Abstract
Background
Perforations and anastomotic leakages of the upper gastrointestinal (GI) tract cause a high morbidity and mortality rate. Only limited data exist for endoscopic vacuum therapy (EVT) in the upper GI tract.
Methods
Fifty-two patients (37 men and 15 women, ages 41–94 years) were treated (12/2011–12/2015) with EVT for anastomotic insufficiency secondary to esophagectomy or gastrectomy (n = 39), iatrogenic esophageal perforation (n = 9) and Boerhaave syndrome (n = 4). After diagnosis, polyurethane sponges were endoscopically positioned with a total of 390 interventions and continuous negative pressure of 125 mm of mercury (mmHg) was applied to the EVT-system. Sponges were changed endoscopically twice per week. Clinical and therapy-related data and mortality were analyzed.
Results
After 1–25 changes of the sponge at intervals of 3–5 days with a mean of 6 sponge changes and a mean duration of therapy of 22 days, the defects were healed in 94.2 % of all patients without revision surgery. In three patients (6 %), EVT failed. Two of these patients died due to hemorrhage related to EVT. Four postinterventional strictures were observed during the follow-up of up to 4 years.
Conclusion
Esophageal wall defects of different etiology in the upper gastrointestinal tract can be treated successfully with EVT, considering that indication for EVT should be weighed carefully. EVT can be regarded as a novel life-saving therapeutic tool.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Failures of the esophageal continuity are diverse and occur as postoperative complications [1], during diagnostic or interventional endoscopy [2], iatrogenic [3], or spontaneously [4, 5]. Esophageal leakages often lead to severe septic conditions which are difficult to treat and give rise to a high morbidity and mortality [6]. Particularly, the incidence of anastomotic insufficiency secondary to esophagectomy or gastrectomy ranges from 5 to 30 % and is associated with a morbidity and mortality rate of 20–50 % [7, 8]. Delays in the treatment for more than 24 h are associated with a threefold increase in mortality rates [4, 9]. Comparative studies between different treatment modalities are rare resulting in lack of clinical evidence for directing treatment modalities [10]. Therefore, therapy differs depending on institutions and primary attending disciplines. In recent years, interventional endoscopy has evolved as an effective alternative to primary surgery in these cases. Endoscopic interventions, including endoscopic suturing, application of metal clips, over-the-scope clips (OTSC) and injection of fibrin glue, can be used for small-sized defects with uncompromised tissue perfusion and in the absence of sepsis [11, 12]. Subsequently, the placement of self-expanding fully or partially covered metal or plastic stents (SEMS or SEPS) has become the first-line therapy in esophageal anastomotic leakages or perforations, if the patient is in a non-septic condition [13–15]. This procedure has helped to avoid the considerable risk of emergency surgery, including two-stage esophageal reconstruction with discontinuity resection and cervical esophagostomy. However, stent therapy might be accompanied by the necessity of additional abscess drainage, local pressure necrosis of the esophagus, stent migration with consequently inadequate defect closure, persistence of the leak or stent ingrowth with the impossibility of a later stent removal. Moreover, success rates after endoscopic stenting vary considerably [16]. The well-established stent therapy is now being challenged increasingly by endoscopic vacuum therapy (EVT), which already has been established successfully for anastomotic leaks after rectal resection [17]. The principles of continuous or intermittent negative pressure lead to decrease of bacterial contamination, secretion, local edema and promotion of perfusion and granulation. By endoscopic insertion of a polyurethane sponge into the defect cavity and transnasal application of external vacuum, defect closure and effective drainage are united. However, data for its application in the upper Gl tract consist of only case reports and small case series. In our department, EVT has become the treatment of choice for perforations and anastomotic leakages of the upper GI tract. We report, to our knowledge, the worldwide largest single-center experience of EVT for substantial esophageal wall defects comprising results with intracavitary and intraluminal variants of EVT in 52 consecutively treated patients with spontaneous, iatrogenic, or postoperative leaks in the upper GI tract. In addition, we propose a treatment algorithm based on these results.
Materials and methods
Seventy-three patients with esophageal leakage were treated during the study period (December 2011–2015) at our institution. Indications and inclusion criteria for EVT in the study cohort were anastomotic leakages, iatrogenic esophageal perforations or Boerhaave syndrome accompanied with no prior or post therapeutic modalities. Twenty-one referred patients with prior stent therapy (n = 11) and prior surgery (n = 10) were excluded from this study. To avoid a bias in patient selection, all patients with an untreated diagnosis of an esophageal wall defect regardless the etiology of the defect were consecutively treated with EVT in the upper GI tract within the study period and prospectively documented and included into this report. Leaks in the upper GI tract were diagnosed by primary endoscopy with application of contrast agent under direct visualization by radioscopy. In addition, every patient received computed tomography (CT) scan of neck, thorax or abdomen depending on the localization of the defect. Defect sizes ranged from small (<10 %), intermediate (10–40 %) to large (>45 %) defect sizes of circumferential involvement of the anastomosis at any esophageal height (Table 1). Except patients suffering from Boerhaave Syndrome, all patients had extraluminal abscesses ranging from pronounced mediastinal abscesses to extraluminal cavities. All included patients received EVT as first-line therapy after diagnosis and did not undergo additional surgery during the course of therapy. Besides the positive vote of the local ethical review committee, written consent was obtained from each patient or their legally authorized representative prior to the initiation of EVT. The primary end point of the study was leak closure. Patients were followed up after discharge from hospital for a median of 162 days (95–1475 days). By review of charts and recent follow-up visits, the following parameters were analyzed: initial interventional or operative procedure, size and localization of the leak, duration of EVT, number and frequency of sponge changes, complications and side effects of EVT, duration of hospitalization, in-hospital mortality and success rate as documented by endoscopy and x-ray contrast study. White blood cell (WBC) count and C-reactive protein (CRP) were recorded as inflammatory markers. Data were expressed as numbers with percentages or as median with range. Statistics were performed using nonparametric tests (Wilcoxon rank sum test).
Endoscopic vacuum therapy (EVT)
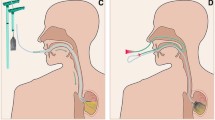
EVT was performed as previously described by our group and as shown in Figs. 1 and 2A [14, 18, 19]. Briefly, depending on the size of the defect, EVT was carried out, either by endoscopic insertion of an open-pore polyurethane sponge (e.g., VivanoMed® Foam, Paul Hartmann AG, Heidenheim, Germany) into the abscess cavity (Figs. 1B, 2C2), or in cases of only small defects without a cavity by endoscopic insertion of a sponge in the lumen of the esophagus covering the leak (Figs. 1A, 2B). With diminishing defect size, sponge placement could be changed from its initial intracavitary position to an intraluminal position. If the defect was initially not wide enough to accommodate the endoscope (<10 mm) and an abscess cavity was suspected, the opening was dilated by endoscopic balloon dilatation (Esophageal Balloon Dilatation Catheter, 10–12 mm, Boston Scientific, Ratingen, Germany) to allow extraluminal inspection. An additional suture loop (L-loop) was placed at the tip of the sponge (Fig. 2A1). This loop was grasped with endoscopic forceps, pulled close to the endoscope, and the sponge drainage system was guided in parallel to the endoscope (“backpack-method”, Fig. 2A2) and placed in the leakage cavity under direct endoscopic visualization (intracavitary) (Fig. 2C2). Moreover, in large leakage cavities or in cavities apart from each other, up to two sponges could be placed separately to allow rapid and sufficient drainage. Controlled continuous negative pressure of 100–125 mmHg, generated by an electronic vacuum pump system (e.g., VivanoTec®, Paul Hartmann AG, Heidenheim, Germany), was applied via a polyvinyl chloride (PVC) gastroduodenal tube (e.g., Covidien™ Salem Sump™, 14 Fr/Ch (4.7 mm) × 114 cm; Covidien™, MA, USA) connected to the sponge (Fig. 2A). Parenteral feeding, a transnasal enteral feeding tube, a percutaneous endoscopic gastrostomy (PEG) or a jejunostomy feeding tube ensured enteral nutrition. The entire procedure was performed under conscious sedation (with midazolam and propofol) or under general anesthesia, depending on the medical condition of the patient in our endoscopy unit. A scheduled change of sponges took place every 3–5 days. At each session, the size of the defect was assessed and treated with an individually prepared sponge, cut to fit the lesion’s dimensions. EVT was stopped when the defect size became too small for further sponge placements, and the defect was lined with surface epithelium (Fig. 2B2, C3). In some cases, complete closure was achieved by clipping the residual defect with an Over-The-Scope Clip (OTSC®; Ovesco Endoscopy AG, Tübingen, Germany). In case of failure of EVT (one patient), the persisting leakage was covered with a fully covered self-expanding nitinol stent (aixstent® OEL, 28/34 mm, Leufen Medical GmbH, Berlin, Germany) in addition to an external drainage.
A shows sponge preparation (A1) and principle of insertion into the esophagus using a forceps in a “backpack-method” (A2). Example of EVT for Boerhaave syndrome (B1–3) and late anastomotic insufficiency (C1–3). EVT treatment resulted in recovery of the mucosal surface with re-epithelialization and closure of the defect
Results
Patient demographics
Between 2011 and 2015, 52 patients (37 men and 15 women) with a median age of 65 years (range 41–94) were treated with EVT for anastomotic insufficiency secondary to esophagectomy (n = 30) or gastrectomy (n = 9), iatrogenic esophageal perforation (n = 9) and Boerhaave syndrome (n = 4). Defects were detected at a median of 8 postoperative days (range 3–25), whereas iatrogenic perforations and Boerhaave syndrome were diagnosed in median on day 5 (range 0–19). The esophageal defects were located 15–40 cm distal to the dental arch, and the defect size of anastomotic insufficiencies ranged from small (<10 %) to large (>45–<90 %) circumferential involvement of the anastomosis accompanied by larger wound cavities.
Endoscopic vacuum therapy (EVT)
In total, 390 polyurethane sponges were inserted in 52 patients with different upper gastrointestinal defects. Overall successful healing of the various defects was achieved in 49 of 52 patients (94.2 %), of which 42 defects healed with vacuum therapy only and without any additional measures. In 7 cases, final complete closure was performed by additional clipping of residual superficial defects with an OTSC. Median duration of EVT was 22 days (range 3–104) with a median number of 6 (range 1–25) sponge insertions per patient. The median changing interval of sponges was 3.5 days (range 3–5). Hospital length of stay was 53 days (range 16–180 days) for patients overall, 60 days (range 16–180) for anastomotic insufficiency, 43 days (range 20–50) for Boerhaave syndrome and 49 days (range 29–105) for iatrogenic perforations. Overall clinical results are summarized in Table 1. After the start of EVT, rapid debridement of the cavity and formation of granulation tissue was visible in all patients. EVT resulted in a reduction of size of the cavity and consecutive closure by granulation tissue. Conversion to a surgical intervention was not needed in any patient. After closure of the leakage, only a small fibrin scar remained (Fig. 2B3, C3).
Inflammatory markers during EVT
All patients showed initially signs of a systemic inflammatory response with elevated WBC and CRP levels. Both inflammatory markers normalized during the course of EVT and showed a significant reduction after therapy compared to pre-treatment (Fig. 3).
Complications and failure
Minor EVT—associated complications occurred in 4.1 % (n = 16) of all performed interventions. Of 390 sponge insertions, 11 sponge dislocations were seen (2.8 %); the sponge dislocation occurred when the leakage was already small and EVT was performed intraluminally due to swallowing and coughing. Five minor bleedings (1.3 %) occurred after sponge removal. None of these bleedings needed additional therapy, and EVT could be successfully continued in all cases.
EVT failed in three patients. Inexplicably, one patient did not show any response to EVT during a course of therapy of 57 days and 16 endoscopic interventions consisting of consecutively performed sponge insertions following anastomotic insufficiency secondary to esophagectomy. The persisting leakage was subsequently covered with a fully covered SEMS in addition to an external drainage.
Two patients, suffering from a late anastomotic insufficiency after distal esophagectomy, died due to fatal hemorrhage during EVT. After initial EVT over a period of 56 and 12 days, respectively, one patient died after acute hemorrhage and subsequent edema of the cerebrum accompanied by the finding of intracranial air inclusion with revealing a cardiac infarction as cause of death but simultaneously a close proximity of the treated insufficiency cavity to a pseudoaneurysm of the right atrium by autopsy. The other patient died due to a fulminant and non-manageable hemorrhage during the third scheduled endoscopic change of the sponge in the endoscopy unit. After successful removal of the sponge from the cavity, a non-manageable, sudden hemorrhage occurred while inserting the new sponge drainage system assuming a rupture of the descending aorta. Despite these two cases, there was subsequently no additional need for termination of EVT due to a close contact of the sponges with cardiovascular structures.
Hospital mortality
A hospital mortality of 9.6 % was observed. In addition to the above-mentioned fatal adverse events, one patient died due to multiorgan failure and two patients died due to severe pneumonia after successful EVT.
Follow-up
During a median follow-up of 162 days (range 95–1475), strictures after EVT were detected in 4 of 47 patients (8.5 %) (Table 1). A total of 3 patients required endoscopic dilation of moderate anastomotic strictures after completion of EVT. However, it remains unclear, if manifestation of strictures in these cases could be ascribed to EVT.
Discussion
This prospective single-center study, comprising 52 consecutive patients, is to our knowledge, the largest patient cohort with upper GI defects treated with EVT, published so far. Since its first description by Wedemeyer et al. [20] and Loske et al. [21, 22], EVT is known to be applicable even with variations in the procedure. The previously published studies, encompassing cohorts ranging from 1 to 35 patients (137 patients in total), have shown success rates ranging from 84 to 100 % [5, 9, 23–30]. Recently, we discussed that EVT in the upper GI tract seems to be not only feasible but superior to previous therapeutic procedures such as surgical revision and stent placement for esophageal defects [14, 19]. Although EVT requires multiple endoscopic procedures (every 3–4 days), its advantages with regard to previous treatment options are the regular visualization of the wound cavity and the optimal drainage provided by the vacuum system. This leads to effective sepsis control and final closure of the defect. In accordance with other reports and studies [3, 20, 22–26, 28, 29, 31, 32], the present study reaffirms the role of EVT as a minimally invasive endoscopic option for leaks of the upper gastrointestinal tract with an extraordinary success rate. Our leak closure rate of 94.2 % was characterized by rapid decrease of inflammation and closure of the defect site by rejection of necrosis and development of granulation tissue starting at the first system change even independently from potential different physiological characteristics of different entities of esophageal leakages. Although, for example, the physiological situation after occurrence of a leakage after gastrectomy if a Roux en Y reconstruction is used is characterized by a smaller output of intestinal juice and bile, the behavior when treated with EVT does not significantly differ from leakages after esophagectomy. Due to the construction of the sponge drainage system with the open tip allowing additional suction capacity beside the attached sponge, only the amount of evacuated fluid differs between these two entities of anastomotic leakages rather than the process of wound closure characterized by the formation of granulation tissue. In regard to this observation, it has to be emphasized that due to EVT followed by a prompt recovery from the sepsis, the limitation and especially duration of recovery and finally closure of the leak are more limited to the respective size of the defect rather than to the septic conditions and potential different physiological characteristics of leakages of different etiology. Previous studies reporting heterogeneous types of upper GI tract leakages reported comparable success rates without procedure-related complications [3, 9, 23–29, 31–33]. Further comparative retrospective studies demonstrated that EVT is superior to surgical revision and stent placement in managing major esophageal leaks, especially in septic patients [19, 25, 26] and showed the benefit of EVT in combination with revision surgery in terms of improved control of septicemia and successful closure of the defects [9, 23, 32]. We implemented EVT as our first-line procedure even in advanced stages of esophageal leakage 4 years ago. Based on our experience and increasing number of cases, we have modified our management algorithm using EVT as gold standard for the treatment of any kind of leak in the upper GI tract allowing small residual fistulas (1–2 cm deep) following EVT to be closed by additional OTSCs.
Our locally established and generally recommended treatment algorithm of defects and leaks in the upper GI tract is based on the algorithm of Schorsch et al. [27] and shown modified in Fig. 4 emphasizing that EVT is not reasonable and should not be performed in patients with complete insufficiency of the anastomotic region after upper GI surgery. An anastomotic leak, leading to a complete discontinuity of the passage, should only be treated by EVT in critically ill patients in terms of a bridging technique allowing later surgical repair since a complete, straightforward healing of such a defect cannot be expected in such cases. Moreover, it seems to be unlikely that patients with no response to EVT show progress in healing after 22 days, which is based on the median duration of therapy leading to defect closure in our study. We recommend that these patients should be evaluated for a different therapy regime.
Treatment algorithm for leakages in the upper gastrointestinal tract (modified according to Schorsch et al. [27])
Nonetheless, Ahrens et al. [31] were the only group reporting about a fatal incidence indirectly related to EVT. One of their patients died of acute severe hemorrhage from an aortoanastomotic fistula after a dilatation procedure following a completed EVT. Since we experienced two severe critical events of fatal hemorrhage in patients suffering from a late anastomotic insufficiency after distal esophagectomy, we strongly recommend EVT for esophageal perforations to be performed combined with a CT scan of the thorax done directly before or after every first endoscopic placement of the sponge to exclude close proximity of the sponge to cardiovascular structures with subsequent risk of erosion bleeding. Even though the autopsy of one patient did not reveal a direct correlation between EVT and the cause of death and an autopsy, on the other patient, was not performed due to the missing consent of the relatives, a coincidence with EVT and the fatal course of therapy has to be assumed in both patients. One of these patients suffered from a squamous cell carcinoma and the other patient from an adenocarcinoma of the distal esophagus. Both patients developed a late anastomotic insufficiency after distal esophagectomy (12 and 18 days after surgery, respectively) assuming to have led to an erosive precondition of adjacent vascular structures. Even though a close contact with cardiovascular structures, but also with the tracheobronchial system, is a common situation in our study cohort, the reported two severe critical events of fatal hemorrhage occurred in patients, who retrospectively showed no intermediate tissue layer between the sponge and a pseudoaneurysm of right atrium and the descending aorta, respectively, defining the close proximity to cardiovascular structures and revealing a major complication risk for EVT in the upper GI tract in these patients. Neither the height nor the quadrant of the defect in the esophagus showed a correlation with these fatal events. Even though the application of a lower negative pressure to the sponge system than the 100–125 mmHg used in this study might theoretically reduce the risk of erosions, no available data concerning lower pressure levels in these particular cases are present in the literature up to date. Therefore, we recommend a critical evaluation of comparable patients in terms of potential different therapy regimes and exit strategies such as stent placement even though procedure-related complications like hemorrhage, migration, and perforation have been reported after placement or removal of endoscopic stents with a mortality rate up to 13 % in the upper GI tract [16]. Our data confirm the initially promising results of EVT that have been reported so far but also strengthen, despite the potentially upcoming euphoria, the assumption that the risk factors of EVT still need to be monitored very closely. Especially the risk of hemorrhage by erosion of major vessels due to, e.g., ongoing inflammatory processes needs to be considered just like a pre- or post-interventional sponge position control by computertomography is indispensable. Therefore, exit strategies, such as stent therapy combined with an external drainage of the septic focus, even by additional surgical intervention, remain valuable options. Although prospective studies for the use of EVT in the upper GI tract are scarce, EVT can already be regarded as a novel life-saving therapeutic tool.
Abbreviations
- GI:
-
Gastrointestinal
- EVT:
-
Endoscopic vacuum therapy
- SEMS:
-
Self-expanding metal stent
- SEPS:
-
Self-expanding plastic stent
- CT:
-
Computed tomography
- OTSC:
-
Over-the-scope clip
- WBC:
-
White blood cell count
- CRP:
-
c-reactive protein
- PVC:
-
Polyvinyl chloride
References
Junemann-Ramirez M, Awan MY, Khan ZM, Rahamim JS (2005) Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on long-term survival in a high volume centre. Eur J Cardiothorac Surg 27:3–7
Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P, Fockens P (2012) Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol 10:603–608
Schorsch T, Muller C, Loske G (2013) Endoscopic vacuum therapy of anastomotic leakage and iatrogenic perforation in the esophagus. Surg Endosc 27:2040–2045
Biancari F, D’Andrea V, Paone R, Di Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E (2013) Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg 37:1051–1059
Kuehn F, Schiffmann L, Janisch F, Schwandner F, Alsfasser G, Gock M, Klar E (2016) Surgical endoscopic vacuum therapy for defects of the upper gastrointestinal tract. J Gastrointest Surg 20:237–243
Alanezi K, Urschel JD (2004) Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 10:71–75
Lang H, Piso P, Stukenborg C, Raab R, Jahne J (2000) Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol 26:168–171
Ikeguchi M, Oka S, Gomyo Y, Tsujitani S, Maeta M, Kaibara N (2001) Postoperative morbidity and mortality after gastrectomy for gastric carcinoma. Hepatogastroenterology 48:1517–1520
Smallwood NR, Fleshman JW, Leeds SG, Burdick JS (2015) The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 30:2473–2480
Schaheen L, Blackmon SH, Nason KS (2014) Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 208:536–543
Bohm G, Mossdorf A, Klink C, Klinge U, Jansen M, Schumpelick V, Truong S (2010) Treatment algorithm for postoperative upper gastrointestinal fistulas and leaks using combined vicryl plug and fibrin glue. Endoscopy 42:599–602
Hampe J, Schniewind B, Both M, Fritscher-Ravens A (2010) Use of a NOTES closure device for full-thickness suturing of a postoperative anastomotic esophageal leakage. Endoscopy 42:595–598
Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M (2008) Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg 12:1168–1176
Mennigen R, Senninger N, Laukoetter MG (2014) Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol 20:7767–7776
van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD (2011) Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 33:1292–1301
Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E (2014) The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 259:852–860
Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW (2008) Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 22:1818–1825
Mennigen R, Senninger N, Laukoetter MG (2015) Endoscopic vacuum therapy of esophageal anastomotic leakage. Gastrointest Endosc 82:397
Mennigen R, Harting C, Lindner K, Vowinkel T, Rijcken E, Palmes D, Senninger N, Laukoetter MG (2015) Comparison of endoscopic vacuum therapy versus stent for anastomotic leak after esophagectomy. J Gastrointest Surg 19:1229–1235
Wedemeyer J, Schneider A, Manns MP, Jackobs S (2008) Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 67:708–711
Loske G, Muller C (2009) Vacuum therapy of an esophageal anastomotic leakage—a case report. Zentralbl Chir 134:267–270
Loske G, Muller C (2009) Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 69:601–602 (author reply 602)
Kuehn F, Schiffmann L, Rau BM, Klar E (2012) Surgical endoscopic vacuum therapy for anastomotic leakage and perforation of the upper gastrointestinal tract. J Gastrointest Surg 16:2145–2150
Bludau M, Holscher AH, Herbold T, Leers JM, Gutschow C, Fuchs H, Schroder W (2014) Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 28:896–901
Brangewitz M, Voigtlander T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J, Manns MP, Schneider AS, Wedemeyer J (2013) Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 45:433–438
Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jurgensen C, Schreiber S, Becker T, Hampe J (2013) Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 27:3883–3890
Schorsch T, Muller C, Loske G (2014) Endoscopic vacuum therapy of perforations and anastomotic insufficiency of the esophagus. Chirurg 85:1081–1093
Wedemeyer J, Brangewitz M, Kubicka S, Jackobs S, Winkler M, Neipp M, Klempnauer J, Manns MP, Schneider AS (2010) Management of major postsurgical gastroesophageal intrathoracic leaks with an endoscopic vacuum-assisted closure system. Gastrointest Endosc 71:382–386
Weidenhagen R, Hartl WH, Gruetzner KU, Eichhorn ME, Spelsberg F, Jauch KW (2010) Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg 90:1674–1681
Wallstabe I, Plato R, Weimann A (2010) Endoluminal vacuum therapy for anastomotic insufficiency after gastrectomy. Endoscopy 42(Suppl 2):E165–E166
Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, Broering DC, Schniewind B (2010) Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy 42:693–698
Heits N, Stapel L, Reichert B, Schafmayer C, Schniewind B, Becker T, Hampe J, Egberts JH (2014) Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg 97:1029–1035
Loske G, Schorsch T, Muller C (2011) Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy 43:540–544
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
M.G. Laukoetter is a member of the expert panel of negative pressure wound therapy of the Paul Hartmann (AG) holding company. He received fees for invited speeches on endoscopic vacuum therapy. Drs. R. Mennigen, P.A. Neumann, S. Dhayat, G. Horst, D. Palmes, N. Senninger and T. Vowinkel have no conflicts of interest or financial ties to disclose.
Additional information
Mike G. Laukoetter and Rudolf Mennigen have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Laukoetter, M.G., Mennigen, R., Neumann, P.A. et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 31, 2687–2696 (2017). https://doi.org/10.1007/s00464-016-5265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5265-3