Abstract
Background
Spontaneous esophageal perforation (Boerhaave’s syndrome) is a highly morbid condition traditionally associated with poor outcomes. The Pittsburgh perforation severity score (PSS) accurately predicts risk of morbidity, length of stay (LOS) and mortality. Operative management is indicated among patients with medium (3–5) or high (> 5) PSS; however, the role of minimally invasive surgery remains uncertain.
Methods
Consecutive patients presenting with Boerhaave’s syndrome with intermediate or high PSS managed via a thoracoscopic and laparoscopic approach from 2012 to 2018 were reviewed. Demographics, clinical presentation, management, and outcomes were analyzed.
Results
Ten patients (80% male) with a mean age of 61.3 years (range 37–81) were included. Two patients had intermediate and eight had high PSS (7.9 ± 2.8, range 4–12). The mean time from onset of symptoms to diagnosis was 27 ± 12 h and APACHE II score was 13.6 ± 4.9. Thoracoscopic debridement and primary repair was performed in eight cases, with two perforations repaired primarily over a T-tube. Laparoscopic feeding jejunostomy was performed in all patients. Critical care LOS was 8.7 ± 6.8 days (range 3–26), while inpatient LOS was 23.1 ± 12.5 days (range 14–46). Mean comprehensive complications index was 42.1 ± 26.2, with grade IIIa and IV morbidity in 60% and 10%, respectively. One patient developed dehiscence at the primary repair, which was managed non-operatively. In-hospital and 90-day mortality was 10%.
Conclusion
Minimally invasive surgical management of spontaneous esophageal perforation with medium to high perforation severity scores is feasible and safe, with outcomes which compare favorably to the published literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In 1724, Hermann Boerhaave described a fatal case of esophageal perforation in a Dutch admiral, Baron von Wassenaer, induced by forceful retching [1]. This eponymous syndrome has traditionally resulted in considerable morbidity and mortality, even when compared with esophageal perforation due to iatrogenic injury or underlying disease [1,2,3]. In the modern era, spontaneous esophageal perforation is associated with a significant risk of major morbidity and mortality. A recent English population-based study including 2564 patients treated from 2000 to 2012, the majority (82%) with Boerhaave syndrome, demonstrated 30% and 39% 30- and 90-day mortality, respectively [3]. There is, however, evidence to suggest improving outcomes in recent years, with reduced 30- and 90-day mortality rates from 37 to 44%, to 25% and 35% in the former report, while the North American PERF study report a mortality rate of just 15% among all-cause esophageal perforations, including 30% Boerhaave’s syndrome.
Improvements in outcomes for patients with esophageal perforation may be attributed to a number of factors including increased centralization of care and advances in perioperative management. In this regard, a strong hospital volume-outcome relationship is evident following esophageal perforation, with reduced 30- and 90-day mortality (33% vs. 23% and 41% vs. 32%) among patients managed in higher volume centers [4]. The significance of the volume-outcome relationship in esophageal perforation appears to be, in part, underpinned by the utilization of surgery where necessary, with improved outcomes associated with increased surgical intervention rates [3, 4], and management in an esophageal cancer center with significant experience in complex elective esophageal surgery [4]. However, the finding of more favorable outcomes even among patients with esophageal perforation managed conservatively or endoscopically, in higher volume centers, highlights the essential role of an experienced multidisciplinary team in the management of this challenging condition [3].
Indeed, a variety of approaches are now available; from conservative treatment and radiologic intervention, to endoluminal stent placement, vacuum therapy and operative lavage, repair, resection, or bypass and reconstruction [5, 6], with management guided by perforation severity. The Pittsburgh perforation severity score (PSS), developed based on experience with 119 patients with esophageal perforation treated at the University of Pittsburgh Medical Center [7], has been recently validated in a large multicenter study, and accurately predicts morbidity, mortality, and length of stay [8]. Importantly, failure of initial conservative management was observed in 19, 29, and 71% of patients with low, medium, and high PSS, respectively, and therefore, operative management is advocated for patients with medium and high PSS, suggestive of significant mediastinal contamination [7].
Despite such welcome advances in the management of esophageal perforation, major morbidity and mortality rates remain high. Minimally invasive surgery may represent a means to reduce the systemic inflammatory response when operative intervention for esophageal perforation is necessary, minimizing postoperative endotoxemia and preserving immune function [9,10,11] while achieving control of sepsis, a concept which has shown promise in emergency general surgery [12, 13]. Furthermore, in the elective setting, two randomized controlled trials now demonstrate the clinical benefit of minimally invasive approaches to esophageal resection, with major improvements in postoperative pulmonary morbidity evident in both the TIME and MIRO studies, and oncologic equivalence [14, 15]. With increasing experience in minimally invasive complex elective esophageal surgery, the application of such approaches to esophageal emergencies is emerging [3, 16,17,18,19,20]. In the largest series to date, Haveman et al. describe thoracoscopic washout and irrigation among eight patients with Boerhaave’s syndrome from 2002 to 2009, with a mortality rate of just 8% [16].
The aim of the present study was to determine the feasibility and safety of minimally invasive surgical management for patients with Boerhaave’s syndrome with medium to high perforation severity scores in an upper gastrointestinal cancer center.
Methods
Patient selection and study design
The Esophageal and Gastric Centre at Mercy University Hospital, Cork, is a high-volume Regional Centre, and a detailed clinicopathologic database is maintained for all patients with a diagnosis of esophageal perforation. Records for consecutive patients with spontaneous esophageal perforation between 2012 and 2018 were assessed for inclusion. Patients with iatrogenic or malignant perforation were excluded, as were those with low perforation severity scores, and those who were managed conservatively. All patients with intermediate or high perforation scores were reviewed.
As per institutional policy, Institutional Review Board approval was not required for this retrospective cohort study, which was conducted in accordance with the principles of the Declaration of Helsinki. Written consent could not be obtained for the use of the included images and video, as the patient in question unfortunately passed away in the community one year postoperatively from an unrelated medical condition. The patient’s family have no objection to the use of this material.
Treatment protocol
Pittsburgh perforation severity score
The PSS was determined for all patients on the basis of preexisting esophageal pathology and findings at the time of presentation. Each variable was assigned points (Supplementary Table 1) for a possible total score of 18 [7, 8], as follows: 1 = age > 75 years, tachycardia (> 100 beats per minute), leukocytosis (> 10 × 109/mL), pleural effusion; 2 = fever (> 38.5 °C), non-contained leak (on contrast swallow or computed tomography), respiratory compromise (respiratory rate > 30 breaths per minute, increasing oxygen requirement or need for mechanical ventilation), time to diagnosis > 24 h; and 3 = esophageal cancer, hypotension.
Perioperative management
All patients were managed in keeping with the recommendations of the Surviving Sepsis Campaign [21], and broad spectrum antibiotic and antifungal medication were commenced on presentation. All patients with spontaneous esophageal perforation and an intermediate or high PSS were resuscitated in a critical care setting and emergent operative intervention was undertaken. Postoperatively, enhanced recovery principles were adhered to, with intraoperative fluid restriction and early mobilization where possible. All patients were monitored in a critical care setting for at least the first 24 postoperative hours and underwent nutritional and physiotherapy assessment at serial postoperative timepoints. A water-soluble contrast study was undertaken on approximately postoperative day 5, with oral clear fluids commenced thereafter among patients with an intact repair without evidence of contrast leak.
Operative technique
The patient is placed in the lateral position according to the position of the esophageal defect on preoperative imaging. One-lung ventilation is established using a double lumen endotracheal tube and on-table endoscopy is performed. Thoracoscopy is established using a five-port technique and a 10 mm 30° lens as demonstrated in Fig. 1. After copious lavage of the pleural cavity and evacuation of food debris (Fig. 2A), the inferior pulmonary ligament is divided, and a diaphragmatic plication suture is placed using the EndoStitch™ suturing device. The necrotic pleura is divided and the posterior mediastinum accessed (Fig. 2B). The edge of the esophageal defect is then debrided to facilitate visualization of viable muscle and mucosa circumferentially (Fig. 2C), after which the defect is repaired primarily using a full-thickness inverting 2-0 polyglactin sutures using EndoStitch™ (Fig. 2D, E). For larger defects closure over a T-tube is undertaken. A wide bore nasogastric drainage tube, posterior mediastinal Jackson-Pratt drain (Fig. 2F), and basal and apical underwater seal chest drains are then placed, and the lung re-inflated. A feeding jejunostomy catheter is then laparoscopically placed for all patients (Fig. 1), with two-point fixation to the anterior abdominal wall, and feeding commenced on the first postoperative day [22]. On-table bronchoscopy and bronchoalveolar lavage is then undertaken for all patients.
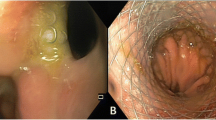
Thoracoscopic and laparoscopic port placement for minimally invasive management of Boerhaave’s syndrome. For thoracoscopic debridement and primary repair, the patient is placed in the lateral decubitus position as shown. One-lung ventilation is established using a double-lumen endotracheal tube (A). For laparoscopic placement of a feeding jejunostomy catheter, the patient is transferred to the supine position and pneumoperitoneum established. A feeding jejunostomy catheter is then percutaneously inserted (B, hollow circle) using the Seldinger technique, and two-point fixation is carried out using EndoStitch™. 10 mm ports are demonstrated in dark grey, and 5 mm ports in light grey
Thoracoscopic debridement and primary repair of the esophageal defect. Thoracoscopic lavage of the pleural cavity and evacuation of gross food debris and contamination is first undertaken (A), after which the inferior pulmonary ligament is divided and a diaphragmatic plication suture placed to facilitate visualization. The pleura is then divided and the posterior mediastinum accessed to expose the esophageal defect (B), which is then debrided to ensure visualization of viable mucosa circumferentially (C). A primary repair is then undertaken, beginning at the apices and working towards the center, using inverted 2–0 interrupted polyglactin sutures placed using EndoStitch™ (D, E). A posterior mediastinal Jackson-Pratt drain is then placed adjacent to the repair (F), in addition to basal and apical underwater seal chest drains, and the lung re-inflated
Statistical analysis
Comorbidity and illness severity were classified according to the Charlson comorbidity index and American Society of Anesthetists’ grade and APACHE II scores, respectively [23,24,25]. Postoperative complications were coded using the Clavien-Dindo classification and comprehensive complications index (CCI) [26, 27]. Pneumonia was defined as per CDC guidelines and postoperative pulmonary complications according to Esophageal Complications Consensus Group (ECCG) criteria [28, 29]. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [30]. Esophageal stricture was defined as dysphagia requiring dilatation at the site of the previous repair [31].
Data were analyzed using GraphPad Prism (v.6.0) for Windows, GraphPad software (San Diego, CA, USA) and SPSS® (v.23.0) software (SPSS, Chicago, IL, USA). Univariable comparisons were performed using linear regression, Student’s t, or Mann–Whitney U tests for continuous or χ2 or Fischer exact test for categorical variables. Multivariable linear regression with a forward stepwise selection procedure was utilized to determine factors independently associated with PSS and clinical outcomes. Data are reported as mean ± standard deviation unless otherwise specified, with the threshold of significance set at p < 0.05.
Results
Patient characteristics
During this period, eleven patients with intermediate (3 [27%]) or high (8 [73%]) PSS were treated at this center. Ten of 11 patients (91%) underwent surgical repair, while one patient with a PSS of 4, a contained leak and < 1 cm defect underwent uncomplicated laparoscopic placement of a feeding jejunotomy catheter, without exploratory thoracoscopy, and is excluded from further analyses.
Clinicopathologic characteristics of the study population (n = 10) are detailed in Table 1. The majority were male (8 [80%]), with a mean ± SD Charlson comorbidity index of 2.7 ± 1.6. Vomiting, chest pain, and dyspnea were the most common presenting features in 10 (100%), 9 (90%), and 6 (60%), respectively, and a mean ± SD time to diagnosis from onset of symptoms of 27 ± 12 h. At presentation, APACHE II score was 13.6 ± 4.9 representing a predicted mortality of 20.9 ± 13.9%, with a mean ± SD score of 7.9 ± 2.8. All patients had a non-contained leak at presentation.
Operative management
Right thoracoscopy was performed in 2 (20%) and left thoracoscopy in 8 (80%), with the majority (8 [80%]) being repaired primarily without a T-tube. All patients underwent laparoscopic placement of a feeding jejunostomy catheter. A laparoscopic decompressing gastrostomy was performed in the first five cases but this procedure was subsequently replaced with simple nasogastric decompression.
Postoperative outcome
Postoperative outcomes are detailed in Table 2. After surgery, the CCI was 42.1 ± 26.2 (range 24.2–100), with a median critical care length of stay (LOS) of 7 (3–26) days, and time to established oral intake of 8 (5–28) days. Five patients (50%) required a further radiological pleural drainage procedure for simple effusions, with three (30%) occurring in the contralateral non-operative pleural space. One patient developed an empyema which was managed with radiological pleural drainage and fibrinolytic therapy [32]. Atrial fibrillation and pneumonia each occurred in five patients (50%), with ARDS occurring in two patients (20%). One patient (10%), who developed dehiscence following repair of a large defect over a T-tube, developed a symptomatic stricture requiring dilatation in follow-up. One patient developed non-occlusive mesenteric ischemia resulting in an in-hospital mortality of 10%.
Pittsburgh perforation severity score (Fig. 3)
PSS was not significantly associated with inpatient LOS (p = 0.08, R2 = 0.38), critical care LOS (p = 0.93, R2 = 0.00), or CCI (p = 0.29, R2 = 0.14). On multivariable regression utilizing all components of the PSS, the presence of hypotension at presentation significantly predicted the overall PSS (p < 0.001, model R2 = 0.81), while PSS components independently predictive of inpatient LOS were age > 75 (p = 0.009), tachycardia at presentation (p = 0.003) and time to diagnosis > 24 h (p = 0.04, model R2 = 0.89).
Pittsburgh perforation severity score and postoperative outcomes. Most patients (80%) exhibited high perforation severity scores (PSS, A). Higher PSS tended to be associated with increased inpatient length of stay (B, p = 0.08, R2 = 0.38), but was not predictive of critical care length of stay (C, p = 0.93, R2 = 0.00) or overall burden of morbidity as determined by the comprehensive complications index (D, p = 0.29, R2 = 0.14), among patients who underwent thoracoscopic debridement and primary repair
Discussion
The development of minimally invasive techniques for the management of esophageal cancer has produced significant reductions in postoperative pulmonary complications, a key driver of overall morbidity among patients undergoing esophagectomy [14, 33, 34]. Furthermore, minimally invasive esophagectomy has been shown to facilitate earlier recovery of health-related quality of life, with reduced pain and improved functional scores at 1 year, as compared with open surgery [34,35,36]. The adoption of minimally invasive techniques in emergency general surgery may reduce postoperative pain, pneumonia and wound infection [37, 38], however few studies to date have evaluated the feasibility and safety of minimally invasive techniques in the management of esophageal emergencies such as Boerhaave’s syndrome. The aim of the present study was to determine short-term outcomes following minimally invasive management of spontaneous esophageal perforation in a tertiary referral esophagogastric unit, where totally minimally invasive esophagectomy (TMIE) is the preferred approach to esophageal resection.
From the present data, a number of key conclusions can be drawn. Firstly, the minimally invasive surgical management of Boerhaave’s syndrome appears both feasible and safe, with outcomes which compare favorably to the published literature. The 10% mortality observed following minimally invasive management, comprising thoracoscopic washout, debridement, and primary repair, with enteral nutrition established by laparoscopic feeding jejunostomy, is promising in a condition that has been associated with mortality rates of up to 40% in modern series [3]. This was achieved in a cohort of high risk patients, with all patients exhibiting a free perforation of the esophagus and 60% showing respiratory compromise at presentation. These data highlight the potential for such approaches to minimize the “second hit” associated with surgical trauma, to reduce the risk of end organ dysfunction and mortality. Furthermore, it is possible that reductions in postoperative pain may facilitate improved ventilation and earlier mobilization, limiting the risk of atelectasis and thromboembolic complications [38].
Secondly, the current data demonstrate the utility of the PSS for stratification of risk among patients with Boerhaave’s syndrome. Patients with a high PSS tended to have an increased postoperative LOS after minimally invasive surgical management. Similarly, a recent English study demonstrated that patients with greater PSS in the context of Boerhaave’s syndrome had an increased risk of post-perforation complications. Notably, this finding was not borne out among patients with perforations of other etiologies, suggesting that the PSS may be of particular value among patients with spontaneous esophageal perforation [39]. Interestingly, from the present data, hypotension at diagnosis may serve as a reliable proxy for PSS to aid rapid clinical decision making, as this factor alone predicted 81% of the variability in PSS between patients.
The Pittsburgh group demonstrated failure of conservative treatment among 19, 29, and 71% of patients with low, intermediate, and high PSS, strengthening the rationale for initial operative management for patients with high scores [7]. Similarly, data from Schweigert et al. advise caution with respect to the routine utilization of endoscopic approaches, with high rates of reintervention and failure of conservative management among patients initially managed with endoscopic stent placement [40]. On the other hand, several series now report the successful endoscopic management of esophageal perforation, with various techniques reported including covered stent placement [5], endoluminal negative pressure sponge therapy [41, 42], and endoscopic ligation with an over-the-scope clip [43, 44] or snare [45]. Thus, while the PSS may represent a useful tool to select patients requiring upfront operative management, further study is needed to identify the subset of patients with medium PSS and minimal pleural contamination who may be safely managed using conservative or endoscopic approaches [3, 6, 18, 46, 47].
A number of limitations are acknowledged. Firstly, given the tertiary referral nature of the study center, the included population may represent a sub-selected cohort of patients who were deemed by referring physicians to be fit for both transfer and intervention. Secondly, the study center represents a high-volume unit for minimally invasive upper gastrointestinal surgery. Considering the significant learning curve associated with the adoption of TMIE [48, 49], the present data may lack generalizability. However, with increasing expertise in minimally invasive esophageal surgery, the application of such techniques in the emergency context will likely increase. Finally, although the small number of patients in the present series may limit the conclusions that can be generated from this report, the rarity of this clinical presentation underscores the need for detailed outcomes reporting to facilitate systematic review and metanalysis. Future collaborative efforts, such as the recent PERF study [5], may be adequately powered to delineate the role of endoscopic and interventional radiology techniques for the management of esophageal perforation.
In conclusion, minimally invasive management of spontaneous esophageal perforation (Boerhaave’s syndrome) is feasible and safe, with postoperative outcomes comparing favorably to existing open and endoscopic techniques.
References
Derbes VJ, Mitchell RE Jr (1955) Hermann Boerhaave’s Atrocis, nec descripti prius, morbi historia, the first translation of the classic case report of rupture of the esophagus, with annotations. Bull Med Libr Assoc 43:217–240
Barrett NR (1946) Spontaneous perforation of the oesophagus; review of the literature and report of three new cases. Thorax 1:48–70
Markar SR, Mackenzie H, Wiggins T, Askari A, Faiz O, Zaninotto G, Hanna GB (2015) Management and outcomes of esophageal perforation: a national study of 2,564 patients in England. Am J Gastroenterol 110:1559–1566
Markar SR, Mackenzie H, Askari A, Faiz O, Hanna GB (2017) Effect of esophageal cancer surgeon volume on management and mortality from emergency upper gastrointestinal conditions: population-based cohort study. Ann Surg 266:847–853
Ali JT, Rice RD, David EA, Spicer JD, Dubose JJ, Bonavina L, Siboni S, O’Callaghan TA, Luo-Owen X, Harrison S, Ball CG, Bini J, Vercruysse GA, Skarupa D, Miller CC III, Estrera AL, Khalil KG (2017) Perforated esophageal intervention focus (PERF) study: a multi-center examination of contemporary treatment. Dis Esophagus 30:1–8
Loske G, Schorsch T, Muller C (2010) Endoscopic vacuum sponge therapy for esophageal defects. Surg Endosc 24:2531–2535
Abbas G, Schuchert MJ, Pettiford BL, Pennathur A, Landreneau J, Landreneau J, Luketich JD, Landreneau RJ (2009) Contemporaneous management of esophageal perforation. Surgery 146:749–755 (discussion 55–56)
Schweigert M, Sousa HS, Solymosi N, Yankulov A, Fernandez MJ, Beattie R, Dubecz A, Rabl C, Law S, Tong D, Petrov D, Schabitz A, Stadlhuber RJ, Gumpp J, Ofner D, McGuigan J, Costa-Maia J, Witzigmann H, Stein HJ (2016) Spotlight on esophageal perforation: a multinational study using the Pittsburgh esophageal perforation severity scoring system. J Thorac Cardiovasc Surg 151:1002–1009
Schietroma M, Carlei F, Cappelli S, Amicucci G (2006) Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg 243:359–363
Schietroma M, Piccione F, Carlei F, Clementi M, Bianchi Z, de Vita F, Amicucci G (2012) Peritonitis from perforated appendicitis: stress response after laparoscopic or open treatment. Am Surg 78:582–590
Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ, vd Peet DL, vd Tol MP, Bonjer HJ, Cuesta MA (2011) The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis 26:53–59
Kohl A, Rosenberg J, Bock D, Bisgaard T, Skullman S, Thornell A, Gehrman J, Angenete E, Haglind E (2018) Two-year results of the randomized clinical trial DILALA comparing laparoscopic lavage with resection as treatment for perforated diverticulitis. Br J Surg 105(9):1128–1134
Schultz JK, Wallon C, Blecic L, Forsmo HM, Folkesson J, Buchwald P, Korner H, Dahl FA, Oresland T, Yaqub S (2017) One-year results of the SCANDIV randomized clinical trial of laparoscopic lavage versus primary resection for acute perforated diverticulitis. Br J Surg 104:1382–1392
Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, Rosman C, van Berge Henegouwen MI, Gisbertz SS, van der Peet DL (2017) Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg 266:232–236
Briez N, Piessen G, Bonnetain F, Brigand C, Carrere N, Collet D, Doddoli C, Flamein R, Mabrut JY, Meunier B, Msika S, Perniceni T, Peschaud F, Prudhomme M, Triboulet JP, Mariette C (2011) Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial—the MIRO trial. BMC Cancer 11:310
Haveman JW, Nieuwenhuijs VB, Kobold JP, van Dam GM, Plukker JT, Hofker HS (2011) Adequate debridement and drainage of the mediastinum using open thoracotomy or video-assisted thoracoscopic surgery for Boerhaave’s syndrome. Surg Endosc 25:2492–2497
Cho JS, Kim YD, Kim JW, Seok H, Kim MS (2011) Thoracoscopic primary esophageal repair in patients with Boerhaave’s syndrome. Ann Thorac Surg 91:1552–1555
Darrien JH, Kasem H (2013) Minimally invasive endoscopic therapy for the management of Boerhaave’s syndrome. Ann R Coll Surg Engl 95:552–556
Landen S, El Nakadi I (2002) Minimally invasive approach to Boerhaave’s syndrome: a pilot study of three cases. Surg Endosc 16:1354–1357
Nakano T, Onodera K, Ichikawa H, Kamei T, Taniyama Y, Sakurai T, Miyata G (2018) Thoracoscopic primary repair with mediastinal drainage is a viable option for patients with Boerhaave’s syndrome. J Thorac Dis 10:784–789
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637
Healy LA, Ryan A, Doyle SL, Bhuachalla EBN, Cushen S, Segurado R, Murphy T, Ravi N, Donohoe CL, Reynolds JV (2017) Does prolonged enteral feeding with supplemental omega-3 fatty acids impact on recovery post-esophagectomy: results of a randomized double-blind trial. Ann Surg 266(5):720–728
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258:1–7
Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB, Griffin SM, Holscher AH, Hofstetter WL, Jobe BA, Kitagawa Y, Kucharczuk JC, Law SY, Lerut TE, Maynard N, Pera M, Peters JH, Pramesh CS, Reynolds JV, Smithers BM, van Lanschot JJ (2015) International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 262:286–294
Center for Disease Control and Prevention: Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event 2017. https://www.cdc.gov/nhsn/PDFs/pscManual/6pscVAPcurrent.pdf
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Ahmed Z, Elliott JA, King S, Donohoe CL, Ravi N, Reynolds JV (2017) Risk factors for anastomotic stricture post-esophagectomy with a standardized sutured anastomosis. World J Surg 41:487–497
Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, Peckham D, Davies CWH, Ali N, Kinnear W, Bentley A, Kahan BC, Wrightson JM, Davies HE, Hooper CE, Lee YCG, Hedley EL, Crosthwaite N, Choo L, Helm EJ, Gleeson FV, Nunn AJ, Davies RJO (2011) Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 365:518–526
Luketich JD, Pennathur A, Franchetti Y, Catalano PJ, Swanson S, Sugarbaker DJ, De Hoyos A, Maddaus MA, Nguyen NT, Benson AB, Fernando HC (2015) Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 261:702–707
van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, Kroese CC, HajMohammad N, Mook S, Vleggaar FP, BorelRinkes IHM, Ruurda JP, van Hillegersberg R (2018) Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg 269(4):621–630
Barbour AP, Cormack OM, Baker PJ, Hirst J, Krause L, Brosda S, Thomas JM, Blazeby JM, Thomson IG, Gotley DC, Smithers BM (2016) Long-term health-related quality of life following esophagectomy: a nonrandomized comparison of thoracoscopically assisted and open surgery. Ann Surg 265(6):1158–1165
Maas KW, Cuesta MA, van Berge Henegouwen MI, Roig J, Bonavina L, Rosman C, Gisbertz SS, Biere SS, van der Peet DL, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ (2015) Quality of life and late complications after minimally invasive compared to open esophagectomy: results of a randomized trial. World J Surg 39:1986–1993
Sanabria A, Villegas MI, Morales Uribe CH (2013) Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database Syst Rev 2:Cd004778
Coccolini F, Catena F, Pisano M, Gheza F, Fagiuoli S, Di Saverio S, Leandro G, Montori G, Ceresoli M, Corbella D, Sartelli M, Sugrue M, Ansaloni L (2015) Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg 18:196–204
Wigley C, Athanasiou A, Bhatti A, Sheikh A, Hodson J, Bedford M, Griffiths EA (2019) Does the Pittsburgh Severity Score predict outcome in esophageal perforation? Dis Esophagus 32:doy109
Schweigert M, Beattie R, Solymosi N, Booth K, Dubecz A, Muir A, Moskorz K, Stadlhuber RJ, Ofner D, McGuigan J, Stein HJ (2013) Endoscopic stent insertion versus primary operative management for spontaneous rupture of the esophagus (Boerhaave syndrome): an international study comparing the outcome. Am Surg 79:634–640
Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, Senninger N, Vowinkel T (2017) Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 31:2687–2696
Kuehn F, Loske G, Schiffmann L, Gock M, Klar E (2017) Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg Endosc 31:3449–3458
Barakat MT, Girotra M, Banerjee S (2018) (Re)building the wall: recurrent Boerhaave syndrome managed by over-the-scope clip and covered metallic stent placement. Dig Dis Sci 63:1139–1142
Musala C, Eisendrath P, Brasseur A, Vincent JL, Cappeliez S, LeMoine O, Deviere J, Lemmers A (2015) Successful treatment of Boerhaave syndrome with an over-the-scope clip. Endoscopy 47(Suppl 1):UCTN:E24-5
Kuwabara J, Watanabe Y, Kojima Y, Higaki N, Ikeda Y, Sato K, Yoshida M, Yamamoto Y, Kikuchi S (2016) Successful closure of spontaneous esophageal rupture (Boerhaave’s syndrome) by endoscopic ligation with snare loops. SpringerPlus 5:921
Ceppa DP, Rosati CM, Chabtini L, Stokes SM, Cook HC, Rieger KM, Birdas TJ, Lappas JC, Kessler WR, DeWitt JM, Maglinte DD, Kesler KA (2017) Development of a multidisciplinary program to expedite care of esophageal emergencies. Ann Thorac Surg 104:1054–1061
Vallbohmer D, Holscher AH, Holscher M, Bludau M, Gutschow C, Stippel D, Bollschweiler E, Schroder W (2010) Options in the management of esophageal perforation: analysis over a 12-year period. Dis Esophagus 23:185–190
Nilsson M, Kamiya S, Lindblad M, Rouvelas I (2017) Implementation of minimally invasive esophagectomy in a tertiary referral center for esophageal cancer. J Thorac Dis 9:S817–S825
Oshikiri T, Yasuda T, Hasegawa H, Yamamoto M, Kanaji S, Yamashita K, Matsuda T, Sumi Y, Nakamura T, Fujino Y, Tominaga M, Suzuki S, Kakeji Y (2016) Short-term outcomes and one surgeon’s learning curve for thoracoscopic esophagectomy performed with the patient in the prone position. Surg Today 47(3):313–319
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Jessie A. Elliott, Louise Buckley, Mohamed Albagir, Antonios Athanasiou, and Thomas J Murphy have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1—Thoracoscopic debridement and primary repair of spontaneous esophageal perforation [video file] (MP4 203200 kb)
Rights and permissions
About this article
Cite this article
Elliott, J.A., Buckley, L., Albagir, M. et al. Minimally invasive surgical management of spontaneous esophageal perforation (Boerhaave’s syndrome). Surg Endosc 33, 3494–3502 (2019). https://doi.org/10.1007/s00464-019-06863-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06863-2