Abstract
Background
The introduction of new technology into the operating room (OR) can be beneficial for patients, but can also create new problems and complexities for physicians and staff. The observation of flow disruptions (FDs)—small deviations from the optimal course of care—can be used to understand how systems problems manifest. Prior studies showed that the docking process in robotic assisted surgery (RAS), which requires careful management of process, people, technology and working environment, might be a particularly challenging part of the operation. We sought to explore variation across multiple clinical sites and procedures; and to examine the sources of those disruptions.
Methods
Trained observers recorded FDs during 45 procedures across multiple specialties at three different hospitals. The rate of FDs was compared across surgical phases, sites, and types of procedure. A work-system flow of the RAS docking procedure was used to determine which steps were most disrupted.
Results
The docking process was significantly more disrupted than other procedural phases, with no effect of hospital site, and a potential interaction with procedure type. Particular challenges were encountered in room organization, retrieval of supplies, positioning the patient, and maneuvering the robot.
Conclusions
Direct observation of surgical procedures can help to identify approaches to improve the design of technology and procedures, the training of staff, and configuration of the OR environment, with the eventual goal of improving safety, efficiency and teamwork in high technology surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The integration of technology into healthcare carries with it a host of new challenges. Although the use of surgical robots has various benefits for surgeons and patients [1, 2], there is also an increased need for intricate problem solving in the OR, a high demand for effective communication [3, 4], and the need to cultivate an environment that suits both the team and technology during robotic assisted surgery (RAS) [5,6,7]. Identifying the obstacles that OR teams experience might lead to more efficient robotic procedures and increase our understanding of human interactions with complex surgical technologies.
Direct observation of the clinical work environment has been used to understand where barriers to optimal system function may exist and assist with identifying opportunities for improvement. The identification of surgical flow disruptions (FDs) or deviations from the optimal course of care that may alter, slow down, or stop the process. [8,9,10] can help identify the challenges that teams experience at a task-specific level, which can guide interventions aimed at improving efficiency and safety towards the most difficult aspects of RAS. The preparation and execution of robot docking involves active participation from the OR team to coordinate the management of the patient, equipment, and technology in the work environment to ensure the robot is positioned correctly for a given procedure, the connection of the robotic arms to the trocars, and the installation of multiple robotic instruments as well as the camera [11]. Numerous pieces of equipment including the CO2 gas canister and tubing for insufflation, the laparoscope and trocars for port placement, the patient cart, power and fiberoptic cables, robotic instruments, and standard OR equipment (OR table, trash cans, anesthesia station, surgical tables) must all be managed when repositioning the robot. This equipment management must be done at a specific time and within a constricted work environment. This requires the team to communicate and coordinate interdependent tasks with a range of hazards that require specific technical skills—skills that go well beyond the surgical training of traditional non-robotic OR staff. Disruptions are typically related to coordination, communication, training, and equipment [12,13,14].
This study aimed to investigate the tasks involved in robot docking by analyzing the rate and content of FDs during various robotic procedure types at multiple hospital sites in an effort to identify the sources of disruption. By deploying the same observational methods across three different sites, we also aimed to differentiate between local health system issues and generalizable observations related to the design of the robot. Finally, by breaking RAS docking down into individual tasks, and exploring qualitative descriptions of disruptions, we sought to identify methods that might improve this process.
Methods
This prospective, direct observation study was conducted between July 2019 and March 2020 across three hospital sites (site 1: 208-bed academic county hospital designated as a level II trauma center; site 2: 133-bed community hospital; site 3: 866-bed non-profit academic hospital designated as a level I trauma center) in Southern California. All research activities were approved by the Institutional Review Boards for all study sites. Site 1 acquired its single Da Vinci Xi robot in 2017, and it is used by general surgery, urology, and gynecology teams. Site 2 had one Si robot that was replaced by an Xi robot that is used daily (up to 10 cases weekly), with a robotic surgical program dating back to 2012. At Site 3 there are 5 da Vinci robots, including 2 Sis and 3 Xis with teaching consoles for training, with a program dating back to at least 2005.
Observer training
Trained human factors researchers conducted each observation following the completion of observer training. The researchers were two full-time research assistants based in the department of surgery at site 3 who visited the other two sites to collect data. Human Factors experts guided and trained each observer in the identification and standardized collection of FDs. The observers were also trained in the basic components of robotic surgery in order to be able to tangibly isolate and describe such disruptive events.
Comprehensive observer training was ensured with both classroom and floor training. Observers were required to review relevant literature, understand general practice guidelines for observing in the OR (e.g., where to stand, what to avoid, who to speak to), and conduct practice observations. The practice observations were broken down into three phases, all performed under the direct supervision of an experienced observer. During phase one, the trainees oriented themselves to the real-time events of both the OR and the general steps in RAS. The trainee was also introduced to the OR staff and any other involved key personnel. During phase two, the trainer and trainee observed three RAS procedures together to practice collecting FDs and become familiar with the data collection tool. Phase three was dedicated to determining inter-rater reliability by having the trainer and trainee simultaneously, yet independently, conduct observations for at least three full RAS procedures. Observers were considered fully trained if, after three full case observations, intra-class correlation coefficients (based on number of observed disruptions per phase) were greater than 0.80, indicating good reliability [15].
Data collection
Following the completion of training, observers individually conducted observations in the OR. All relevant RAS cases were pre-identified on a monthly basis by scanning the surgical schedule and recording a list of procedures. All procedures observed were conducted with the Da Vinci Xi surgical robot, with the exception of one procedure at site 2, which was performed with the Si robot. Observers attended those cases that fit within their allotted work hours and schedule. Observers used Microsoft Surface Pro tablets configured with a customized data collection tool developed using Microsoft Excel to collect data. The data collection tool divided procedures into five phases, as opposed to the four phases previously used in similar research [8, 9, 16], to more clearly distinguish between task demands throughout the procedure. Phases consisted of phase 1—patient in the room to insufflation, phase 2—insufflation to surgeon on console (including docking), phase 3—surgeon on console to surgeon off console, phase 4—surgeon off console to patient closure, and phase 5—patient closure to patient leaves the operating room. During each procedure, FDs were recorded into the appropriate phase, and a narrative, time-stamp, and classification (based off of a robot-specific FD taxonomy [8]) were also recorded. Hospital, OR number, robot model, procedure, and whether the case involved trainees was logged as well as the age, sex, body mass index, and American Society of Anesthesiologists (ASA) physical status classification of the patient.
Each FD was categorized into one of ten categories: communication, coordination, environment, equipment, external factors, other, patient factors, surgical task considerations, training, or unsure. The categorization system is modeled after previous studies [9], as well as the examples provided for each FD category (Table 1).
Once in the OR, observers remained as unobtrusive as possible. They stood at an appropriate vantage point in the room without getting in the way of team members. Once an appropriate time presented itself, observers introduced themselves to the circulating nurse and informed them of the reason for their presence. Observers did not directly engage in conversations with operating room staff, however, if a staff member approached them with any questions/comments they would respond.
Statistical analysis
SPSS (version 25, IBM Corporation) was used to conduct the statistical analyses. Analysis of Variance (ANOVA) was used to compare FD rates between sites overall and for each phase of surgery. Mixed model ANOVA with repeated measures was used to determine if there was an effect of phase, hospital site, or an interaction between phase and site. When the assumption of sphericity was violated, the p value of Greenhouse–Geisser was reported. Procedure type was not included in the mixed model ANOVA due to sample size limitations, however FD rates for gynecological and hernia procedures were compared using t-test analyses. These two categories allowed us to compare abdominal versus pelvic procedures, which have different implications related to robot docking. We would have liked to compare all procedure types but only gynecological and hernia procedures were compared due to sample size limitations. Three procedures were excluded from this analysis because they included additional gynecological components as well as hernia or general surgery components, therefore they did not qualify into only one procedure category. Significance was assessed at the alpha = 0.05 level.
Development of work-system flow
A work-system flow diagram was developed based on educational videos about the Da Vinci Xi Surgical System, the Da Vinci Xi System User Manual, and observations and narratives. The diagram outlined the tasks involved with phase two of RAS procedures. An analysis of the written description of FDs within phase 2 of each procedure was conducted to select the disruptions that pertained to the work-system flow. The selected FDs were matched to the corresponding step of the work-system flow, which revealed the distribution of FDs across all steps.
Results
Forty-five robotic-assisted procedures were observed (Table 2). The average procedure length was 3.33 h (95% CI 3.01–3.65 h). Gynecological procedures averaged 41.57 FDs/h (95% CI 32.15–51.02 FDs/h) and hernia procedures averaged 40.59 FDs/h (95% CI 35.82–45.36 FDs/h). During phase 2, gynecological procedures averaged 51.32 FDs/h (95% CI 35.40–67.24 Fds/h) and hernia procedures averaged 45.03 FDs/h (95% CI 36.99–53.07 FDs/h). These procedures did not have significantly different FD rates overall, t(16) = 0.202, p = 0.843 (adjusted for unequal variance), or during phase 2, t(30) = 0.860, p = 0.397.
Phase and site
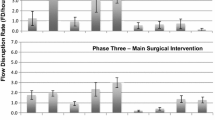
The procedures observed at site 1 had an average FD rate of 37.42/h (95% CI 30.24–44.60 FDs/h), at site 2 an average of 40.19/h (95% CI 35.80–44.58 FDs/h), and at site 3 an average of 42.83/h (95% CI 37.53–48.13 FDs/h) (Fig. 1). ANOVA demonstrated average FD rates were not significantly different between the sites, F(2, 42) = 0.80, p = 0.456. Two procedures were removed from the analysis due to incomplete data.
For phase 2 (docking), site 1 averaged 44.62 FDs/h (95% CI 31.95–57.28 FDs/h), Site 2 averaged 49.54 FDs/h (95% CI 40.44–58.64 FDs/h), and site 3 averaged 53.52 FDs/h (95% CI 42.37–57.28 FDs/h) (Fig. 1). ANOVA demonstrated there was no significant difference in FD rate among sites during phase two, F(2, 44) = 0.573, p = 0.568.
A mixed model ANOVA demonstrated a significant effect of phase [F(3, 125) = 2.72, p = 0.045] but no main effect of site (p = 0.462) and no interaction effect (p = 0.591) of flow disruption rate (FDs/h). The average FD rate for phase 1 was 39.99 FDs/h (95% CI 32.45–47.53 FDs/h), phase 2 was 49.22 FDs/h (95% CI 43.33–55.08 FDs/h), phase 3 was 38.00 FDs/h (95% CI 34.53–41.40 FDs/h), phase 4 was 41.69 FDs/h (95% CI 35.94–47.44 FDs/h) and phase 5 was 33.77 FDs/h (95% CI 26.81–40.73 FDs/h) (Supplemental Figure S1). Pairwise comparisons using Least Significant Difference and applying Bonferroni correction, demonstrated significant differences between phase 2 and phases 3 (p = 0.015) and 5 (p = 0.019), and no significant difference between phases 1 and 4 (p > 0.05). Phases 1 and 4 were not significantly different than any other phase (p > 0.05).
Categorization of flow disruptions
A total of 1229 FDs were observed during phase two. Coordination FDs were most common across all sites occurring at an average of 27.13 FDs/h (95% CI 23.89–30.37 FDs/h) and making up an average of 55% of recorded FDs. Communication FDs were the second most common (14%), occurring at an average of 6.45 FDs/h (95% CI 5.02–7.89 FDs/h). The remaining categories of FDs occurred at 4.58 FDs/h or less (Supplemental Figure S2). Patient factors, surgical task considerations, other, and uncategorized (unsure) disruptions each represented less than 1 FD/h. Site 2 experienced the highest rate of equipment FDs (4.2 FDs/h) while site 3 experienced a relatively even distribution of communication (5.78 FDs/h), environment (6.17 FDs/h), external (6.56 FDs/h), and training disruptions (5.89 FDs/h).
Work-system flow
The tasks in phase two included preparing the OR and patient for robot docking via instrument preparation, insufflation and laparoscopy, and positioning the patient and patient bed followed by docking the robot. Substeps are listed under the more complex tasks (Fig. 2). While this is not a complete list of all tasks, and the order of the task steps vary based on the surgical procedure and surgical team, this flow diagram serves as a visual to identify at what point, and why, FDs were occuring within the second phase of surgery.
Docking-related flow disruptions
The task that was most disrupted during phase two was docking the robot. Observers noted a total of 96 FDs during this task; this represented 38.10% of docking-related disruptions. The most common FDs were coordination and environment FDs related to obstructions or collisions while the robot was moving towards the patient. Organizing the room for the case (44 FDs), preparing the robot for docking (41 FDs) and retrieving instruments and supplies (40 FDs) were the other tasks commonly disrupted. Coordination and environment FDs such as adjustment of the OR bed and patient in preparation for docking, team members leaving the room to retrieve missing supplies, scrub techs missing a tool or having an incorrect or broken tool, and cord hazards generated a high percentage of the total FDs collected (Table 3).
Discussion
The equipment and processes required to appropriately, safely, and efficiently dock a surgical robot in a limited physical space and under time pressure creates several unique challenges for surgical team members. The unique technical and non-technical challenges during this part of the surgery were reflected in a higher rate of surgical FDs in comparison to all other phases of care, replicating findings from previous studies [8, 9, 13, 14]. This held true in the current study across sites and surgical procedure types, suggesting the systemic challenges associated with the preparation and execution of docking in RAS are related to the design of the robot rather than the skills of the teams studied. Building on our prior studies, which focused on quantification and classication of FDs, we were able to utilize the qualitative FD descriptions to explore the specific docking challenges observed across sites. The most common FDs were generated by organizing the room for a robotic procedure, retrieving instruments and supplies, preparing for robot docking, and docking the robot. The technical complexity, physical size, and basic design of the robotic system increases demands on the surgical team to successfully coordinate the technology and equipment within the work environment. Through direct observation of FDs, it may be possible to identify work-system interventions aimed to mitigate these challenges.
The majority of FDs primarily manifested during four of the seven major steps associated with the docking phase of RAS as defined by our work system flow diagram: (1) room organized for robotics case, (2) retrieval of instruments and supplies, (3) preparation for robot docking, and (4) docking the robot. Organizing the room prior to docking requires the arrangement of robotic and non-robotic equipment in preparation for the surgical procedure. The most frequently recorded FDs related to traffic in and out of the OR and the positioning of cords on the floor, both of which disrupt the arrangement of robotic (patient cart, vision cart, surgical console) and non-robotic (surgical tables, booms, anesthesia station) equipment. The movement of people and equipment in a relatively concentrated space is exacerbated by the need to retrieve missing, incorrect or broken instruments and supplies. While this is a common problem in surgery [17, 18], it can be especially disruptive as RAS equipment is often stored outside of the OR, due to the size of the equipment and the lower frequency of robotic procedures. Team members who have to leave to deal with instrument and supply problems are also unable to contribute to the other necessary preparation and docking tasks. Relative expense means that inventories are kept low, increasing the risks of delays from broken or unsterilized equipment. This increases the pressure on sterile processing departments (SPDs) to be accurate, efficient, and fast in cleaning robotic instruments; the responsibility of OR staff to be able to prepare equipment and recognize omissions or breakages early; and the need for close collaboration between the OR and SPD [19].
Patient and robot positioning were also a challenge. RAS procedures require specific height, location and patient positioning adjustments [20]. This requires expertise and foreknowledge in the requirements for surgery, and in maneuvering patients into position, operating the bed appropriately, then securing patients for the surgery [21]. Moving the robot towards the patient was the most disrupted task, caused by the robot being obstructed by or colliding with equipment (monitors, IV poles and drips, lights, and booms, the patient bed, anesthesia equipment, surgical tables, robotic equipment, trash cans, and cords on the floor). This is especially challenging, as the driver (usually OR staff) has to listen to the surgeon for guidance while simultaneously steering and avoiding obstacles. Often the driver is unsure of where the robot should dock in relation to the surgical anatomy. We noted damage to the robot, equipment, injury to team members or the patient, and a delay in the docking process.
By taking a closer look at how teams interact with the robot and OR environment during multiple types of procedures at several hospitals, we have a greater understanding of why the docking process is highly disrupted, regardless of procedure or site. Space limitations, inadequate room configuration, lack of specialized training, and inadequate team communication and coordination are all factors that likely had a negative impact on the second phase of RAS. Addressing these disruptions can be broadly organized into workspace management—that is, configuring the layout of the OR to reduce the potential for collisions/obstructions while allowing for staff movement and access to instruments and supplies; teamwork and coordination, to ensure appropriate supplies, deal with problems, and to maneuver the robot and patient into place safely; and specialized robotic skills, training and cognitive support to ensure staff are able to prepare and perform to the specific requirements of RAS. Role-specific training could enable team members to sharpen their understanding of the unique instruments, supplies, bed and patient positioning for different RAS procedures. The concept of assigning a “leader” nurse who is responsible for organizing training has been proposed but not empirically tested, however we believe this new role could lead to opportunities for improving task training [22]. Team-based training could improve communication and coordination skills that would particularly benefit the task of docking the robot, but would likely have a positive impact on the entire phase. Reconfiguring or altering OR design and space allocation is another chance to improve the second phase of RAS. A reorganization of current equipment may allow for the safer movement of human and equipment traffic, and the possibility of more robotic supplies being stored in the room. Human movement patterns during RAS have been studied, and could guide the reorganization process [5].
We attribute the higher rates of FDs found here, in comparison to prior studies, to using a smaller number of dedicated and more carefully trained observers, who may have been more cued in on the types of FDs to look for given our prior work. The three different organizations also predisposed higher numbers of FDs since one had seen a recent increase in the robotic services there, diluting the expertise, while the other two had lower volumes and thus less extant expertise. One particular strength of our FD method is that it uses both quantitative ‘counts’ of FDs and the qualitative free-text note that describes each FD as it is observed. The qualitative data were used here both to ensure there were no false positives, and to provide the specific events that were used in the final detailed analysis in Table 3. For example, we reviewed bed-related flow disruption, specifically because adjusting the bed can be a trial & error process. In doing so, we found miscommunications between surgeons and anesthesia providers; instances where the room had not been set up in the first place so the table had to be physically moved with the patient on it; disruptions where the robot needed to be undocked and re-docked to find the right position; and equipment limitation issues that prevented optimal positioning. While the focus of prior studies has been on quantitative and statistical analysis, the mixed methods and qualitative data employed here provides a richer understanding of RAS challenges, and specific areas for improvement. Interviews or focus groups, guided by these findings, could also provide a deeper perspective.
For practicing RAS surgeons, we offer a number of suggestions. Not all options will be available to you, but some certainly are. First, recognize that efficiency and safety in RAS are not just about precise individual technical skill, but also about leading and supporting the rest of the team. Docking needs multiple people with a shared understanding to coordinate in sequence. Rely on and encourage leadership from your more experienced staff. New team members will need more guidance and support. Preparing with a briefing, and training as a team will make this more effective. As you prepare to dock, remind the team of the docking sequence and requirements, and ensure everyone has a role before you start. Announcing the start of the docking process and the completion of key tasks, verbal acknowledgements from the time (“readbacks”) would also enhance team coordination. Having a key word or phrase that will halt the process might also be beneficial. Specificity in language and communication particularly in relation to positioning will also make the process smoother. Having a well-organized room of sufficient size and organization of the operating room also makes a difference. Locate equipment in places that are appropriate for the case, and make sure clear paths are available for staff and equipment to traverse when needed. Also, anticipating that the robot can interfere with overhead booms, and power cables on the floor may allow you to create a movement path to avoid damage to the robot or other equipment. This clear path might even be marked out on the floor. Making sure a team member is there to look out for either floor or overhead problems as the robot is moved would also be valuable. Consistency of roles during docking allows team members to become familiar with specific tasks which will also be beneficial. Finally, having opportunities to review performance as a team or even practice together in simulation might contribute to a continuous learning cycle that will enhance the process. This may be informed by translating models from other high risk industries [23].
A number of prior studies have related FDs to specific outcomes including morbidity and death [24], and major failure escalations [25] in congenital heart surgery; surgical errors in cardiac surgery [10], and increased workload that reduces technical performance [26]. However, the relationship between FDs and outcomes is not direct. Damage to a robot after clashing with an overhead boom might lead to multiple case cancellations, which may or may not affect patient outcome measures or the course of care for an individual patient. A damaged robot that lead to a patient injury would be a significant safety event, but might not make a statistical difference to outcome rates. In most cases, docking issues make no detectable difference to patient outcomes. However, while patient outcomes are paramount, they are not the only outcome of importance. For many teams, addressing FDs would create a smoother, more efficient process that can reduce OR time, enhance teamwork and job satisfaction while also reducing the risks of multiple complications. Thus, FDs are best seen in the context of a complex adaptive system [27], which may not be directly reducible to process-outcome relationships, but which suggest a very broad range of clinical implications for this work.
Though we feel that a larger sample might not demonstrate different FDs, it might enable a stronger comparison between sites, revealing differences in how individual robotic programs or specialties overcome obstacles. Opportunity sampling meant that there was an imbalance in sampling across sites, as procedure types were not controlled for, so differences according to anatomical site were not included in the mixed model analysis. Though extensive work was conducted to prepare and train observers, and it is difficult to see other ways to collect these data—direct observation carries a range of challenges [28]. Certainly it is possible that some of the observers missed or did not perceive or record certain FDs. However, the training process used for observation supported high levels of inter-rater reliability, and the clinical implications were not a focus of this study. Additionally, factors such as surgeon experience with RAS, or patient considerations (e.g., BMI, complexity of case, etc.) were not included in this analysis, but could have provided additional context about the individual factors that impact RAS docking. A comparison between the Da Vinci Si and Xi was not included in this analysis, since only a single procedure was performed with the Si. Future studies could focus on the effects of surgical experience, procedure type and room size on FD rate and content during docking. We plan to observe more RAS procedures for a further analysis of the effect of procedure type on FDs, particularly to compare docking by surgical site (abdominal versus pelvic regions), and to explore the effects of different interventions, including teamwork, task design, and workspace management.
Conclusion
Robotic-assisted procedures are complex processes that create unique challenges for OR teams. Through our prospective observational study of FDs during RAS, we have identified robot docking as a highly disrupted part of RAS, with several tasks within the docking process commonly generating disruptions. Organizing the room for a robotic procedure, retrieving instruments and supplies, preparing the robot for docking, and docking the robot generated the most flow disruptions. Workspace management, improved teamwork and coordination, and the development and support of RAS-specific skills might help reduce these problems and make the docking process safer and smoother. Analyzing FDs during RAS can help bridge the gap between teams and technology in surgery, and might lead to safer and more efficient robotic procedures.
References
Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann SurgOncol 16:1480–1487
Ashrafian H, Clancy O, Grover V, Darzi A (2017) The evolution of robotic surgery: surgical and anaesthetic aspects. BJA: Br J Anaesth 119:i72–i84
Cunningham S, Chellali A, Jaffre I, Classe J, Cao C (2013) Effects of experience and workplace culture in human-robot team interaction in robotic surgery: a case study. Int J Soc Robot 5:75–88. https://doi.org/10.1007/s12369-012-0170-y
Schiff L, Tsafrir Z, Aoun J, Taylor A, Theoharis E, Eisenstein D (2016) Quality of communication in robotic surgery and surgical outcomes. JSLS: J SocLaparoendoscSurg. https://doi.org/10.4293/JSLS.2016.00026
Ahmad N, Hussein AA, Cavuoto L, Sharif M, Allers JC, Hinata N, Ahmad B, Kozlowski JD, Hashmi Z, Bisantz A (2016) Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int 118:132–139
Myklebust MV, Storheim H, Hartvik M, Dysvik E (2020) Anesthesia professionals’ perspectives of teamwork during robotic-assisted surgery. AORN J 111:87–96
Randell R, Honey SA, Hindmarsh J, Alvarado N, Greenhalgh J, Pearman A, Long A, Cope A, Gill A, Gardner P (2017) A realist process evaluation of robot-assisted surgery: integration into routine practice and impacts on communication, collaboration and decision-making. Health ServDeliv Res 5:1–140
Catchpole KR, Hallett E, Curtis S, Mirchi T, Souders CP, Anger JT (2018) Diagnosing barriers to safety and efficiency in robotic surgery. Ergonomics 61:26–39
Catchpole K, Perkins C, Bresee C, Solnik MJ, Sherman B, Fritch J, Gross B, Jagannathan S, Hakami-Majd N, Avenido R (2016) Safety, efficiency and learning curves in robotic surgery: a human factors analysis. SurgEndosc 30:3749–3761
Wiegmann DA, Elbardissi AW, Dearani JA, Daly RC, Sundt TM (2007) Disruptions in surgical flow and their relationship to surgical errors: an exploratory investigation. Surgery 142:658–665
Iranmanesh P (2009) Set-up and docking of the da Vinci surgical system: prospective analysis of initial experience. Int J Med Robot Comput Assist Surg. https://doi.org/10.1002/rcs.288
Weber J, Catchpole K, Becker AJ, Schlenker B, Weigl M (2018) Effects of flow disruptions on mental workload and surgical performance in robotic-assisted surgery. World J Surg 42:3599–3607
Weigl M, Weber J, Hallett E, Pfandler M, Schlenker B, Becker A, Catchpole K (2018) Associations of intraoperative flow disruptions and operating room teamwork during robotic-assisted radical prostatectomy. Urology 114:105–113
Dru CJ, Anger JT, Souders CP, Bresee C, Weigl M, Hallett E, Catchpole K (2017) Surgical flow disruptions during robotic-assisted radical prostatectomy. Can J Urol 24:8814–8821
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Jain M, Fry BT, Hess LW, Anger JT, Gewertz BL, Catchpole K (2016) Barriers to efficiency in robotic surgery: the resident effect. J Surg Res 205:296–304
Neyens DM, Bayramzadeh S, Catchpole K, Joseph A, Taaffe K, Jurewicz K, Khoshkenar A, San D (2019) Using a systems approach to evaluate a circulating nurse’s work patterns and workflow disruptions. Appl Ergon 78:293–300
Cohen TN, Cabrera JS, Sisk OD, Welsh KL, Abernathy JH, Reeves ST, Wiegmann DA, Shappell SA, Boquet AJ (2016) Identifying workflow disruptions in the cardiovascular operating room. Anaesthesia 71:948–954
Catchpole K, Bisantz A, Hallbeck MS, Weigl M, Randell R, Kossack M, Anger JT (2019) Human factors in robotic assisted surgery: lessons from studies ‘in the wild.’ Applied Ergon 78:270–276
Takmaz O, Asoglu MR, Gungor M (2018) Patient positioning for robot-assisted laparoscopic benign gynecologic surgery: a review. Eur J ObstetGynecolReprodBiol 223:8–13
Prielipp RC, Weinkauf JL, Esser TM, Thomas BJ, Warner MA (2017) Falls from the OR or procedure table. AnesthAnalg 125:846–851
Randell R, Honey S, Alvarado N, Greenhalgh J, Hindmarsh J, Pearman A, Jayne D, Gardner P, Gill A, Kotze A (2019) Factors supporting and constraining the implementation of robot-assisted surgery: a realist interview study. BMJ Open 9:e028635
Souders CP, Catchpole KR, Wood LN, Solnik JM, Avenido RM, Strauss PL, Eilber KS, Anger JT (2017) Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. https://doi.org/10.1007/s00268-017-3936-4
de Leval MR, Carthey J, Wright DJ, Reason JT (2000) Human factors and cardiac surgery: a multicenter study. J ThoracCardiovascSurg 119:661–672
Catchpole KR, Giddings AE, de Leval MR, Peek GJ, Godden PJ, Utley M, Gallivan S, Hirst G, Dale T (2006) Identification of systems failures in successful paediatric cardiac surgery. Ergonomics 49:567–588
Weigl M, Stefan P, Abhari K, Wucherer P, Fallavollita P, Lazarovici M, Weidert S, Euler E, Catchpole K (2016) Intra-operative disruptions, surgeon’s mental workload, and technical performance in a full-scale simulated procedure. SurgEndosc 30:559–566
Braithwaite J, Churruca K, Ellis LA, Long J, Clay-Williams R, Damen N, Herkes J, Pomare C, Ludlow K (2017) Complexity science in healthcare—aspirations, approaches, applications and accomplishments: a white paper. Australian Institute of Health Innovation, Macquarie University, Sydney
Catchpole K, Neyens DM, Abernathy J, Allison D, Joseph A, Reeves ST (2017) Framework for direct observation of performance and safety in healthcare. BMJ Publishing Group Ltd, London
Funding
This project was funded under Grant Number HS026491-01 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (KC, JA, TC). The authors are solely responsible for this document’s contents, findings, and conclusions, which do not necessarily represent the views of AHRQ. Readers should not interpret any statement in this report as an official position of AHRQ or of HHS. None of the authors has any affiliation or financial involvement that conflicts with the material presented in this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jennifer Anger is on the advisory board for Axonics. Lucy Cofran, Tara Cohen, Myrtede Alfred, Falisha Kanji, Eunice Choi, Stephen Savage, and Ken Catchpole have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cofran, L., Cohen, T., Alfred, M. et al. Barriers to safety and efficiency in robotic surgery docking. Surg Endosc 36, 206–215 (2022). https://doi.org/10.1007/s00464-020-08258-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08258-0