Abstract
Background
Operating room (OR) turnover time, time taken between one patient leaving the OR and the next entering, is an important determinant of OR utilization, a key value metric for hospital administrators. Surgical robots have increased the complexity and number of tasks required during an OR turnover, resulting in highly variable OR turnover times. We sought to streamline the turnover process and decrease robotic OR turnover times and increase efficiency.
Methods
Direct observation of 45 pre-intervention robotic OR turnovers was performed. Following a previously successful model for handoffs, we employed concepts from motor racing pit stops, including briefings, leadership, role definition, task allocation and task sequencing. Turnover task cards for staff were developed, and card assignments were distributed for each turnover. Forty-one cases were observed post-intervention.
Results
Average total OR turnover time was 99.2 min (95% CI 88.0–110.3) pre-intervention and 53.2 min (95% CI 48.0–58.5) at 3 months post-intervention. Average room ready time from when the patient exited the OR until the surgical technician was ready to receive the next patient was 42.2 min (95% CI 36.7–47.7) before the intervention, which reduced to 27.2 min at 3 months (95% CI 24.7–29.7) post-intervention (p < 0.0001).
Conclusions
Role definition, task allocation and sequencing, combined with a visual cue for ease-of-use, create efficient, and sustainable approaches to decreasing robotic OR turnover times. Broader system changes are needed to capitalize on that result. Pit stop and other high-risk industry models may inform approaches to the management of tasks and teams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical services are a significant contributor to a hospital’s profitability [1]. It is also one of the most costly, requiring equipment, supplies, personnel and building resources. Consequently, the proportion of time surgery is being actively conducted—known as Operating Room (OR) utilization—is a key performance metric for surgical suites [2]. Turnover time, defined as the time taken between one patient leaving the room and the next entering, can have a considerable impact on OR utilization and thus on profitability [3].
Introduction of new technology into an OR suite poses challenges beyond the clinical skills to required to successfully and safely conduct surgery. Increasingly, complex surgical automation, such as surgical robots, leads more complex requirements, requiring better skills, teamwork, improved utilization of available resources, and coordination of all critical elements to minimize delay. Consequently, OR turnover times for robotic surgery can be longer than those of more traditional surgery and can be highly unreliable, varying between institutions and within a single institution [4, 5]. Long, inefficient and unpredictable OR turnovers reduce optimal robot use, impact surgical productivity, affecting the ability to treat patients, with potentially serious financial implications [6]. Given the significant cost of initial investment ($1–2.3 million), annual maintenance costs ($100,000–150,000) and the cost per case in disposable instruments, this may eventually threaten the existence of robotic services as a viable treatment option. A recent randomized-controlled trial by Anger et al. [7] compared the costs and outcomes of laparoscopic versus robotic sacrocolpopexy. The study found that the costs of robotic sacrocolpopexy are higher than laparoscopic (initial cost $19,616 vs. $11,573, p < 0.001 and at 6 weeks, $20,898 vs. $12,170, p < 0.001). In addition, the short-term outcomes and complications were similar between the robotic and laparoscopic. Improving the efficiency and consistency of turnover time for robotic cases will help hospitals to ensure the optimal use of their robots and will yield considerable financial benefits and secure the future of this nascent specialty [8].
In a 2007 paper exploring handoffs from surgery to intensive care—another time-critical task requiring complex coordination of people and equipment—Catchpole et al. [9]. were able to improve safety and efficiency by modeling the process on motor racing pit stops. We sought to improve the turnover time for robotic surgery by addressing the complex interactions required between people, technology, and the environment using a similar model in robotic OR suites within a single institution.
Materials and methods
Problem identification
This prospective study was conducted in a tertiary care, teaching institution with approximately 900 beds that performs over 500 robotic surgery cases per year. The need for a formal ethics committee review was waived, given the work met exemption criteria as a quality improvement project that did not involve human subjects research. Direct observation of 45 baseline robotic OR turnovers was performed by observers with basic human factors and medical training. Room ready time (RRT)—from when the patient exited the OR until the surgical technician was ready to receive the next patient—was measured for each case. To determine RRT, the surgical technician was asked to inform the observer when they were completely ready and no additional instruments or equipment needed to be retrieved. Observer confirmed this as the scrub technician stopped organizing items on their table or asking the OR nurses for more equipment. They were scrubbed and waiting for the entry of the patient. During ten robotic turnovers, detailed notes were taken on the number of staff present, roles of the staff, time intervals required for tasks and the coordination and communication problems they encountered.
Based on these observations, work patterns of staff were assessed for inefficiencies and task overlap. To accomplish this, we conducted several informational meetings at multiple organizational levels. We met with the Robotic Surgery Improvement group on two occasions, the OR staff on one occasion, and multiple times with the robotic surgery lead (R.A.). These meetings confirmed that the roles for OR staff during turnover were neither well-defined nor specifically assigned to individual staff. Variation in the number of staff available to turn a given room over, which ranged from two to five people, further complicated task allocation, team work, and coordination. This meant that staff was often unaware of what tasks needed to be completed, and in what order, or what had been done and what else needed to be done. This led to duplication of some tasks and omission of others, causing further delays.
Intervention development
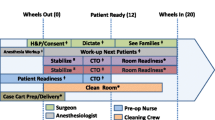
We recognized these challenges were analogous to previous research that applied pit stop models to improve handoffs [9]. Applying the same model here, role definition (who does what), task allocation (what they do), task sequence (the order in which they do them), leadership (who is in charge), briefings (does everyone know what is happening?), and involvement (are we using everyone we have available today?) were recognized as offering means for improvement (Table 1).
A meeting of all those involved in robotic surgery (surgeons, anesthesiologists, nursing staff, and administrators) provided a focus for robotic OR turnover improvement efforts. The robotic surgery manager composed a list of the turnover tasks, which were vetted and approved by OR staff and were allocated to one of four cards. The cards were color-coded, laminated and left in the robotic surgery OR suites (Table 2). Staff was told where to find the cards and how to use them. Roles included “circulator,” “robotic support,” “scrub technician,” “environmental service (EVS) 1,” and “EVS2”. These pre-assigned roles were specified on cards mounted in the OR. All assignments were unambiguous. When four staff members were available, each took one card. If only two staff were available, each took two cards. This way, it was clear who should be doing which tasks, thereby reducing overlap or task omissions.
Evaluation
Room ready time (RRT), already available for 45 cases pre-intervention, was collected for 41 cases post-intervention. Total turnover time (TTT) was defined as the time from first patient exiting to second patient entering the OR and was obtained from the electronic medical record for those observed cases. RRT and TTT results were compared pre- and post-intervention, initially 1 month after intervention to ensure appropriate implementation (n = 9), and then 3-month post-intervention to evaluate sustainability and overall effect (n = 32). Staff involved in the study included: 15 RNs, 15 surgeons, 8 scrub technicians, 2 environmental services staff and 26 anesthesiologists.
One-way ANOVAs were used to assess the effect of the intervention on TTT and RT, with Tukey’s HSD post hoc comparison used to compare between pre-intervention, post-intervention month 1 and month 3. This was performed in SPSS v23.
Results
Cases observed during the pre-intervention and post-intervention stages were pelvic surgery cases in the fields of urology, gynecology, and colorectal surgery, all requiring similar equipment, which controlled for any confounding that might be caused by more complex cases, such as cardiac, that require a substantial amount of additional equipment. Pre-intervention (n = 45) mean TTT was 99.2 min (95% CI 88.0–110.3). One month after the intervention (n = 9), mean TTT was 42.8 min (95% CI 27.2–58.6). After 3 months (n = 32), mean TTT was 53.2 min (95% CI 48.0–58.5). The ANOVA demonstrates a significant effect of the intervention (F = 24.1, p < 0.0001), with significant differences between pre- and post-month 1 (p < 0.0001) and pre- and post-month 3 (p < 0.0001), but not between post-months 1 and 3. Figure 1, top panel, shows the mean TTT pre-and post-intervention. Figure 2 demonstrates the annotated run-time chart for TTT. The reduction in duration and improvement in consistency is clearly observable, with a slight upwards creep in month 3, which then reduces again.
Average RRT was 42.2 min (n = 45, 95% CI 36.7–47.7) before the intervention, which was reduced to 21.0 min (n = 9, 95% CI 17.6–24.3) after 1 month and 27.2 min at 3 months (n = 32, 95% CI 24.7–29.7). The ANOVA demonstrates a significant effect of the intervention (F = 12.52, p < 0.0001), with a significant difference between pre- and post-month 1 (p < 0.005) and pre- and post-month 3 (p < 0.0001), with no significant difference between post-month 1 and post-month 3. Figure 1, bottom panel, shows the mean RRT pre- and post-intervention. Figure 3 demonstrates the annotated run-time chart for RRT, again demonstrating a reduction in duration and improvement in consistency, with a very marked upward trend just before the intervention clearly addressed in the post-intervention phases.
Discussion
Both TTT and RRT significantly decreased after 1 month and were sustained at 3 months. Our intervention was based on the increasingly valuable concept of a “system” approach to improvement that provides a perspective beyond the natural, but usually fallacious, view that human performance is solely defined by individual motivation and intent. The pit stop model recognized that making explicit what needs to be done, providing a clear structure to the individual tasks, embedding visibility for shared tasks, providing clear leadership, and utilizing all human resources available can be an extremely effective improvement approach.
Our results are similar to a 2011 study by Rebuck et al. that evaluated a handful of interventions to improve overall efficiency for robot-assisted laparoscopic radical prostatectomy (RALRP), including decreasing intraoperative time, as well as OR turnover. They found that, by using the same staff members and parallel task processes, they could reduce TTT from 43.0 (n = 26) to 30.9 min (n = 29) (p = 0.005). However, in contrast to our study, multiple and less cost-effective improvements measures to increase efficiency were also implemented concurrently, including a dedicated anesthesia team for RALRP, confounding their findings. Further, their work only focused on a single robotic surgery type, decreasing the complexity of the turnover and increasing staff constancy and task familiarity. Our study has a broader case mix, numerous staff (including trainees), and an explicit, low-cost, less obviously confounded intervention.
It was noteworthy that the benefits were generally sustained between the first and third month, since the sustainment of similar interventions can be a challenge. Certainly, by month 3, there appeared to be a waning of the original effect, suggesting that sustainment is also a requirement here, with continuing quality checks and in-service education to maintain performance. Moreover, if this model were to be applied in the outpatient setting or ORs with shorter cases, it is logical to assume that additional cases may be completed as a result of the time saved from more efficient turnovers. Indeed, we wonder whether such a model could benefit other OR turnover teams. The intervention and maintenance of the pit stop model to reduce turnover time require few resources and may be widely applicable across surgical settings and types.
In order to maximize utilization and thus realize the true financial benefit, TTT should align more closely with RRT. This disparity—which suggests an empty, fully prepared room awaiting a patient—is related to preoperative area delays (e.g., missing labs, missing forms, changes to the surgery consent form), late arrival of the anesthesiologist or surgeon, change of anesthesiologist, scheduling issues in which the second case surgeon was unable to arrive earlier than scheduled if the first case finished early, and patient delays. These were outside the scope of this study, yet the TTT decreased more than anticipated from the decrease in the RRT alone. This suggests that our process changes also influenced performance outside the OR. Since ORs were ready faster, preoperative staff may have been motivated to ensure their patients were ready earlier. Physicians may also have begun to realize the benefits, especially given that the OR turnover time was not only shorter, but more predictable, thus arriving earlier and allowing the next case to commence sooner. Regardless of these additional effects, the removal of the OR as the rate-limiting step provides impetus for efficiency benefits in these other areas to be realized.
We are aware that this is a relatively small-scale pilot study in one institution, and thus there are a range of limitations. This was not a controlled study, and thus, though the robotic surgery service had been in place for some time, and it seems distinctly unlikely, it is not possible to entirely discount the notion that the observed improvements may purely be attributable to staff experience. Similarly, since our sample was not case-controlled, and indeed there was an expansion of robotic surgery into other specialties, it is possible that this accounts for the improvement. However, it would be difficult to see that the strength of the effect—a near halving of RRT and TTT—could be entirely attributable to case mix. Finally, we were not able to fully realize our aims of providing more care at less cost. Given that the surgical case types evaluated in the study were relatively long, the time saved in turnovers did not directly allow additional cases to be performed. However, it may have reduced OR staff overtime payments. A larger study could allow for temporal and case controls, and a fuller assessment of financial benefits.
Improvement in turnover times and associated increase in OR utilization requires multi-level and multi-factorial considerations. A broadly applicable, simple intervention based on strategies used in other high stakes, time pressured industries, and previously translated into health care, can provide the basis for considerable, and sustainable improvements in OR efficiency. We intend to pursue a more comprehensive multi-center, controlled trial of this intervention to replicate and better understand how systems approaches to complex tasks can improve the delivery of robotic surgery.
References
Cima RR, Brown MJ, Hebl JR et al (2011) Use of lean and six sigma methodology to improve operating room efficiency in a high-volume tertiary-care academic medical center. J Am Coll Surg 213:83–92 (discussion 93–84)
Donham RTBM, Hebl JR et al (1997) Glossary of times used for scheduling and monitoring of diagnostic and therapeutic procedures. AORN J 66(601–60):6
Tyler DC, Pasquariello CA, Chen CH (2003) Determining optimum operating room utilization. Anesth Analg 96:1114–1121 (table of contents)
Rebuck DA, Zhao LC, Helfand BT et al (2011) Simple modifications in operating room processes to reduce the times and costs associated with robot-assisted laparoscopic radical prostatectomy. J Endourol/Endourol Soc 25:955–960
Urrego H, Sanni A, Toro JP et al (2014) Out by 3: 30—a study in robotic bariatric surgery efficiency. Bariatr Surg Pract P 9:55–60
Stahl JE, Sandberg WS, Daily B et al (2006) Reorganizing patient care and workflow in the operating room: a cost-effectiveness study. Surgery 139:717–728
Anger JT, Mueller ER, Tarnay C et al (2014) Robotic compared with laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol 123:5–12
Nayeemuddin M, Daley SC, Ellsworth P (2013) Modifiable factors to decrease the cost of robotic-assisted procedures. AORN J 98:343–352
Catchpole KR, de Leval MR, McEwan A et al (2007) Patient handover from surgery to intensive care: using Formula 1 pit-stop and aviation models to improve safety and quality. Paediatr Anaesth 17:470–478
Funding
National Institute of Biomedical Imaging and Bioengineering (1R03EB017447, Catchpole/Anger).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Catchpole has no direct conflicts of interest to disclose; however, he has received research funding from Medtronic Ltd, and received funding to attend a meeting unrelated to this Project at Intuitive Surgical headquarters. Drs. Eilber and Anger have no direct conflicts of interest to disclose; however, they are investigators for ASTORA Women’s Health LLC, and investigators and expert witnesses for Boston Scientific Corporation. Drs. Souders, Wood, Solnik, and Strauss and Ray Avenido, RN have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Souders, C.P., Catchpole, K.R., Wood, L.N. et al. Reducing Operating Room Turnover Time for Robotic Surgery Using a Motor Racing Pit Stop Model. World J Surg 41, 1943–1949 (2017). https://doi.org/10.1007/s00268-017-3936-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3936-4