Abstract
Background
Laparoscopic gastrectomy (LG) is increasingly applied in locally advanced gastric cancer (LAGC) after neoadjuvant chemotherapy (NC). However, there is no study to comprehensively evaluate the clinicopathological, prognostic, and laboratory data such as nutrition, immune, inflammation-associated indexes, and tumor markers between LG and open gastrectomy (OG) for LAGC following NC.
Methods
The clinicopathological, prognostic, and laboratory data of LAGC patients with clinical stage of cT2-4aN1-3M0 who underwent gastrectomy after NC were retrospectively collected. The effects of LG and OG were compared after propensity score matching (PSM).
Results
This study enrolled 148 cases, of which 110 cases were included after PSM. The LG group had a shorter length of incision (P < 0.001) and was superior to OG group in terms of blood loss (P < 0.001), postoperative first flatus time (P < 0.001), and postoperative first liquid diet time (P = 0.004). No significant difference was found in postoperative complications (P = 0.482). Laboratory results showed that LG group had less reduced red blood cells (P = 0.039), hemoglobin (P = 0.018), prealbumin (P = 0.010) in 3 days after surgery, and less reduced albumin in 1 day (P = 0.029), 3 days (P = 0.015), and 7 days (P = 0.035) after surgery than the OG group. The systemic immune-inflammation index and systemic inflammatory response index were not significantly different between the two groups. As for oncological outcomes, there were no significant differences in postoperative tumor markers of CEA (P = 0.791), CA199 (P = 0.499), and CA724 (P = 0.378). The 5-year relapse-free survival rates (P = 0.446) were 46.9% and 43.3% in the LG and OG groups, with the 5-year overall survival rates (P = 0.742) being 46.7% and 52.1%, respectively; the differences were not statistically significant. Multivariate Cox regression analysis revealed that tumor size ≥ 4 cm (P = 0.021) and the absence of postoperative adjuvant chemotherapy (P = 0.012) were independent risk factors for overall survival.
Conclusions
LG has faster gastrointestinal recovery, better postoperative nutritional status, and comparable oncological outcomes than OG, which can serve as an alternative surgical method for LAGC patients after NC.
Similar content being viewed by others
Background
Gastric cancer is the fifth most common cancer and the fourth leading cause of cancer-related mortality worldwide [1]. In China, approximately 70% of gastric cancer patients are at an advanced stage of cancer when their diagnoses are confirmed. The primary treatment for locally advanced gastric cancer (LAGC) is complete surgical resection with D2 lymph node dissection. Laparoscopic surgery has been widely performed for gastric cancer since the first reported case of laparoscopic distal gastrectomy [2]. The results of the CLASS-01 [3,4,5] and KLASS-02 trial [6, 7] showed that laparoscopic surgery for distal gastric cancer was safe and feasible; therefore, the 2021 National Comprehensive Cancer Network guidelines recommended laparoscopic surgery for LAGC.
The MAGIC trial indicated that perioperative chemotherapy with epirubicin, cisplatin, and infused fluorouracil for operable adenocarcinomas of stomach or lower esophageal could decrease tumor stage and significantly improve the 5-year progression-free survival and 5-year overall survival (OS) [8]. The EORTC40954 [9], FNCLCC FFCD [10], and RESOLVE [11] trials have successively confirmed the safety and efficacy of neoadjuvant chemotherapy (NC) for LAGC. However, NC can cause tissue fibrosis and edema, which may influence surgical procedures and tissue healing. Li et al. conducted a randomized controlled trial (RCT) confirming the short-term effects of laparoscopic distal gastrectomy for LAGC after NC [12]; however, long-term outcomes are ambiguous because of limited evidence. And most retrospective studies have differences in baseline data. In addition, there is no study to synthetically compare the laboratory indexes such as nutritional status, immune-inflammation conditions, and tumor markers between the two surgical methods, which is of great significance to comprehensively reflect the influence of different surgical methods on patients. Therefore, after balancing the difference in baseline data by propensity score matching (PSM), we comprehensively compared the clinicopathological, laboratory, and prognostic data of the two groups, so as to confirm the non-inferiority of laparoscopic gastrectomy (LG) to open gastrectomy (OG) for LAGC patients following NC.
Methods
Patient selection
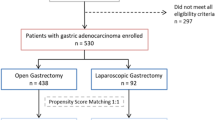
This study included patients with LAGC who received surgery after NC between January 2012 and September 2020 in the Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The first OG and LG for LAGC patients after NC were conducted at January 2012 and August 2012, respectively. NC was conducted on LAGC patients with a clinical tumor stage of cT2-4aN1-3M0. The treatment regimens included FOLFOX (oxaliplatin, leucovorin, and 5-fluorouracil), SOX (oxaliplatin and TS-1), XELOX (oxaliplatin and capecitabine), and FLOT (docetaxel, oxaliplatin, leucovorin and fluorouracil), which were selected based on the Chinese Society of Clinical Oncology (CSCO) clinical guidelines of gastric cancer [13] and the tolerance of patients. The following were the criteria for inclusion: (1) clinical tumor stage of cT2-4aN1-3M0, (2) underwent surgery after NC. And the following were the criteria for exclusion: (1) palliative gastrectomy, (2) diagnosis of any other malignant tumor in the past 5 years and (3) incomplete clinicopathological or follow-up data. All patients were separated into two groups: those who received LG (LG group) and those who underwent OG (OG group) after NC. The flow chart for the selection of cases is shown in Fig. 1. The Tongji Medical College Ethics Committee of Huazhong University of Science and Technology ratified the protocol of this study and the approved number is UHCT-IEC-SOP-016–03-01. The informed consent was signed by all patients to have their data used for the study.
Surgical procedure
All surgeries were performed by experienced surgeons of the Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Based on previous literatures [4, 7, 12], and physician experience, LG is only considered for patients who meet the following conditions: (1) without abdominal operation before, (2) patients could tolerate laparoscopic surgery, and (3) without obvious metastasis and R0 resection is possible. The criteria for OG are as follows: (1) patients could tolerate surgery, and (2) without obvious metastasis and R0 resection is possible. For patients according to the above criteria, the benefits and risks of the two surgical methods would be fully explained by the surgical physician, and the final decision was made by the patients. The performance of proximal gastrectomy, distal gastrectomy, or total gastrectomy was depended on the tumor location. All patients underwent standard gastrectomy with D2 lymphadenectomy and standard reconstruction based on the Japanese gastric cancer treatment guidelines (5th edition) [14]. Reconstruction after proximal gastrectomy was performed via esophagogastrostomy, while reconstruction after total gastrectomy was performed via Roux-en-Y esophagojejunostomy. Distal gastrectomy reconstruction was performed via standard Roux-en-Y gastrojejunostomy or Billroth II gastrojejunal anastomosis depending on the surgeon’s preference. All reconstruction processes were performed in an open manner. Postoperative adjuvant chemotherapy was routinely applied to all patients unless they could not put up with it due to serious adverse effects. The postoperative adjuvant chemotherapy regimens included XELOX, SOX, FOLFOX, and S-1. For patients who achieved R0 resection after NC, postoperative adjuvant chemotherapy was carried out consistent with the original regimens if NC was effective. If the disease progressed after NC, subsequent chemotherapy regimens would be discussed through multidisciplinary team. Postoperative adjuvant chemotherapy and NC were 8 cycles in total and were adjusted according to the patient’s disease condition and tolerance.
Data collection and outcome assessment
Demographic data, involving age, sex, body mass index, Charlson Comorbidity Index (CCI) [15], and American Society of Anesthesiologists (ASA) score, were collected from all patients. Enhanced abdominal computed tomography (CT) was performed before NC to evaluate the clinical tumor stage and after NC to assess the chemotherapy response. The American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th) [16] was used to define the clinical tumor stage, and the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) was applied to evaluate the chemotherapy response [17].
A standard clinical pathway was used for management of all patients. Liquid diet was started after first flatus, and patients were discharged after the absence of complications. Data related to surgery, including the length of incision, operative time, estimated blood loss, time to first flatus, time to first liquid diet, postoperative complications, and length of postoperative hospital stay, as well as pathological characters, were collected. The Clavien–Dindo classification system was applied to grade postoperative complications, which were defined as incidents happening within 30 days after surgery [18, 19]. Tumor regression grade was evaluated according to AJCC standard established by College of American Pathologists (CAP) [20], and pathological stage was defined according to the AJCC Cancer Staging Manual (8th) [16]. A 30-day mortality was defined as death due to any cause that occurred within 30 days after surgery.
Laboratory data such as hemocytes, hemoglobin (Hb), total protein (TP), albumin (Alb), and prealbumin (PAB) at 1 day before surgery and 1 day, 3 days, and 7 days after surgery were collected. To avoid the potential influence of inconsistent preoperative baseline levels, the reduced value was calculated as preoperative value subtracted postoperative value to evaluate the outcomes of different surgical methods. The systemic immune-inflammation index (SII) and systemic inflammatory response index (SIRI) were adopted to assess the immune and inflammation conditions at 1 day before surgery and 1 day, 3 days, and 7 days after surgery. The SII was calculated as neutrophil count × platelet count/lymphocyte count. The SIRI was calculated as neutrophil count × monocyte count/lymphocyte count. Tumor markers including CEA, CA199, and CA724 at 1 week before surgery and 1 month after surgery were collected to evaluate the short-term oncological outcomes of two surgical methods.
Follow-up
Postoperative follow-up was performed every 3–6 months for the first 2 years and every 6–12 months thereafter via outpatient clinics or telephonic interviews. During follow-up, patients’ complete blood count, liver and kidney function, and tumor markers were examined. Chest-, abdomen-, and pelvis-enhanced CT were examined once every 6 months. Gastroscopy was conducted once every year. The last follow-up date was December 30, 2022. After excluding the cases with incomplete clinicopathological or follow-up data, all 148 cases included in this study achieved complete follow-up, and the median follow-up period was 48.0 (3.0–128.8) months. Median follow-up period in LG group was 44.0 (3.2–119.0) months and was 50.0 (3.0–128.8) months in OG group. Relapse-free survival (RFS) was defined as the duration from surgery to recurrence, and OS was defined as the duration from surgery to death. Local recurrence refers to the recurrence at the original surgical site, while distant metastasis refers to the metastasis of organs or parts other than the original surgical site, such as the liver, peritoneum, lung, and bone.
Propensity score matching
PSM was conducted by matching patients who underwent LG after NC with those who underwent OG after NC to eliminate differences in baseline statistics. Seven covariates (age, sex, CCI, ASA score, clinical stage, tumor size, and resection type) were chosen to calculate the propensity score. PSM was conducted by one-to-one nearest neighbor method, and the caliper was set on 0.1.
Statistical analysis
Quantitative variables were presented as mean ± standard deviation, and the differences were compared with independent t-test. Quantitative variables with high deviation were expressed as medians (interquartile range), and the differences were compared with Mann–Whitney U-test. Categorical variables were expressed as frequencies with the differences compared by chi-square test or Fisher’s exact test. Standardized difference was calculated to compare the balance of baseline data before and after PSM. Kaplan–Meier analysis was used to generate the survival curves, and the differences were evaluated by log-rank test. Univariate and multivariate analyses of risk factors for RFS and OS were conducted by the Cox proportional hazards regression model. The cutoff point of the continuous variables associated with survival was determined using the median. The multivariate Cox proportional hazards regression model included variables with P < 0.1 in the univariate analysis. Statistical analysis was conducted by SPSS software (SPSS 20.0, Chicago, IL, USA). R (version 4.0.4) was used for PSM. GraphPad Prism software (version 8.0, USA) was used for image processing. P < 0.05 was considered statistically significant.
Results
Demographic and clinicopathological characteristics
As shown in Fig. 1, 148 patients who underwent gastrectomy after NC were enrolled in the study based on inclusion and exclusion criteria. The LG and OG groups consisted of 80 and 68 participants, respectively. The resection type and anastomotic methods were significantly different before matching. After PSM, 55 patients remained in each group, and the baseline data were comparable between the two groups (Table 1). The LG group included 45 men (81.8%) and 10 women (18.2%) with a mean age of 56.9 ± 9.2 years. The OG group included 46 men (83.6%) and 9 women (16.4%) with a mean age of 57.4 ± 10.8 years.
As shown in Table 2, the most common NC regimens were FOLFOX and SOX in both of the groups. According to the RECIST criteria, 3 patients (2.7%) achieved complete response (CR), 48 patients (43.6%) achieved partial response (PR), 55 patients (50.0%) had stable disease (SD), and 4 patients (3.6) had progressed disease (PD) after NC. Grade 3/4 adverse events of chemotherapy happened in 9.1% (10/110) of patients during the NC period based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) [21]. The most common adverse events were neutropenia and myelosuppression. No NC-related death was observed. The postoperative adjuvant chemotherapy portion of the LG group and OG group was 81.8% (45/55) and 83.6% (46/55), respectively, and the difference was not statistically significant (P = 0.801).
The short-term surgical outcomes
The variables related to the short-term surgical outcomes are shown in Table 3. Compared with the OG group, the LG group had a shorter length of incision (6.4 ± 3.2 cm vs. 19.1 ± 4.5 cm, P < 0.001) and less blood loss (121.7 ± 71.5 ml vs. 180.0 ± 89.4 ml, P < 0.001), but the operative time was longer (282.0 ± 64.5 min vs. 254.6 ± 74.2 min, P = 0.041). The proportion of R0 resection (90.9% vs. 90.9%, P = 1.000) and number of lymph node dissection (LND) (22.5 ± 6.0 vs. 24.5 ± 9.2, P = 0.180) were not significantly different between the two groups. As for postoperative recovery, it was shown that the LG group had a shorter time to first flatus (3.4 ± 1.2 days vs. 4.5 ± 1.8 days, P < 0.001) and shorter time to first liquid diet (4.3 ± 1.8 days vs. 5.4 ± 2.0 days, P = 0.004) than those of the OG group, but there was no significant difference in the median postoperative hospital stay (10.0 days vs. 11.0 days, P = 0.291).
The overall incidence of postoperative complications was 20.9% (23/110), with 10 patients (18.2%) in the LG group and 13 patients (23.6%) in the OG group; the difference was not statistically significant (P = 0.482) (Table 4). Grade III or higher postoperative complications occurred in 7 patients (6.4%), including intraperitoneal hemorrhage in 1 patient (OG), delayed gastric emptying in 1 patient (LG), duodenal stump fistula in 1 patient (LG), anastomotic stenosis in 1 patient (OG), respiratory failure in 2 patients (both OG), and cerebral embolism in 1 patient (OG). All these complications were managed by conservative treatment or reoperation as appropriate. No 30-day mortality was recorded.
Perioperative nutritional and immune-inflammation status
In order to comprehensively figure out the influence of different surgical methods on LAGC patients following NC, we collected perioperative laboratory data to analyze the nutritional and immune-inflammation status of patients during the perioperative period. By calculating the reduction of red blood cells (RBCs) and Hb, we found that LG group had less reduced RBCs (P = 0.039) and Hb (P = 0.018) in 3 days after surgery (Fig. 2A–B), which confirmed that LG group had less blood loss than OG group during surgery. This may be attributed to the smaller incision size, better visualization of anatomical structures, and more precise hemostasis achieved by laparoscopic surgery. The reduced Alb was also calculated and found to be less in LG group both in 1 day (P = 0.029), 3 days (P = 0.015), and 7 days (P = 0.035) after surgery (Fig. 2D). PAB is a good indicator for short-term nutritional status due to its short half-life period [22, 23]. Our results showed that LG group had less reduced PAB in 3 days after surgery (P = 0.010) (Fig. 2E). All above results indicated that LG is less harmful to the nutritional status of LAGC patients after NC. The SII and SIRI are generally acknowledged indicators for the immune-inflammation status of patients [24, 25]. By calculating the SII and SIRI before surgery and 1 day, 3 days, and 7 days after surgery, we found no significant difference between the two groups (Fig. 3), which further proved the safety of LG for LAGC patients after NC.
Perioperative nutritional indexes changes in the LG and OG groups. Reduced value was calculated as the value at 1 day before surgery subtracted that of 1 day, 3 days, or 7 days after surgery. LG, laparoscopic gastrectomy; OG, open gastrectomy; RBC, red blood cell; Hb, hemoglobin; TP, total protein; Alb, albumin; PAB, prealbumin
Perioperative immune-inflammation status of the LG and OG groups. The SII was calculated as neutrophil count × platelet count/lymphocyte count. The SIRI was calculated as neutrophil count × monocyte count/lymphocyte count. LG, laparoscopic gastrectomy; OG, open gastrectomy; SII, systemic immune-inflammation index; SIRI, systemic inflammatory response index
Oncological outcomes
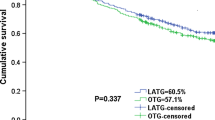
To evaluate the short-term oncological outcomes of different surgical methods, the data of tumor markers at a week before surgery and 1 month after surgery were collected. The results showed no significant difference between the two groups (Fig. 4). Regarding the long-term oncological outcomes, we recorded and analyzed the recurrence and survival status of patients after a median follow-up of 48.0 (3.0–128.8) months. The overall recurrence within 3 years occurred in 24 (43.6%) and 28 (50.9%) patients in the LG and OG groups, respectively (P = 0.445). And there were no significant differences in local recurrence (P = 0.768) and metastasis (P = 0.550) between the two groups. The 5-year RFS and 5-year OS rates in the LG group were 46.9% and 46.7%, respectively, and the corresponding rates in the OG group were 43.3% and 52.1%, respectively. No significant difference was found in RFS (P = 0.446) or OS (P = 0.742) (Table 5). The Kaplan–Meier analysis showed no significant difference in RFS and OS both before and after PSM (Figs. 5 and 6).
Risk factors of RFS and OS
Univariate Cox regression analysis showed that surgical procedures were not relevant to RFS (P = 0.448) or OS (P = 0.742). The risk factors related to RFS were tumor size ≥ 4 cm, lymph-vascular invasion, nerve invasion, R1 resection, clinical response of RECIST being SD or PD, ypTNM stages III or IV, and the absence of postoperative adjuvant chemotherapy. The risk factors related to OS were tumor size ≥ 4 cm, lymph-vascular invasion, nerve invasion, R1 resection, clinical response of RECIST being SD or PD, ypTNM stages III or IV, and the absence of postoperative adjuvant chemotherapy (Table 6). Multivariate Cox regression analysis showed that tumor size ≥ 4 cm (P = 0.021) and the absence of postoperative adjuvant chemotherapy (P = 0.012) were independent risk factors of OS (Table 7).
Discussion
Surgery is the first-line treatment for LAGC. The principle of surgery is the complete resection of tumor and adequate lymphadenectomy. The JCOG0912 trial [26], KLASS01 trial [27], and CLASS02 trial [28] have proved the safety and feasibility of LG for early gastric cancer. Similarly, the CLASS01 trial [3,4,5] and KLASS02 trial [6, 7] observed that laparoscopic distal gastrectomy is safe and feasible for LAGC. However, the participants included in the above studies were gastric cancer patients who did not receive NC. Most previous retrospective studies on the outcomes of laparoscopic surgery for LAGC after NC showed significant differences in baseline data [29,30,31]. And seldom have studies compared the nutritional status, immune-inflammation conditions, and tumor markers between the two different surgical methods. To the best of our knowledge, this is the first study to comprehensively evaluate the short- and long-term outcomes of laparoscopic surgery for LAGC after NC with clinicopathological, prognostic, and laboratory data after balancing the differences between the two groups with PSM. Our results confirmed the safety and efficacy of LG in LAGC patients following NC. What’s more, we found that LG had faster gastrointestinal recovery, better postoperative nutritional status than OG, which allows LG to have extensive application in the background of enhanced recovery after surgery (ERAS).
Local tumors are prone to tissue edema, exudation, and fibrosis after NC, which may affect the identification of anatomic spaces and increase the difficulty of surgery as well as the risk of postoperative complications [32]. However, the FNCLCC FFCD trial showed that the occurrence rate of complications in surgery following NC group was 25.7% (28/109), which was not significantly different from 19.1% (21/110) in the surgery-only group for resectable gastroesophageal adenocarcinoma [10]. Previous studies showed that the occurrence rate of postoperative complications in LAGC patients who did not receive NC was 14.1–38.0% [3, 6, 33,34,35]. The overall incidence of postoperative complications in our study was 20.9%, which indicated that NC did not obviously increase the incidence of postoperative complications.
Laparoscopic surgery involves a smaller incision and can effectively avoid tissue pull injury caused by open surgery. In addition, the visual magnification of laparoscopy allows a clearer surgical field and improves visualization of anatomical layers, resulting in less intraoperative damage to the surrounding tissues. Our study showed that patients in LG group had less blood loss during surgery and had less reduced Alb and PAB after surgery, which indicated a better postoperative nutritional status. This could be due to less damage to the gastrointestinal tract, which allows faster recovery of gastrointestinal function and earlier time to first liquid diet. Related study reported that the faster recovery of postoperative nutritional status in laparoscopic surgery patients may also be associated with less postoperative pain, early-stage physical exercise, and smaller stress response [36]. Meanwhile, Alb and PAB were reported to be closely related to the prognosis of cancer patients [37,38,39,40], which indicated that LG might have potential benefit of improving prognosis. Above all, these advantages allow LG to have promising application in the era of minimally invasive surgery and ERAS [41, 42].
The number of LND is closely related to postoperative pathological stage and prognosis evaluation of gastric cancer. Some lymph nodes retreat after NC, which may lead to a reduced number of LND. Therefore, a large bias may arise when predicting prognosis using the ypTNM staging system. The lymph node ratio (LNR) has been proved to be a more stable and accurate prognostic indicator than N stage in previous studies [43], and it is usually unaffected by the number of LND in predicting the prognosis of gastric cancer patients. Therefore, LNR is a promising prognostic indicator to replace the ypTNM staging system for LAGC after NC [44, 45]. In addition, several large RCTs confirmed that the number of LND by laparoscopic surgery was similar to that of open surgery for LAGC [3, 6, 46]. However, it is unknown whether LG can achieve the same number of LND as that of OG for LAGC after NC because of the fact that tissue exudation and edema caused by NC may affect the surgical process. Our results showed that the two surgical methods have similar number of LND, which is consistent with the results of Li et al. [12] and Fujisaki et al. [47]. Therefore, LG for LAGC patients after NC is safe and feasible in terms of lymphadenectomy.
Long-term follow-up findings in CLASS-01 [5] and KLASS-02 trials [7] showed that LG was not inferior to OG in the treatment of LAGC. With regard to the oncological outcomes of LG for LAGC after administration of NC, our study indicated that LG was comparable to those of OG both in the efficacy of decreasing tumor markers and long-term RFS and OS, which is consistent with previous literature reports [30, 31, 47]. Moreover, it is unclear whether postoperative adjuvant chemotherapy will further improve the prognosis of LAGC after NC. Our results showed that the absence of postoperative adjuvant chemotherapy was an independent risk factor to the OS of LAGC patients after NC. Therefore, it is necessary for LAGC patients following NC to receive postoperative adjuvant chemotherapy to achieve the best survival benefit as long as they are able to tolerate adverse effects.
Above all, this study made a comprehensive evaluation and proved the non-inferiority of LG to OG for LAGC patients following NC. However, as this was a retrospective study of single center, it had limited sample size and could not avoid the inherent bias of the retrospective study design. Besides, LG is a novel surgical method emerging in recent years. The widespread application of LG is later than traditional OG, especially for LAGC patients after NC. Therefore, the follow-up time of LG was shorter than that of OG, which may affect the long-term survival results between the two groups. However, the median follow-up time in both groups was greater than 36 months, which provides preliminary evidence for the oncologic safety of LG. Certainly, we will keep on following up and look forward to conducting multicenter prospective RCT to derive more rigorous and accurate conclusions.
Conclusions
In conclusion, LG has a shorter length of incision, faster gastrointestinal recovery, better postoperative nutritional status, and comparable oncological outcomes than OG and can serve as an alternative surgical method for LAGC patients after NC.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LAGC:
-
Locally advanced gastric cancer
- NC:
-
Neoadjuvant chemotherapy
- RCT:
-
Randomized controlled trial
- LG:
-
Laparoscopic gastrectomy
- OG:
-
Open gastrectomy
- PSM:
-
Propensity score matching
- CCI:
-
Charlson Comorbidity Index
- ASA:
-
American Society of Anesthesiologists
- AJCC:
-
American Joint Committee on Cancer
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- CT:
-
Computed tomography
- LND:
-
Lymph node dissection
- RFS:
-
Relapse-free survival
- OS:
-
Overall survival
- LNR:
-
Lymph node ratio
- SII:
-
Systemic immune-inflammation index
- SIRI:
-
Systemic inflammatory response index
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressed disease
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–8.
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34:1350–7.
Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321:1983–92.
Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg. 2021;157(1):9–17.
Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann Surg. 2019;270:983–91.
Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol. 2020;38:3304–13.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European organisation for research and treatment of cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–8.
Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081–92.
Li Z, Shan F, Ying X, Zhang Y. E JY, Wang Y, Ren H, Su X, Ji J: Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg. 2019;154:1093–101.
The Chinese Society of Clinical Oncology Guidelines Working Committee: The Chinese Society of Clinical Oncology (CSCO) Clinical guidelines of gastric cancer (2018). People's Medical Publishing House; 2018.
Japanese Gastric Cancer Association: Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24:1-21.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
The American Joint Committee on Cancerr: The Eighth edition of American Joint Committee on Cancerr (AJCC) Cancer Staging Manual. Springer; 2017.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–26.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, Guillem JG, Paty PB, Goodman KA, Wu A, Gollub M, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106:dju248.
The National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2009.
Bernstein LH, Leukhardt-Fairfield CJ, Pleban W, Rudolph R. Usefulness of data on albumin and prealbumin concentrations in determining effectiveness of nutritional support. Clin Chem. 1989;35:271–4.
Dellière S, Cynober L. Is transthyretin a good marker of nutritional status? Clin Nutr. 2017;36:364–70.
Shen YJ, Qian LQ, Ding ZP, Luo QQ, Zhao H, Xia WY, Fu YY, Feng W, Zhang Q, Yu W, et al. Prognostic value of inflammatory biomarkers in patients with stage I lung adenocarcinoma treated with surgical dissection. Front Oncol. 2021;11:711206.
Abbate V, Barone S, Troise S, Laface C, Bonavolontà P, Pacella D, Salzano G, Iaconetta G, Califano L, Dell’AversanaOrabona G. The combination of inflammatory biomarkers as prognostic indicator in salivary gland malignancy. Cancers (Basel). 2022;14:5934.
Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Misawa K, Takagi M, Takagane A, Teshima S, et al. Randomized phase III trial of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer (JCOG0912). J Clin Oncol. 2019;37:4020–4020.
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–13.
Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, et al. Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: the CLASS02 multicenter randomized clinical trial. JAMA Oncol. 2020;6:1590–7.
Li Z, Shan F, Wang Y, Li S, Jia Y, Zhang L, Yin D, Ji J. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer after neoadjuvant chemotherapy: safety and short-term oncologic results. Surg Endosc. 2016;30:4265–71.
Wang N, Zhou A, Jin J, Huang H, Zhang Y, Chen Y, Zhao D. Open vs. laparoscopic surgery for locally advanced gastric cancer after neoadjuvant therapy: short-term and long-term survival outcomes. Oncol Lett. 2020;20:861–7.
Khaled I, Priego P, Soliman H, Faisal M, Saad Ahmed I. Oncological outcomes of laparoscopic versus open gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a retrospective multicenter study. World J Surg Oncol. 2021;19:206.
An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH. Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol. 2012;19:2452–8.
Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23:323–31.
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27:286–94.
Li B, Yu-Hong Wong I, Siu-Yin Chan F, Chan KK, Lai-Yin Wong C, Law TT, Yat-Yin Kwok J, Law S. Comparison of laparoscopic versus open gastrectomy for gastric cancer. Surg Oncol. 2020;35:14–21.
Xu D, Li J, Song Y, Zhou J, Sun F, Wang J, Duan Y, Hu Y, Liu Y, Wang X, et al. Laparoscopic surgery contributes more to nutritional and immunologic recovery than fast-track care in colorectal cancer. World J Surg Oncol. 2015;13:18.
Zhang Y, Chen Q, Lu C, Yu L. Prognostic role of controlling nutritional status score in hematological malignancies. Hematology. 2022;27:653–8.
He MM, Fang Z, Hang D, Wang F, Polychronidis G, Wang L, Lo CH, Wang K, Zhong R, Knudsen MD, et al. Circulating liver function markers and colorectal cancer risk: a prospective cohort study in the UK Biobank. Int J Cancer. 2021;148:1867–78.
Zu H, Wang H, Li C, Xue Y. Preoperative prealbumin levels on admission as an independent predictive factor in patients with gastric cancer. Medicine (Baltimore). 2020;99:e19196.
Chiang HC, Lin MY, Lin FC, Chiang NJ, Wang YC, Lai WW, Chang WL, Sheu BS. Transferrin and prealbumin identify esophageal cancer patients with malnutrition and poor prognosis in patients with normal albuminemia: a cohort study. Nutr Cancer. 2022;74:3546–55.
Zhao EH, Ling TL, Cao H. Current status of surgical treatment of gastric cancer in the era of minimally invasive surgery in China: opportunity and challenge. Int J Surg. 2016;28:45–50.
Yamagata Y, Yoshikawa T, Yura M, Otsuki S, Morita S, Katai H, Nishida T. Current status of the “enhanced recovery after surgery” program in gastric cancer surgery. Ann Gastroenterol Surg. 2019;3:231–8.
Jiang Q, Zeng X, Zhang C, Yang M, Fan J, Mao G, Shen Q, Yin Y, Liu W, Tao K, Zhang P. Lymph node ratio is a prospective prognostic indicator for locally advanced gastric cancer patients after neoadjuvant chemotherapy. World J Surg Oncol. 2022;20:261.
Chen S, Liu Y, Huang L, Chen CM, Wu J, Shao ZM. Lymph node counts and ratio in axillary dissections following neoadjuvant chemotherapy for breast cancer: a better alternative to traditional pN staging. Ann Surg Oncol. 2014;21:42–50.
Zhu K, Jin H, Li Z, Gao Y, Zhang Q, Liu X, Yu J. The prognostic value of lymph node ratio after neoadjuvant chemotherapy in patients with locally advanced gastric adenocarcinoma. J Gastric Cancer. 2021;21:49–62.
Wei Y, Yu D, Li Y, Fan C, Li G. Laparoscopic versus open gastrectomy for advanced gastric cancer: a meta-analysis based on high-quality retrospective studies and clinical randomized trials. Clin Res Hepatol Gastroenterol. 2018;42:577–90.
Fujisaki M, Mitsumori N, Shinohara T, Takahashi N, Aoki H, Nyumura Y, Kitazawa S, Yanaga K. Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc. 2021;35:1682–90.
Acknowledgements
The authors thank Editage for editing grammar, spelling, and other common errors.
Funding
This study was supported by National Natural Science Foundation of China (No. 81702386, 82072736) and Natural Science Foundation of Hubei Province (No.2021CFB566).
Author information
Authors and Affiliations
Contributions
T.K., Y.Y., Z.P. and Z.C. designed this study. Z.C., Z.P., Y.J., J.Q., S.Q. and M.G. joined the acquisition, analysis and interpretation of data. Z.C. and Y.J. wrote the main manuscript text. Z.P., K.A., L.W., Z.X., Y.Y. and T.K. made critical revision of the manuscript for important intellectual content. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Union Hospital, Huazhong University of Science and Technology, and the approved number is UHCT-IEC-SOP-016–03-01. The informed consent was signed by all patients to have their data used for the study. All procedures were conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Zhang, P., Yu, J. et al. Laparoscopic versus open gastrectomy for locally advanced gastric cancer after neoadjuvant chemotherapy: a comprehensive contrastive analysis with propensity score matching. World J Surg Onc 21, 350 (2023). https://doi.org/10.1186/s12957-023-03221-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03221-4