Abstract
Background
It remains inconclusive whether laparoscopic gastrectomy (LG) has better long-term outcomes when compared with open gastrectomy (OG) for elderly gastric cancer (EGC). We attempted to explore the influence of the immune prognostic index (IPI) on the prognosis of EGCs treated by LG or OG to identify a population among EGC who may benefit from LG.
Methods
We included 1539 EGCs treated with radical gastrectomy from January 2007 to December 2016. Propensity score matching was applied at a ratio of 1:1 to compare the LG and OG groups. The IPI based on dNLR ≥ cut-off value (dNLR) and sLDH ≥ cut-off value (sLDH) was developed, characterizing two groups (IPI = 0, good, 0 factors; IPI = 1, poor, 1 or 2 factors).
Results
Of the 528 EGCs (LG: 264 and OG: 264), 271 were in the IPI = 0 group, and 257 were in the IPI = 1 group. In the entire cohort, the IPI = 0 group was associated with good 5-year overall survival (OS) (p = 0.001) and progression-free survival (PFS) (p = 0.003) compared to the IPI = 1 group; no significant differences in 5-year OS and PFS between the LG and OG groups were observed. In the IPI = 1 cohort, there was no significant difference in OS or PFS between the LG and OG groups across all tumor stages. However, in the IPI = 0 cohort, LG was associated with longer OS (p = 0.015) and PFS (p = 0.018) than OG in stage II EGC, but not in stage I or III EGC. Multivariate analysis showed that IPI = 0 was an independent protective factor for stage II EGC receiving LG, but not for those receiving OG.
Conclusion
The IPI is related to the long-term prognosis of EGC. Compared with OG, LG may improve the 5-year survival rate of stage II EGC with a good IPI score. This hypothesis needs to be further confirmed by prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer (GC) is the fifth most frequently diagnosed cancer and the third leading cause of cancer deaths in both sexes worldwide [1]. Approximately 60% of cancer incidence and 70% of cancer-related deaths occur in patients over 65 years old [2]. Surgery is still the preferred treatment for cancer, but elderly patients often have higher morbidity and mortality from postoperative complications than younger patients, due to multiple organ dysfunction and poor immune status. Several studies have indicated that LG has short-term advantages, such as less intraoperative blood loss, shorter operation time, and fewer postoperative complications, compared to traditional OG [3]. Some researchers have attributed these advantages to the lower impact on the inflammatory immune response induced by LG [4, 5]. Thus far, the long-term influence of LG on EGC is uncertain, rendering it necessary to explore the factors influencing the long-term efficacy of LG in EGC.
The inflammatory immune response is an important mechanism for tumor immune escape, which may lead to tumor progression [6]. Increased levels of pretreatment inflammatory markers, such as neutrophils, platelets and sLDH, are associated with a poor cancer prognosis [7, 8]. Moreover, elevated circulating immunoinflammatory factors (IL-6, CRP), which are induced by the systemic inflammatory response under surgical stress, have been confirmed to be risk factors for tumor recurrence [6]. Okholm et al. [9] found that LG seemed to attenuate the inflammatory immune response compared to OG, indicating the degree of systemic inflammatory response mediated by surgical stress varies depending on the surgical approach.
Recently, Mezquita et al. [10] showed that a composite index (LIPI), based on pretreatment circulating-derived neutrophil-to-lymphocyte ratio (dNLR) and serum lactate dehydrogenase (sLDH), could be useful for identifying patients who might benefit from immunotherapy in nonsmall cell lung cancer. In this study, we attempted to combine pretreatment circulating dNLR and sLDH to generate a new gastric immune prognosis index (IPI) to investigate whether the IPI is associated with the long-term prognosis of EGC. Furthermore, we explored if the IPI is able to identify a population among EGC patients who might benefit from LG.
Materials and methods
Patients
A retrospective analysis was performed on all patients who underwent radical gastrectomy in Fujian Medical University Union Hospital (FMUH) from January 2007 to December 2016. The inclusion criteria were (1) age ≥ 65 years, (2) gastric adenocarcinoma was histologically confirmed, (3) no distant metastasis, (4) D2 or modified D2 lymph node dissection was performed, and (5) postoperative pathology confirmed R0 resection. The exclusion criteria were: (1) preoperative neoadjuvant chemotherapy, (2) nonradical (R1/2) resection, (3) concurrent with other malignant tumors, (4) autoimmune diseases and recent steroid therapy, (5) pathological T stage was T4b, or (6) incomplete case records. Initially, LG was performed in patients diagnosed with cT1N0M0 to cT2N0M0 gastric cancer. The indications for LG were then gradually extended to all stages of disease up to and including cT4N2M0 [11]. The surgical approaches were independently chosen by the patients after being fully informed of the advantages and disadvantages of LG and OG [12, 13]. All surgical procedures, including the scope of lymph node dissection, were performed according to the guidelines of the Japanese Gastric Cancer Association [14]. Staging was performed according to the corresponding 8th edition of the AJCC Staging Manual [15]. In this study, patients with stage II/III disease were treated according to the East Asia standard (D2 gastrectomy followed by postoperative adjuvant chemotherapy) [16,17,18], even though perioperative chemotherapy is the preferred treatment option for these patients in Western countries [19]. Adjuvant postoperative chemotherapy based on 5-fluorouracil (mostly oxaliplatin with either Xeloda or S-1) was recommended for all patients with stage II or more advanced GC [20]. This study was approved by the Ethics Committee of Union Hospital of Fujian Medical University.
Definitions
Elderly gastric cancer (EGC) was defined as cases involving patients ≥ 65 years of age [21]. Postoperative complications were classified according to the Clavien–Dindo classification system [22]. Serum samples were collected and assayed (including WBC, neutrophils, sLDH, and albumin level) within 7 days before surgery. dNLR was calculated as follows: absolute neutrophil count/(white blood cell concentration—absolute neutrophil count) [23]. ROC curve analysis was used to determine the cut-off values of dNLR and sLDH. The IPI scores were calculated based on the dNLR ≥ cut-off value(dNLR) and sLDH ≥ cut-off value(sLDH), characterizing two groups (IPI = 0, good, 0 factors; IPI = 1, poor, 1 or 2 factors).
Follow-up
A postoperative follow-up assessment was performed every 3 months for 2 years and then every 6 months during years 2–5. Most routine follow-up appointments included a physical examination, laboratory testing [including cancer antigen (CA) 19–9, CA72-4, and carcinoembryonic antigen (CEA) level measurements], chest radiography, and abdominopelvic ultrasonography or computed tomography, along with an annual endoscopic examination. The final follow-up evaluation was conducted in June 2019, and the median follow-up time was 65.8 months (3.7–150.9 months). Overall survival (OS) was defined as the time from surgery to death from any cause or to the time of censoring on the date of the last follow-up, and progression-free survival (PFS) was defined as the time from surgery to the time of disease progression or death from any cause.
Propensity score matching
To reduce bias in comparisons between groups, an one-to-one propensity score matching analysis was performed between the LG and OG groups based on the estimated propensity scores of each patient [24]. The propensity scores were estimated using a logistic regression model and the following covariates: age, sex, BMI, blood vessel invasion, and tumor TNM stage.
Statistical analysis
Descriptive statistics were used to summarize cohort characteristics and distributions of dNLR and sLDH. The optimal cut-off of the dNLR and sLDH values was assessed by receiver operating characteristic (ROC) analysis. The data are presented as the mean ± standard deviation for continuous variables and as a number for categorical variables. Correlations between categorical variables were analyzed using Chi-squared tests, and continuous variables were analyzed using Student’s t tests. Survival curves were estimated using the Kaplan–Meier method, and the log-rank test was used to determine significance. Variables associated with OS and PFS were identified using multivariate Cox regression models. All statistical analyses were performed using SPSS for Windows version 26.0 (SPSS Inc. Chicago, IL, USA) and R ver. 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, with a significance level set at p < 0.05.
Results
Patient characteristics
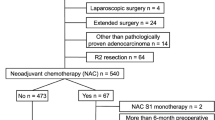
The study flow diagram is shown in Fig. 1. Overall, 1539 EGCs treated with LG or OG in our department between January 2007 and December 2016 were enrolled, 1256 patients underwent LG and 283 OG. Using one-to-one propensity score matching, 264 pairs of LG and OG patients were included in the final analysis (Table 1): 417 were male (78.98%), and 111 were female (21.02%). The median age was 71 years (interquartile range: 68–75 years). The distribution of TNM stage was 119 patients (22.54%) in stage I, 120 patients (22.73%) in stage II, and 289 patients (54.73%) in stage III. The preoperative mean values of dNLR and sLDH were 1.81 ± 0.89 and 170.68 ± 42.26 IU/L, respectively. The dNLR and sLDH cut-off values chosen according to the ROC curve analysis were 1.88 and 190.5 IU/L, respectively (Figure S1). The values allowed two populations to be identified: IPI = 0 (good immune status, 0 factors), which included 271 patients (51.33%) and IPI = 1 (poor immune status, 1 or 2 factors), which included 257 patients (48.67%).
The relationship between the IPI score and clinicopathological factors is shown in Table S1. There were no significant differences in age, sex, surgical approach, type of gastrectomy, digestive tract reconstruction, tumor site, tumor size or histology between the IPI = 0 and IPI = 1 groups, but there were statistically significant differences in the pathological stage (p = 0.026), especially in lymph node stage (p = 0.004).
Short-term outcomes
Table S2 summarizes the intraoperative details and postoperative rehabilitation for the two groups. Compared with the OG group, the LG group had a significantly shorter operating time (entire cohort: 167.10 vs. 252.79 min, p < 0.001; IPI = 0 cohort: 168.26 vs. 246.89 min, p < 0.001; IPI = 1 cohort: 165.10 vs. 259.30 min, p < 0.001) and less estimated blood loss (entire cohort: 77.90 vs. 212.57 mL, p < 0.001; IPI = 0 cohort: 75.17 vs. 199.64 mL, p = 0.001; IPI = 1 cohort: 82.60 vs. 238.67 mL, p < 0.001). There was no significant difference between the OG and LG groups in number of LNs retrieved. In terms of postoperative rehabilitation, compared with the OG group, the LG group had shorter time to start bedside activity, shorter time to remove abdominal drainage, and shorter postoperative hospital stay (all p < 0.05). There were no significant differences between the LG and OG groups in time to first flatus, time to remove gastric tube or time to fluid diet (all p > 0.05).
The details of postoperative complications for the two groups are shown in Table S3. There were no significant differences in local complications between the LG and OG groups, but the incidence of systemic complications tended to be more common in the OG group (37.88% vs. 41.67%, p = 0.335). The most common local complication was surgical site infection (LG: 3.79% vs. OG: 5.68%, p = 0.310), while the most common systemic complication was pulmonary inflammation or infection (LG: 26.51% vs. OG: 26.14%, p = 0.942).
Long-term outcomes
Prognostic value of IPI score in the entire cohort
For the entire cohort, the 5-year overall survival rate and progression-free survival rate was 54.3% and 55.8%, respectively. There were no significant differences in OS (56.6% vs. 52.0%, p = 0.166) or PFS (57.4% vs. 50.6%, p = 0.072) between the LG and OG groups (Figures S2 and S3). The IPI = 0 group had significantly better OS (61.2% vs. 46.9%, p = 0.001) and PFS (60.1% vs. 47.5%, p = 0.003) compared to those in the IPI = 1 group (Figures S4 and S5).
The univariate and multivariate analyses affecting OS and PFS in the entire cohort are shown in Table S4. In the univariate analysis, the variables of age, type of gastrectomy, reconstruction method, tumor location, tumor size, differentiation, vascular invasion, T stage, N stage, pathological stage, dNLR, sLDH, and IPI score had significant effects on OS (all p < 0.05). Multivariate analysis revealed that IPI = 1 was an independent risk factor for OS (HR 1.351; 95% CI 1.05–1.73; p = 0.018). Other independent risk factors included age ≥ 75 years, stage II tumor, and stage III tumor (all p < 0.05). In terms of PFS, IPI = 1 was also an independent risk factor for PFS (HR, 1.339; 95% CI 1.03–1.74; p = 0.029). In addition, OG, tumor ≥ 5.0 cm, vascular invasion, stage II tumor, and stage III tumor were also independent risk factors affecting PFS (all p < 0.05).
Survival difference between LG and OG groups stratified by IPI score and TNM stage
To evaluate the effect of IPI on the prognosis of EGC with different surgical approaches, we compared the OS and PFS between the LG and OG groups in different IPI subpopulations. With regard to IPI = 1, there was no significant difference in 5-year OS (p = 0.733) or PFS (p = 0.784) between the LG and OG groups at all stages (Figures S6 and S7). However, with regard to the IPI = 0, the 5-year OS (p = 0.040) and PFS (p = 0.048) were significantly better in the LG group than those in the OG group, especially for those with stage II disease (OS: p = 0.015 and PFS: p = 0.018, respectively) (Figs. 2 and 3).
Moreover, the prognostic value of the IPI score at different tumor stages was explored. In univariate analysis, IPI = 0 was significantly associated with good OS and PFS in stage II patients but not in stage I or III patients. The multivariate analysis showed that only IPI = 0 was an independent protective factor for both OS (HR 1.995; 95% CI 1.12–3.57; p = 0.020) and PFS (HR 2.025; 95% CI 1.25–3.59; p = 0.045) in stage II EGC (Table S5). Further multivariate analyses for stage II patients in the LG group revealed that, in the stage II disease, IPI = 0 was an independent protective factor for OS (HR 4.171; 95% CI 1.66–10.49; p = 0.002) and PFS (HR 5.27; 95% CI 1.38–20.06; p = 0.015) in the LG group but not for those in the OG group. (Table 2).
Discussion
Our study showed that the IPI score based on preoperative dNLR and sLDH was closely related to the long-term prognosis of EGC. A good IPI score was associated with an advantageous clinical outcome for EGC. Comparison of survival differences between the LG and OG groups revealed that the LG group had a significantly better prognosis than the OG group in IPI = 0 patients but not IPI = 1 patients or the entire patient cohort. Stratified analysis according to TNM stage further indicated that only stage II EGC with IPI = 0 could benefit from LG. Therefore, we speculate that LG might improve the long-term outcome of stage II EGC with a good IPI score. To our knowledge, this is the first study to correlate the influence of preoperative IPI score on long-tern prognosis with the surgical approach in EGC.
The inflammatory immune response has been associated with prognosis in a variety of malignant tumors. dNLR, which covers monocytes and other granulocyte subsets, has been considered an indicator of the inflammatory immune response [23]. In recent years, many researchers have reported that the preoperative dNLR provides comparable or better values than NLR in predicting prognosis in many kinds of cancer, such as melanoma, bladder, kidney, and breast cancers [25].
In addition to dNLR, another classic indicator of the inflammatory immune response is sLDH. Elevated sLDH is associated with a poor prognosis in several cancer types [26, 27]. Previous studies in lung cancer report that sLDH is associated with shorter survival when increasing from 1 to 2.5 times baseline [28]. In melanoma, sLDH is thought to be a potential efficacy predictor for patients treated with PD-1 and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) inhibitors [29, 30]. In gastric cancer, Sun et al. reported that a high level of sLDH is an independent risk factor for long-term prognosis in patients with diffuse and undifferentiated disease [31].
Mezquita et al. create a new prognostic index based on the preoperative levels of dNLR (> 3.0) and sLDH (> upper limit of normal, ULN) and correlate it with resistance to immune checkpoint inhibitor therapy in patients with advanced nonsmall cell lung cancer [10]. The thresholds of dNLR and sLDH were different among studies. Diem [29] and Weide [32] set the threshold of sLDH at ULN and 2ULN, respectively, to predict the prognosis of melanoma. In Ferrucci PF’s [33] study on predicting the prognosis of melanoma, the threshold of dNLR was 3.0, while in other tumor types, M. J. Proctor et al. [23] set the threshold of dNLR at 2.0. Although the thresholds were different, these studies all suggested that dNLR and sLDH were important markers of cancer prognosis. ROC analysis, which combines the sensitivity and specificity of the indicators to predict event occurrence, is one of the most widely used methods in the literature [34]. In this study, the IPI score was generated based on the cut-off values of dNLR (1.88) and sLDH (190.5 IU/L) determined by ROC curve analysis. We used this IPI score to stratify our EGC population into 2 cohorts: good and poor. Our results showed that a good IPI score was significantly associated with better OS and PFS than a poor IPI score but only among patients receiving LG and not those receiving OG. Stratified analysis according to TNM stage further revealed that the survival benefit of a good IPI score was only observed in stage II patients who received LG (Figures S8–S11). These results provided support that a good IPI score might predict benefit from LG.
Since the first reported LG for gastric cancer in 1994 [35], many studies have demonstrated the short-term advantages of laparoscopic surgery over open surgery [3]. Our data showed a significantly shorter operating time, less estimated blood loss, shorter time to start bedside activity, shorter time to remove abdominal drainage, and shorter postoperative hospital stay for the LG group compared with the OG group (all p < 0.05). In terms of postoperative complications, there were no differences in local complications between the LG and OG groups, but the incidence of systemic complications tended to be more common in the OG group (37.88% vs. 41.67%, p = 0.335). All these short-term outcomes were consistent with the results of previous studies [36, 37]. Therefore, considering the trauma caused by surgery, laparoscopic surgery may be optimal for minimizing surgical trauma in EGC. In recent years, several studies of LG in elderly patients have been published, but most of these studies were focused on the short-term advantages of the laparoscopic approach [38, 39]. Our previous studies [11, 37] showed no difference in long-term survive between the LG and OG group, which was consistent with the results reported in most literature [40]. In this study, we also found that there was no significant difference in 5-year OS or PFS between the LG and OG groups among the entire cohort.
Systemic immune inflammation status is associated with the density of immune cells in the tumor microenvironment, which leads to prognostic values of systemic inflammation in gastric cancer [41]. It remains unclear whether the prognostic value of preoperative circulatory inflammatory factors in elderly cancer patients can be influenced by surgical approaches. Zhao et al. [5] reported that LG had less of a change in the inflammatory immune response, which was indicated by lower concentrations of increasing circulatory inflammatory factors after surgery when compared with OG. Okholm et al. [9] reviewed seven studies that addressed the postoperative immunological status in patients with GC, they found that all studies showed a lower postoperative immune response in patients with LG, indicating that laparoscopic-assisted surgery has immunological advantages when compared with open surgery. In this study, we observed better OS and PFS for the LG group compared to the OG group in patients with a good IPI but not those with a poor IPI. Therefore, we hypothesized that in EGC with a good IPI, receiving LG might give rise to a lower inflammatory immune response and better prognosis than in those receiving OG. Stratified analysis according to TNM stage further indicated that the survival advantage of LG only in stage II patients with a good IPI score.
It is well accepted that the prognosis of stage I GC is relatively good, with a 5-year overall survival of over 90% [42]. Many studies [43,44,45] have shown that except for pathological indicators such as TNM staging and lymphatic vessel invasion, other factors, including chemotherapy have limited influence on the prognosis of stage I GC, even though the benefit of adjuvant chemotherapy has been confirmed in stage II and stage III GC [46, 20]. In addition, the inflammatory immune response induced by the tumor was lower in stage I GC than that in advanced cancer [47], which might be associated with improved prognosis. Thus, IPI may not have a predictive role in stage I patients. In stage III patients, due to the relatively advanced T/N staging, the effect of immune status on prognosis might have been attenuated by T/N staging and failed to reach the statistical difference. Our data indicate that the prognosis of stage III GC with good IPI tended to be better than that with poor IPI , but the difference was not statistically significant (Figure S4, 5-year OS: 39.6% vs 29.5%, Figure S5, 5y PFS: 36% vs 27.4%; p > 0.05).
For the first time, we applied IPI to predict the long-term prognosis in EGC. Our data showed that IPI was a good prognostic indicator for stage II patients and suggested that stage II EGC with good IPI might benefit from receiving LG. Therefore, stage II EGC with a good IPI could be recommended as the candidate population for LG. This finding might provide reference for accurate and individualized selection of surgical approaches according to the immune status of patients in future clinical work. However, the internal mechanism needs further study.
This study has several limitations. First, this was a single-center retrospective study and thus may have been subject to selection bias. Second, despite the large size of the cohort in this study, the number of events was insufficient to divide the patients into training and validation sets for internal validation, and the results of this study need to be further verified by external data. Third, patients with neoadjuvant chemotherapy were excluded since the number of patients was quite low in our center, especially for those with age greater than 65 years. This could be attributed to the safety and feasibility of LG were not confirmed by randomized controlled trials in patients with neoadjuvant chemotherapy until 2019 [48]. Fourth, the effects of postoperative adjuvant chemotherapy on the immune status and prognosis were not investigated in this study. Finally, since sLDH was not routinely detected after surgery in our center, it was impossible to compare the concentration change in dNLR and sLDH after surgery. Future studies may objectively compare the changes in concentrations of various inflammatory immune markers including sLDH and dNLR, after surgery.
In conclusion, a good preoperative IPI score correlated with better long-term outcomes for EGC. In patients with a good IPI score, LG had significant survival advantages compared to those in OG, especially for those with stage II disease, suggesting that stage II EGC with a good IPI score might be recommended as a candidate population for LG. This hypothesis needs to be further confirmed by prospective studies with large sample sizes.
References
Rawla P, Barsouk A (2019) Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 14(1):26–38
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ (2016) Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 263:28–35
Janež J, Korać T, Kodre AR, Jelenc F, Ihan A (2015) Laparoscopically assisted colorectal surgery provides better short-term clinical and inflammatory outcomes compared to open colorectal surgery. Arch Med Sci 11(6):1217–1226
Ma Z, Bao X, Gu J (2016) Effects of laparoscopic radical gastrectomy and the influence on immune function and inflammatory factors. Exp Ther Med 12(2):983–986
Cai H, Zhang Y, Meng F, Cui C, Li H, Sui M, Zhang H, Lu S (2019) Preoperative serum IL6, IL8, and TNF-α may predict the recurrence of hepatocellular cancer. Gastroenterol Res Pract 2019:6160783
Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J, Munekage M, Uemura S, Tsujii S, Tamura T, Yatabe T, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K (2019) Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer 22(4):684–691
Zhang J, Zhang HY, Li J, Shao XY, Zhang CX (2017) The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget 8(40):68837–68846
Okholm C, Goetze JP, Svendsen LB, Achiam MP (2014) Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol 49(9):1027–34
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, Felip E, Zerón-Medina J, Garrido P, Brosseau S, Zalcman G, Mazieres J, Caramela C, Lahmar J, Adam J, Chaput N, Soria JC, Besse B (2018) Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 4(3):351–357
Lu J, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M (2015) Short- and long-term outcomes after laparoscopic versus open total gastrectomy for elderly gastric cancer patients: a propensity score-matched analysis. J Gastrointest Surg 19(11):1949–1957
Li Z, Shan F, Wang Y, Li S, Jia Y, Zhang L, Yin D, Ji J (2016) Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer after neoadjuvant chemotherapy: safety and short-term oncologic results. Surg Endosc 30(10):4265–4271
Alhossaini RM, Altamran AA, Cho M, Roh CK, Seo WJ, Choi S, Son T, Kim HI, Hyung WJ (2020) Lower rate of conversion using robotic-assisted surgery compared to laparoscopy in completion total gastrectomy for remnant gastric cancer. Surg Endosc 34(2):847–852
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver.3). Gastric Cancer 14(113–23):13
Amin MB (2016) AJCC cancer staging manual, 8th edn. Springer, New York, p 2016
Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M (2020) Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer 24:492
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G (2016) Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 34(12):1350–1357
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzén F, Noh SH, CLASSIC Trial Investigators (2012) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379(9813):315–321
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Trial Participants MAGIC (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355(1):11–20
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29(33):4387–4393
Nashimoto A (2013) Current status of treatment strategy for elderly patients with gastric cancer. Int J Clin Oncol 18(6):969–970
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ (2012) A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 107(4):695–699
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424
Duan J, Pan L, Yang M (2018) Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: a meta-analysis. Medicine (Baltimore) 97(49):e13340
Wang ZX, Yang LP, Qiu MZ, Wang ZQ, Zhou YX, Wang F, Zhang DS, Wang FH, Li YH, Xu RH (2018) Prognostic value of preoperative serum lactate dehydrogenase levels for resectable gastric cancer and prognostic nomograms. Oncotarget 7(26):39945–39956
Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, Barni S (2015) Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 54(7):961–970
Inomata M, Hayashi R, Tanaka H, Shimokawa K, Tokui K, Taka C, Okazawa S, Kambara K, Ichikawa T, Yamada T, Miwa T, Kashii T, Matsui S, Tobe K (2016) Elevated levels of plasma lactate dehydrogenase is an unfavorable prognostic factor in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer, receiving treatment with gefitinib or erlotinib. Mol Clin Oncol 4(5):774–778
Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J (2016) Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 114(3):256–261
Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, Kapiteijn EW, de Groot JW, Soetekouw P, Jansen RL, Fiets E, Furness AJ, Renn A, Krzystanek M, Szallasi Z, Lorigan P, Gore ME, Schumacher TN, Haanen JB, Larkin JM, Blank CU (2014) Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 63(5):449–458
Sun X, Sun Z, Zhu Z, Guan H, Zhang J, Zhang Y, Xu H, Sun M (2014) Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients. PLoS ONE 9(3):e91068
Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di Giacomo AM, Brenner N, Kähler K, Heinzerling L, Gutzmer R, Bender A, Gebhardt C, Romano E, Meier F, Martus P, Maio M, Blank C, Schadendorf D, Dummer R, Ascierto PA, Hospers G, Garbe C, Wolchok JD (2016) Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 22(22):5487–5496
Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P, Savoia P, Mandalà M, Simeone E, Valpione S, Altomonte M, Spagnolo F, Cocorocchio E, Gandini S, Giannarelli D, Martinoli C (2016) Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 27(4):732–738
Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M (2017) Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 26(4):611–619
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4(2):146–148
Ding Z, Jiang L, Zhang K, Huang R (2018) Short- and long-term outcomes of conversion in laparoscopic gastrectomy for gastric cancer. J BUON 23(4):1004–1012
Wang JB, Zhong Q, Chen QY, Lin GT, Liu ZY, Huang XB, Xie JW, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Zheng CH, Huang CM, Li P (2020) Well-designed retrospective study versus small-sample prospective study in research based on laparoscopic and open radical distal gastrectomy for advanced gastric cancer. Surg Endosc 34(10):4504–4515
Hoshino N, Fukui Y, Hida K, Sakai Y (2019) Short-term outcomes of laparoscopic surgery for colorectal cancer in the elderly versus non-elderly: a systematic review and meta-analysis. Int J Colorectal Dis 34(3):377–386
Khalilov ZB, Kalinichenko AY (2017) Laparoscopic surgery for colon cancer in elderly patients. Khirurgiia (Mosk) 3:86–89
Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Song KY, Ryu SY (2014) Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol 32(7):627–633
Choi Y, Kim JW, Nam KH, Han SH, Kim JW, Ahn SH, Park DJ, Lee KW, Lee HS, Kim HH (2017) Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer 20(4):602–611
Park JH, Ryu MH, Kim HJ, Ryoo BY, Yoo C, Park I, Park YS, Oh ST, Yook JH, Kim BS, Kang YK (2016) Risk factors for selection of patients at high risk of recurrence or death after complete surgical resection in stage I gastric cancer. Gastric Cancer 19(1):226–233
Kito T, Yamamura Y, Kojima H (1990) Factors in the prognosis of early gastric cancer. Gan To Kagaku Ryoho 17(1):15–21
Onodera H, Tokunaga A, Yoshiyuki T, Kiyama T, Kato S, Matsukura N, Masuda G, Tajiri T (2004) Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology 51(55):82–5
Scartozzi M, Galizia E, Verdecchia L, Berardi R, Graziano F, Catalano V, Giordani P, Mari D, Silva RR, Marmorale C, Zingaretti C, Cascinu S (2006) Lymphatic, blood vessel and perineural invasion identifies early-stage high-risk radically resected gastric cancer patients. Br J Cancer 95(4):445–449
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–20
Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, Xiao J, Alexeeff S, Corley D, Weltzien E, Castillo AL, Caan BJ (2017) Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results From the C SCANS Study. JAMA Oncol 3(12):e172319
Li Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, Ren H, Su X, Ji J, (2019) Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg 154(12):1093–1101
Acknowledgements
We are grateful to the Chinese Gastric Cancer Association, the Chinese Society of Laparo-Endoscopic Surgery, and the Chinese Society of Gastrointestinal Surgery for their scientific support.
Funding
This study was funded by the Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171), Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant Number: 2018Y9041), the second batch of special support funds for Fujian Province innovation and entrepreneurship talents (2016B013), National Nature Science Foundation of China (No. 81871899), and Special Fund for Clinical Research of Wu Jieping Medical Foundation (No. 320.6750.17511).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Drs. Guo-Sheng Lin, Xiao-Yan Huang, Jun Lu, Dong Wu, Hua-Long Zheng, Bin-Bin Xu, Chao-Hui Zheng, Ping-Li, Jian-Wei Xie, Jia-Bin Wang, Jian-Xian Lin, Qi-Yue Chen, Long-Long Cao, Mi-Lin, Ru-Hong Tu, Guang-Tan Lin, Ze-Ning Huang, Ju-Li Lin, and Chang-Ming Huang, have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
464_2021_8461_MOESM1_ESM.tif
Supplementary file 1—Fig. S1 Comparison of the areas under the receiver operating characteristic (ROC) curves among the seven immunoinflammatory markers for elderly gastric cancer (TIF 1966 kb)
464_2021_8461_MOESM2_ESM.tif
Supplementary file 2—Fig. S2 Comparison of overall survival between the LG and OG groups according to pathological stage in entire cohort. A, entire cohort. B, stage I. C, stage II. D, stage III (TIF 8520 kb)
464_2021_8461_MOESM3_ESM.tif
Supplementary file 3—Fig. S3 Comparison of progression-free survival between the LG and OG groups according to pathological stage in entire cohort. A, entire cohort. B, stage I. C, stage II. D, stage III (TIF 8520 kb)
464_2021_8461_MOESM4_ESM.tif
Supplementary file 4—Fig. S4 Comparison of overall survival based on immune prognostic index in entire cohort. A, entire cohort. B, stage I. C, stage II. D, stage III (TIF 8507 kb)
464_2021_8461_MOESM5_ESM.tif
Supplementary file 5—Fig. S5 Comparison of overall survival based on immune prognostic index in entire cohort. A, entire cohort. A, entire cohort. B, stage I. C, stage II. D, stage III (TIF 8507 kb)
464_2021_8461_MOESM6_ESM.tif
Supplementary file 6—Fig. S6 Comparison of overall survival between the LG and OG groups according to pathological stage in IPI=1 subpopulation. A, patients with IPI = 1. B, stage I patients with IPI = 1. C, stage II patients with IPI = 1. D, stage III patients with IPI = 0 (TIF 8533 kb)
464_2021_8461_MOESM7_ESM.tif
Supplementary file 7—Fig. S7 Comparison of progression-free survival between the LG and OG groups according to pathological stage in IPI = 1 subpopulation. A, patients with IPI = 1. B, stage I patients with IPI = 1. C, stage II patients with IPI = 1. D, stage III patients with IPI = 1 (TIF 8532 kb)
464_2021_8461_MOESM8_ESM.tif
Supplementary file 8—Fig. S8 Comparison of overall survival based on immune prognostic index in patients receiving LG. A, patients receiving LG. B, stage I patients receiving LG. C, stage II patients receiving LG. D, stage III patients receiving LG (TIF 8525 kb)
464_2021_8461_MOESM9_ESM.tif
Supplementary file 9—Fig. S9 Comparison of overall survival based on immune prognostic index in patients receiving OG. A, patients receiving OG. B, stage I patients receiving OG. C, stage II patients receiving OG. D, stage III patients receiving OG (TIF 8526 kb)
464_2021_8461_MOESM10_ESM.tif
Supplementary file 10—Fig. S10 Comparison of progression-free survival based on immune prognostic index in patients receiving LG. A, patients receiving LG. B, stage I patients receiving LG. C, stage II patients receiving LG. D, stage III patients receiving LG (TIF 8524 kb)
464_2021_8461_MOESM11_ESM.tif
Supplementary file 11—Fig. S11 Comparison of progression-free survival based on immune prognostic index in patients receiving OG. A, patients receiving OG. B, stage I patients receiving OG. C, stage II patients receiving OG. D, stage III patients receiving OG (TIF 8525 kb)
Rights and permissions
About this article
Cite this article
Lin, GS., Huang, XY., Lu, J. et al. A good preoperative immune prognostic index is predictive of better long-term outcomes after laparoscopic gastrectomy compared with open gastrectomy for stage II gastric cancer in elderly patients. Surg Endosc 36, 1814–1826 (2022). https://doi.org/10.1007/s00464-021-08461-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08461-7