Abstract
Background and study aims
The management plan for gastric indefinite for neoplasia is undetermined, and endoscopic forceps biopsy might be inconclusive in ascertaining whether a resection is required. This study aimed to evaluate the clinical outcomes of endoscopic submucosal dissection (ESD) for gastric indefinite for neoplasia and to identify the factors highly predictive of true neoplasia.
Patients and methods
This retrospective study was conducted in a single, tertiary, referral hospital between November 2008 and December 2015. A total of 109 gastric indefinite for neoplasia lesions from endoscopic forceps biopsy that were resected by ESD were included in the study. The clinical outcomes and endoscopic factors for prediction of true neoplasia were analyzed.
Results
A total of 99 patients (90.8%) were diagnosed with definite neoplasia after ESD and were classified as category 3 (n = 42), category 4 (n = 50), and category 5 (n = 7) according to the revised Vienna classification. The mean age of the patients was 65.8 ± 9.8 years. The mean lesion size was 10.7 ± 6.1 mm. The patient population predominantly consisted of male patients (70.6%). The en bloc and complete endoscopic resection rates were 98.2% and 94.5%, respectively. Factors associated with true neoplastic lesions were male sex (odds ratio [OR] 8.596, p = 0.008) and lesion size ≥ 5 mm (OR 11.355, p = 0.003). Factors associated with category 4–5 were male sex (OR 3.165, p = 0.021) and erosive change (OR 2.841, p = 0.031).
Conclusions
Endoscopic resection for indefinite for neoplasia with larger lesions size and erosive changes, especially in males, should be considered when possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the advancement of endoscopic instruments, subtle mucosal changes can be detected. Although an endoscopic forceps biopsy is a useful method to differentiate between benign and malignant lesions, pathologic diagnosis may be inconclusive especially in borderline situations. Interobserver variability in the pathologic diagnosis of gastric epithelial neoplasia, especially between Japanese and Western pathologists, is a longstanding problem. The Japanese pathologists emphasize nuclear, cytologic, and glandular architectural abnormalities to diagnose carcinoma. In contrast, Western pathologists emphasize the presence of invasion. The diagnostic concordance rate between a Western viewpoint and a Japanese viewpoint was 37% for gastric epithelial neoplasia [1]. The Vienna classification of gastrointestinal epithelial neoplasia was developed to decrease the differences between Western and Japanese pathologists [1, 2]. After using Vienna classification, the diagnostic concordance rate was increased to 71% for gastric epithelial lesions [1].
According to the Vienna classification, an indefinite for neoplasia was classified as category 2 and used when a pathologist was unable to decide whether a lesion is neoplastic or not [1, 2]. For gastric indefinite for neoplasia, follow-up examination is recommended because of the uncertain nature of the lesion [1, 2]. However, from previous studies on follow-up examinations for gastric indefinite for neoplasia, 26–47% of lesions were diagnosed as true neoplastic lesions [3, 10]. The diagnostic discrepancy between endoscopic forceps biopsy and resected specimen was reported to be 20.1–76.3% [4, 5]. Therefore, endoscopic resection may be required in some patients diagnosed with indefinite for neoplasia. An endoscopic mucosal resection or endoscopic submucosal dissection (ESD) has been used to remove gastric epithelial or subepithelial neoplasia. In particular, by using ESD technique, > 90% of lesions can be removed by en bloc maneuver [4, 6]. Although no sufficient evidence has been available to support ESD as a diagnostic modality for gastric indefinite for neoplasia, it may be a useful diagnostic or therapeutic tool for gastric indefinite for neoplasia, which can be removed endoscopically.
This study aimed to evaluate the outcomes of ESD for gastric indefinite for neoplasia from endoscopic forceps biopsy and to analyze the associated factors predictive of true neoplasia (categories 3–5 according to the revised Vienna classification).
Materials and methods
Patients
The medical records of patients who underwent ESD at the Pusan National University Yangsan Hospital in the Republic of Korea between November 2008 and December 2015 were reviewed retrospectively. During the study period, a total of 1901 gastric epithelial neoplastic lesions were resected by ESD. Pathologic diagnoses were classified according to the revised Vienna classification: category 1 (negative for neoplasia), category 2 (indefinite for neoplasia), category 3 (low-grade adenoma/dysplasia), category 4 (high-grade adenoma/dysplasia, noninvasive carcinoma, suspicious for invasive carcinoma, and intramucosal carcinoma), and category 5 (submucosal invasion by carcinoma) [2]. Among the 1901 gastric epithelial neoplastic lesions, category 3 (n = 1076) and categories 4–5 (n = 716) from endoscopic forceps biopsy were excluded. Finally, a total of 109 category 2 (indefinite for neoplasia) lesions were enrolled and analyzed (Fig. 1). Prior to ESD procedure, written informed consent was obtained from all patients. The present study was approved by the ethics committee of the institutional review board (Institutional Review Board no. 05-2018-091).
Procedure
Diagnostic or therapeutic endoscopy was performed using a standard single-channel endoscope (GIF-H260, GIF-H260Z, or GIF-HQ290; Olympus Optical, Tokyo, Japan) or a 2-channel endoscope (GIF-2TQ260M; Olympus Optical, Tokyo, Japan). One or two endoscopic forceps biopsy samples were obtained before ESD. During ESD procedure, conscious sedation using intravenous midazolam (0.05 mg/kg) and meperidine (50 mg) was usually performed. During ESD, the first step is creating a marking of 1–2 mm outside the lesion using electrosurgical knives. Then, a solution containing a mixture of normal saline, epinephrine, and indigo carmine was injected into the submucosa, and a circumferential incision/submucosal dissection was performed using electrosurgical knives (needle or insulation-tipped electrosurgical knife). After removal of a lesion, preventive coagulation for all visible vessels was done in the artificial ulcer bed [4,5,6,7] (Fig. 2).
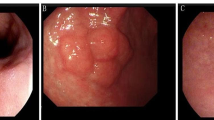
A case of a 39-year-old female who underwent endoscopic submucosal dissection. A A 15-mm-sized depressed mucosal lesion at the posterior angle (black arrow). B Submucosal dissection was performed. C En bloc resection was performed. Blue circle indicates tumor margin. D A histology of endoscopic forceps biopsy. Severe inflammatory cells and atypical cells were seen (red circle). A pathologist could not determine whether the lesion is malignant or not (× 100). E Characteristic histologic picture of well-differentiated carcinoma from endoscopic submucosal dissection (yellow circle) (× 40). F The well-differentiated adenocarcinoma invades the muscularis mucosa, not through the submucosa. (Color figure online)
Clinical and endoscopic factors
All clinical data were reviewed by an endoscopist (CW Choi, M.D., PhD). The location of the lesions was classified as the lower third, middle third, or upper third of the stomach [8]. The maximal diameter of a lesion was measured via a pathologic examination of resected specimen. Erythematous and whitish color changes and the color of the lesions with the background mucosa were compared. Nodularity was checked when irregularly raised or nodular mucosa was present. The submucosal fibrosis was recorded after confirming the presence of fibrosis during dissecting submucosa. The endoscopic extent of atrophic gastritis was measured using the Kimura–Takemoto classification system: mild (normal to closed type 2), moderate (closed type 3 to open type 1), and severe (open type 2 to open type 3) [9]. The procedure time was calculated from the marking to the completion of preventing coagulation after the removal of the lesion.
The resected specimens were stretched, pinned, and fixed with formalin. Specimens that were resected in a piecemeal fashion were reconstructed as accurately as possible. Fixed specimens were then sectioned at 2-mm intervals. En bloc resection was defined as a resection in a single piece of the lesion. Endoscopic complete resection was defined as the absence of tumors cells at the margins of an en bloc-resected specimen (Fig. 3).
A case of finally diagnosis from indefinite for neoplasia to early gastric cancer (a 67-year-old woman). A, B Conventional endoscopic image: the depressed lesion located at cardia with surface redness (black arrows). C Magnifying endoscopy with narrow-band imaging finding of the lesion. A distinct demarcation line (red arrows) is detected between the background mucosa and the depressed lesion. Within the demarcation line, it shows irregular microvascular pattern plus irregular microsurface pattern. D En block resected specimen. (Color figure online)
Statistical analyses
Univariate analysis using either a Chi-square test or the Fisher’s exact test for categorical variables or the Student’s t test for continuous variables was performed. The variables with p < 0.05 in the univariate analysis were included for the multivariable analysis using multiple logistic regression models. A p value of < 0.05 was considered to be statistically significant. Calculations were performed using the Statistical Package for the Social Sciences (SPSS) version 21.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Figure 1 shows a summary of the final diagnosis and treatment results of the 109 patients. A total of 99 patients (90.8%) were diagnosed with definite neoplasia and were classified as category 3 (n = 42), category 4 (n = 50), and category 5 (n = 7). There were 46 early gastric cancers (EGCs). Of those, 39 were category 4 (mucosal cancer) and 7 were category 5 (submucosal cancer). Each of histological types was classified into well-differentiated types (n = 33) and moderately differentiated types (n = 6) in category 4. In category 5, there were well-differentiated types (n = 3), moderately differentiated types (n = 3), and signet ring cell carcinoma (n = 1). Among the category 4 lesions, one patient needed an additional operation, and two additional ESD were performed during follow-up because of local recurrence. Among the category 5 lesions, additional operations were performed on 4 patients (no evidence of lymph node metastasis after operation). Three patients refused additional operation, and no evidence of recurrence was found during follow-up examinations (Fig. 1; Table 1).
The patients’ mean age was 65.8 ± 9.8 years. The mean lesion size was 10.7 ± 6.1 mm. The patient population consisted predominantly of male patients (77/109, 70.6%). The most predominant location was the lower third of the stomach (85/109, 78.0%). The en bloc and complete endoscopic resection rates were 98.2% and 94.5%, respectively. Delayed bleeding after ESD occurred in 8.3% of the patients. Perforations during ESD procedure occurred in 2 patients (1.8%), which were closed successfully by endoscopic clips without operation (Table 1).
Associated factors with true neoplastic lesions (categories 3–5) were analyzed (Table 2). After univariate and multivariate analyses, male sex (odds ratio [OR] 8.596, 95% confidence interval [CI] 1.755–42.088, p = 0.008) and lesion size ≥ 5 mm (OR 11.355, 95% CI 2.298–56.109, p = 0.003) were significant. Associated factors with categories 4–5 (high-grade neoplasia and early gastric cancer [EGC]) were analyzed additionally (Tables 2, 3). After univariate analysis, male sex, submucosal fibrosis, erythema, depression, and erosion were significant. After multivariate analysis, male sex (OR 3.165, 95% CI 1.192–8.399, p = 0.021) and erosive change (OR 2.841, 95% CI 1.101–7.324, p = 0.031) were significant.
Discussion
The nature of gastric indefinite for neoplasia remains to be fully elucidated. When a pathologist cannot decide whether the tissue from endoscopic forceps biopsy is truly neoplastic or not, they usually use indefinite for neoplasia or atypia as diagnosis. From previous other reports, 26–47% of lesions may be true neoplastic lesions after follow-up examination [3, 10]. In the present study, ESD of feasible gastric epithelial lesions was conducted for endoscopic resection. After endoscopic resection, the pathologic diagnosis rates of true neoplasia and categories 4–5 (high-grade dysplasia or EGC) were 90.8% and 52.2%, respectively. The discrepancy between endoscopic forceps biopsy and resected specimen had been reported to be 20.1–76.3% of lesions [4, 5]. Endoscopic forceps biopsy is a simple diagnostic method for gastrointestinal epithelial lesions. However, in some instances, an endoscopic forceps biopsy may be inconclusive to diagnose EGC. Several possible reasons may be present for discrepancy. The first reason may be that target biopsy for neoplastic lesion is not performed. The high-grade dysplasia or invasive cancer may exist focally within background low-grade dysplasia [5]. For an EGC of signet ring cell carcinoma without surface changes, the target biopsy may be more difficult because it may spread subepithelially [11]. The second reason may be that the dysplastic lesion may be too subtle to be determined whether it is neoplastic or not. In this regard, increasing the number of biopsies or larger size of biopsy specimens may improve the diagnostic accuracy of endoscopic forceps biopsy. A previous study compared the results of conventional and jumbo forceps biopsy, which showed that the increasing number of biopsies was more important than forceps size [12]. A previous prospective study conducted by Graham et al. reported that the first biopsy yielded a correct diagnosis only in 70% of patients with gastric cancer and three additional biopsy specimens increased the yield to > 95% [13]. However, in recent years, with the advancement of endoscopic instruments, the minute EGC < 5 mm can be detected [14]. For small EGC lesions, because bleeding from a previous biopsy may obscure the lesion to the target next biopsy, the first target biopsy is the most important [15]. If the proper target biopsy was done, the diagnostic yield of the first biopsy was reported to be 92.3% [15]. In the present study, the mean lesion size was 10.7 mm. Obtaining > 4 biopsy samples is difficult, and submucosal fibrosis caused by multiple biopsy may be an obstacle for subsequent ESD. Therefore, we usually performed endoscopic forceps biopsy 1–2 times according to the endoscopists’ decision.
The management plan for gastric indefinite for neoplasia is yet to be determined. Previously, although follow-up is needed because of the uncertain nature of the lesion [1, 2], a recommendation of optimal surveillance interval is absent. In recent years, endoscopic resection for adenomatous polyps of any size or gastric polyps is suggested when possible [16]. Therefore, the management plan for gastric indefinite for neoplasia should be individualized. However, endoscopic resection for all cases of gastric indefinite for neoplasia is unnecessary. In the present study, the associated factors with true neoplastic lesions were evaluated. Significant factors were male sex, a lesion size ≥ 5 mm, and erosive changes. However, the reason why male sex is considered a risk factor remains unclear. An epidemiologic study reported that gastric cancer incidence rates are two- to threefolds higher in men than in women [17] and the annual age-standardized incidence rates of gastric cancer are 65.9/100,000 in men in Korea [18]. Well-known endoscopic findings associated with EGC are larger lesion size and surface abnormalities such as depressive morphology, erythematous color compared with the surrounding mucosa, erosive change, and nodular surface pattern [4, 5, 10, 12, 19,20,21]. If pathologic diagnosis of gastric indefinite for neoplasia is reported for lesions with highly suspicious of endoscopic findings of EGC, complete resection is usually recommended regardless of lesion size when possible. In the present study, 90.8% of resected lesions were diagnosed as definite neoplasia (38.5% category 3 and 52.3% categories 4–5). Therefore, if indefinite for neoplasia lesions have highly suspicious endoscopic findings of EGC, endoscopic resection of lesions is a valuable treatment option rather than a regular follow-up examination. In our hospital, if these risk factors are present and endoscopic resection is feasible, endoscopic resection is strongly recommended after discussion with the patient. If the patient does not want to undergo endoscopic resection, or if there are no risk factors, follow-up endoscopy is performed for re-biopsy after 3 months and 6 months. During the waiting period, diagnosis and treatment of Helicobacter pylori infection are performed. Because a previous report has shown that H. pylori eradication can suppress the progression of adenomatous lesions to some degree or regress [22].
The present study has several limitations. First, selection bias may be present because of a retrospective analysis of medical chart review. Most of the patients were referred from other hospitals or medical clinics. Because the sample size is small, we cannot generalize the present study results. Second, we could use only the endoscopic findings of lesion diameter and macroscopic appearances of conventional white light endoscopy. Image-enhanced endoscopy with magnification might improve the target endoscopic forceps biopsy. However, not all endoscopic examinations can use image-enhanced endoscopy with magnification. Although advanced image-enhanced endoscopy with magnification may be useful, conventional white light endoscopic findings are still important.
In summary, the recommended simple follow-up examination for gastric indefinite for neoplasia is inappropriate for patients with suspicious endoscopic findings of EGC or true neoplasia. We should keep in mind that the discrepancy between endoscopic forceps biopsy and resection may exist. This study showed highly successful outcomes of ESD for gastric indefinite for neoplasia from endoscopic forceps biopsy. Some patients had submucosal invasive or lymphovascular invasive EGC. If simple follow-up examinations were recommended for EGC patients, they might miss the chance for endoscopic resection. Furthermore, repeated follow-up examination with biopsy may burden the patients’ financial, physical, and psychological strains. When possible, endoscopic resection for these lesions should be considered for lesion size ≥ 5 mm and surface changes such as erosion, especially in males.
References
Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Flejou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H (2000) The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47:251–255
Dixon MF (2002) Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 51:130–131
Yu CH, Jeon SW, Kim SK, Lee HS, Heo J, Kwon YH, Kim GY, Kim SZ, Bae HI (2014) Endoscopic resection as a first therapy for gastric epithelial atypia: is it reasonable? Dig Dis Sci 59:3012–3020
Ryu DG, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Nam HS (2017) Clinical outcomes of endoscopic submucosa dissection for high-grade dysplasia from endoscopic forceps biopsy. Gastric Cancer 20:671–678
Choi CW, Kim HW, Shin DH, Kang DH, Hong YM, Park JH, Park SB, Cho M, Lee JH (2014) The risk factors for discrepancy after endoscopic submucosal dissection of gastric category 3 lesion (low grade dysplasia). Dig Dis Sci 59:421–427
Kim JH, Nam HS, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Hwang SH, Lee SH (2017) Risk factors associated with difficult gastric endoscopic submucosal dissection: predicting difficult ESD. Surg Endosc 31:1617–1626
Choi CW, Kang DH, Kim HW, Park SB, Kim S, Cho M (2012) Endoscopic submucosal dissection as a treatment for gastric adenomatous polyps: predictive factors for early gastric cancer. Scand J Gastroenterol 47:1218–1225
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Mihara M, Haruma K, Kamada T, Komoto K, Yoshihara M, Sumii K, Kajiyama G (1999) The role of endoscopic findings for the diagnosis of Helicobacter pylori infection: evaluation in a country with high prevalence of atrophic gastritis. Helicobacter 4:40–48
Goo JJ, Choi CW, Kang DH, Kim HW, Park SB, Cho M, Hwang SH, Lee SH (2015) Risk factors associated with diagnostic discrepancy of gastric indefinite neoplasia: Who need en bloc resection? Surg Endosc 29:3761–3767
Kim H, Kim JH, Lee YC, Kim H, Youn YH, Park H, Choi SH, Noh SH, Gotoda T (2015) Growth patterns of signet ring cell carcinoma of the stomach for endoscopic resection. Gut Liver 9:720–726
Jeon HK, Ryu HY, Cho MY, Kim HS, Kim JW, Park HJ, Kim MY, Baik SK, Kwon SO, Park SY, Won SH (2014) A randomized trial to determine the diagnostic accuracy of conventional vs. jumbo forceps biopsy of gastric epithelial neoplasias before endoscopic submucosal dissection; open-label study. Gastric Cancer 17:661–668
Graham DY, Schwartz JT, Cain GD, Gyorkey F (1982) Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 82:228–231
Shimizu S, Tada M, Kawai K (1995) Early gastric cancer: its surveillance and natural course. Endoscopy 27:27–31
Iishi H, Tatsuta M, Okuda S (1985) Endoscopic diagnosis of minute gastric cancer of less than 5 mm in diameter. Cancer 56:655–659
Committee ASoP, Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Sharaf R, Shergill AK, Cash BD, DeWitt JM (2015) The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 82:1–8
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Jemal A, Center MM, DeSantis C, Ward EM (2010) Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomark Prev 19:1893–1907
Cho SJ, Choi IJ, Kim CG, Lee JY, Kook MC, Park S, Ryu KW, Lee JH, Kim YW (2011) Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy 43:465–471
Park DI, Rhee PL, Kim JE, Hyun JG, Kim YH, Son HJ, Kim JJ, Paik SW, Rhee JC, Choi KW, Oh YL (2001) Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy 33:501–506
Goldstein NS, Lewin KJ (1997) Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol 28:127–133
Suzuki S, Gotoda T, Suzuki H, Kono S, Iwatsuka K, Kusano C, Oda I, Sekine S, Moriyasu F (2015) Morphologic and histologic changes in gastric adenomas after Helicobacter pylori eradication: a long-term prospective analysis. Helicobacter 20:431–437
Acknowledgements
Hyeong Seok Nam and Cheol Woong Choi share first authorship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
HS Nam, CW Choi, SJ Kim, DH Kang, HW Kim, SB Park, and DG Ryu have no conflicts of interest or financial ties to disclose.
Ethical approval
Written informed consent was obtained from all patients prior to the procedure. The study was approved by the ethics committee of the Institutional Review Board.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nam, H.S., Choi, C.W., Kim, S.J. et al. Endoscopic submucosal dissection for gastric indefinite for neoplasia: which lesions should be resected?. Surg Endosc 33, 3976–3983 (2019). https://doi.org/10.1007/s00464-019-06686-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06686-1