-Oudshoorn K (2011) MICE: multivariate imputation by chained equations in R. J Stat Softw 45:1–67
Boyd-Carson H, Doleman B, Herrod PJJ et al (2019) Association between surgeon special interest and mortality after emergency laparotomy. Br J Surg 106(7):940–948
Whelehan DF, McCarrick CA, Ridgway PF (2020) A systematic review of sleep deprivation and technical skill in surgery. Surgeon 18(6):375–384
Qadri AH, Sproule S, Girling L et al (2020) Effect of daytime versus night-time on outcome in patients undergoing emergent neurosurgical procedures. J Neurosurg Anesthesiol 32(4):315–322
Koda N, Oshima Y, Koda K et al (2020) Surgeon fatigue does not affect surgical outcomes: a systematic review and meta-analysis. Surg Today 51:659
van der Pas MH, Haglind E, Cuesta MA et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14(3):210–218
Patel K, Abbassi O, Tang CB et al (2020) Completely minimally invasive esophagectomy versus hybrid esophagectomy for esophageal and gastroesophageal junctional cancer: clinical and short-term oncological outcomes. Ann Surg Oncol 28:702
Author information
Authors and Affiliations
Contributions
PHP, SAR, SJM: study concept, data analysis, drafting of manuscript, final review. VT, HM: data analysis, study concept, final review.
Corresponding author
Ethics declarations
Disclosures
Dr. Philip Pucher receives consulting fees from Fundamental Surgery and declares no conflicts of interest. Dr. Saqib Rahman, Dr. Hugh Mackenzie, Dr. Vanessa Tucker, and Dr. Stuart Mercer declare no financial ties or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pucher, P.H., Rahman, S.A., Mackenzie, H. et al. Feasibility of laparoscopy and factors associated with conversion to open in minimally invasive emergency major abdominal surgery: population database analysis. Surg Endosc 36, 4499–4506 (2022). https://doi.org/10.1007/s00464-021-08803-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08803-5