Abstract
Background
Treatment with endoscopic submucosal dissection (ESD) for gastric category 3 lesion (low grade dysplasia, LGD) diagnosed by endoscopic forceps biopsy (EFB) is controversial.
Aims
The purpose of the present study was to validate the use of ESD for gastric LGD diagnosed by EFB and to evaluate predictable factors for pathologic upgrade diagnosis to category 4 (high grade dysplasia, HGD) or 5 (early gastric cancer, EGC) lesions.
Methods
Between November 2008 and October 2011, a retrospective analysis of a prospective database was conducted at a single tertiary referral center. A total of 218 ESD procedures were carried out for gastric LGD lesions identified by EFB. The under-diagnosis rate by EFB and the predictable factors for upgrade diagnosis to category 4 or 5 lesions were analyzed.
Results
Pathologic discrepancy between EFB and surgical resection was 20.1 % (44/218). Thirty eight lesions (17.4 %) were diagnosed HGD or EGC by ESD. Gastric HGD lesions were 14 cases (6.4 %) and EGC lesions were 24 cases (23 mucosal and 1 submucosal cancer) (11.0 %). Multivariate analysis revealed that lesion diameter more than 1 cm (OR 3.496 [95 % CI 1.375–8.849]), surface redness (OR 6.493 [95 % CI 2.557–16.666]) and nodular surface (OR 2.762 [95 % CI 1.237–6.172]) were significant risk factors.
Conclusions
Endoscopic resection can be recommended if a LGD lesion has risk factors such as a size of 1 cm or greater, surface redness or surface nodulariy. For lesions without the risk factors, follow-up endoscopy may be recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer remains a major problem worldwide. The intestinal type of gastric cancer develops through a cascade of well-defined and recognizable precursors (inflammation–metaplasia–dysplasia–carcinoma sequence) [1]. Gastric adenoma/dysplasia is regarded as a precancerous lesion and the risk of carcinoma generally increases with the histological grade of the dysplasia (low grade to high grade dysplasia) [2, 3]. To date, the natural history of gastric adenoma/dysplasia is still uncertain. Previous reports have strongly suggested that high grade dysplasia (category 4 in the Vienna classification) is highly predictive of invasive carcinoma, which either coexists or appears within a short time after biopsy [4, 5]. Therefore, from the revised Vienna classification, high grade dysplasia should be removed by endoscopic resection [6]. However, data on the clinical significance of low-grade adenoma/dysplasia (category 3 in the Vienna classification) are still scant. Even though low-grade adenoma/dysplasia lesions are commonly found in everyday practice, there are no international recommendations to guide. Correct diagnosis and grading of dysplasia are critical, because the reported progression rates of dysplasia to gastric cancer vary greatly, from 0 to 73 % per year [4, 7–10]. Moreover, gastric adenoma and well-differentiated adenocarcinoma are often difficult to differentiate based on the histopathological findings of biopsy specimens. Correct diagnosis and grading of dysplasia can predict both the risk of malignant transformation and the risk of metachronous gastric cancer. Standardization of the low grade dysplasia lesions and focusing on patients with the risk of progression of higher risk lesions may be cost-effective.
Endoscopic submucosal dissection (ESD) is a useful procedure for the treatment of early gastric neoplasms because it facilitates en bloc resection [11, 12]. ESD allows for a higher en bloc resection rate for larger lesions, and thus a pathological diagnosis can be made more accurately than other endoscopic techniques [11, 13]. A recent multicenter study conducted with more than 1,000 patients with gastric neoplasm reported that the ESD-related complication rate was relatively low [14]; however, evidence regarding the appropriate treatment strategy are still insufficient to decide whether gastric low grade adenoma/dysplasia, which progresses slowly to invasive neoplasia, should be observed with regular follow-ups or should be removed by ESD/EMR. In particular, the validation of ESD for gastric low grade adenoma/dysplasia has been equivocal.
We performed ESD for gastric category 3 lesions (low grade dysplasia, LGD) identified by endoscopic forceps biopsy (EFB) and aimed to determined predictive factors for gastric category 4 or 5 lesions (high grade dysplasia, HGD or early gastric cancer, EGC).
Patients and Methods
Patients
Between November 2008 and October 2011, 413 ESD procedures were carried out in Pusan National University Yangsan Hospital in Korea. After exclusion, 218 cases of gastric LGD identified by endoscopic forceps biopsy (EFB) were included in this study. A prospective database was retrospectively analyzed for this study. Exclusion criteria were adenocarcinoma or HGD identified by EFB. Written informed consent for the endoscopic submucosal dissection was obtained from all patients before the procedure.
ESD Procedure
Two endoscopists (CW Choi and HW Kim) were involved in the diagnostic and therapeutic endoscopy. Each endoscopist had performed more than 2,000 diagnostic endoscopies and at least 50 ESD procedures at the beginning of the study. We performed ESD in three steps; first, normal saline with epinephrine and indigocarmine mixture was injected into the submucosal layer to elevate the lesion from the muscularis propria after marking around the lesion; then, precutting the mucosa surrounding the lesion was performed with the use of an electrosurgical generator (ERBE VIO 300D, Endocut I mode, Effect 3, duration 2; Erbe Co, Tubingen, Germany) by a flex knife or an insulation-tipped electrosurgical knife 2 (IT 2); lastly, the connective tissue of the submucosa beneath the lesion was dissected with an IT 2 or a flex knife with coagulation current (Swift coagulation 60W, ERBE VIO 300D). A proton pump inhibitor (pantoprazole, 40 mg/d) was intravenously administered for 2 days after ESD and then orally for 2 months for all patients.
Clinicopathologic Factors
Baseline characteristics and endoscopic findings were assessed. Each endoscopic report was reviewed to determine the maximum diameter and macroscopic appearance of the lesions. Endoscopic photographs were reviewed in all cases. One endoscopist (CW Choi) reviewed and analyzed the data. The Paris classification [15] was used to define the gross types of the superficial lesions, which were classified into elevated, flat, or depressed. The surface redness, erosion, nodularity, ulceration, submucosal fibrosis and location of the lesions were also evaluated. Surface redness was defined as red discoloration on the mucosal surface of the lesion compared to the surrounding mucosa. Surface nodularity was defined as the presence of irregularly raised or nodular mucosa. Lesions with ulcerations or scarring secondary to previous ulceration (converging folds or deformity of the muscularis propria, or fibrosis in the submucosa) were regarded as ulcerations. The location of lesions was described using the Japanese Classification of Gastric Cancer [16]. In this system, the gastric area is divided into three equal sections: upper, middle, and lower.
All of the endoscopic forceps biopsy samples and resected tissue slides were blindly reviewed by two pathologists (DH Shin and JH Lee). Discordant cases were reevaluated under multi headed microscope to reach agreement. The resected specimens were stretched, pinned, and fixed with formalin. Piecemeal-resected specimens were reconstructed as much as possible. The fixed specimen was sectioned at 2-mm intervals. All of the lesions were classified as gastrointestinal epithelial neoplasia according to Vienna classification, i.e. low grade adenoma/dysplasia in category 3 lesion, high grade adenoma/dysplasia or noninvasive carcinoma in category 4 lesion, and intramucosal carcinoma or submucosal carcinoma or beyond in category 5 (Table 1) [17].
The “under-diagnosis” rate was defined as the proportion of lesions diagnosed as high grade dysplasia or adenocarcinoma (category 4 or 5) after ESD.
Statistical Analysis
Univariate analysis with chi-square test or Fisher’s exact test for categorical variables and Student’s t test for continuous variables were performed. Variables with p < 0.05 in univariate analysis were included in a forward stepwise multiple logistic regression model to identify independent associated risk factors for early gastric cancer. A value of p < 0.05 indicated statistical significance. Statistical calculations were performed with SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
During the study period, 218 ESD procedures were performed for patients with gastric LGD lesions diagnosed by EFB. Clinical baseline features of the enrolled patients are shown in Table 2. The mean age was 62.00 ± 9.27 years. The most predominant location was the lower third (177/218, 81.2 %). The most common gross type was the elevated type (155/218, 71.1 %).
After ESD, the en bloc resection rate and complete resection rate were 96.8 % (211/218) and 95.4 % (208/211), respectively. The perforation rate and delayed bleeding rate was 0.4 % (1/218) and 2 % (4/218), respectively. Microperforation was resolved by conservative care and bleeding was successfully controlled by endoscopic intervention. No surgical intervention was needed.
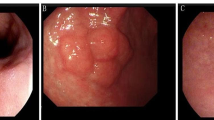
After resection, the pathologic discrepancy rate between EFB and ESD was 20.1 % (44/218). The under-diagnosis rate was 17.4 % (38/218). The over-diagnosis rate was 2.8 % (6/218). Among under-diagnostic lesions, HGD lesions were 14 cases (6.4 %) and EGC lesions were 24 cases (11.0 %, 23 intra-mucosal carcinoma and one submucosal lesion). Among resected specimens, the HGD or EGC lesions focally exist (0.2–1.6 cm, range) within background low grade dysplasia (Fig. 1). During the follow-up, two cases of local recurrence (0.9 %) which was associated with incomplete resection were reported.
A case of upgrade diagnosis to high grade dysplasia. a Conventional endoscopic image: the lesion located at the antrum greater curvature-posterior wall side. b Narrow band image. c Endoscopic submucosal dissection (ESD) specimen (long diameter 3.2 cm). d Gross picture fixed with formalin (blue line indicates low grade dysplasia, red line indicates carcinoma). e Characteristic histological pictures of low grade dysplasia from ESD specimen. f Characteristic histological pictures of carcinoma from ESD specimen
Comparison analysis between ESD pathologies (category 1–3 vs. category 4–5) showed that there were significant differences in lesion diameter, surface redness and surface nodularity (p = 0.001, <0.001 and 0.009 respectively) but not in other clinical variables (Table 3).
Multivariate analysis for the associated risk factors for category 4–5 lesions after ESD was performed. After adjustment for patient age, sex and macroscopic type, lesion diameter more than 1 cm (OR 3.496 [95 % CI 1.375–8.849]), surface redness (OR 6.493 [95 % CI 2.557–16.666]) and nodular surface (OR 2.762 [95 % CI 1.237–6.172]) were identified as significant risk factors (Table 4).
Discussion
Endoscopic resection is strongly recommended for HGD (category 4 lesion), but therapeutic guidelines have not yet been established for gastric LGD (category 3 lesion). Although an endoscopic biopsy is the best method to diagnose the dysplastic lesions, sometimes it is difficult to rule out malignancy because the histopathology of the lesion is not homogeneous. Therefore, an initial finding of a LGD cannot exclude the presence of a HGD or a focus of carcinoma in an unsampled part of the lesion. Long-term follow-up studies have demonstrated that LGD lesions do not progress rapidly to HGD or carcinoma, thus some authors have advocated a management approach of scheduled endoscopic surveillance and re-biopsy [7, 8, 18]. However, others have suggested removal of the lesions because of the histological discrepancy between forceps biopsy and resected specimens [19, 20]. Recent studies showed the underdiagnosis rate up to 37–40 % [19, 20]. The discrepancy rate in the present study was 20.1 % (44/218). The reasons for the difficulty in making an accurate diagnosis based on the initial biopsy specimen are as follows: (1) the structural atypia of both adenoma and well-differentiated adenocarcinoma is too subtle to detect in a small biopsy specimen, (2) cancer sometimes exists focally in the lesion and sampling error might occur, and (3) the regeneration of tissue showing atypia induced by gastritis induces histological modification [19]. On examination of resected specimens in the present study, HGD or EGC lesions exist either focally or intermixed within background LGD lesions. Therefore, sample error from EFB might be the major cause of the underdiagnosis.
Conventional endoscopy with biopsy may be insufficient to make a precise diagnosis of dysplastic lesions. Recently, endoscopic imaging techniques, such as magnifying narrow-band imaging (NBI) endoscopy, were reported to be capable of predicting histologic characteristics of EGC [21] and the histologic severity of gastritis [22]. Yao et al. [23] reported that the finding of a white opaque substance on magnified endoscopy with NBI differentiated non-invasive neoplasm with a sensitivity of 94 % and a specificity of 96 %. Although the use of an image-enhanced endoscope (IEE) is a promising method to improve the diagnostic accuracy of non-invasive neoplasm, it is not clear yet whether it is clinically useful, because of expert bias. To be a useful adjunctive for the diagnosis of gastric dysplasia, further studies are warranted.
Many risk factors associated with malignant transformation of dysplasia were reported. Lesion diameter more than 1 cm [24] and the surface appearance of dysplasia such as depressed morphology [5], erythema and surface erosions [3], are reported as associated with malignancy. In the present study, we observed a significant correlation with lesion size, surface redness and surface nodularity.
In this study, the under-diagnosis rate in gastric LGD was 17.4 %. Previous prospective long-term follow-up studies have indicated that the 5-year gastric cancer incidence in gastric LGD ranges from 17 to 30 %, and non-invasive neoplasm is considered to be a premalignant lesion [25]. Current knowledge also indicates that gastric LGD is not only a premalignant lesion, but that malignancies are detected in 11.4 % of cases. Endoscopic mucosal resection (EMR) with a snare enables accurate histopathological diagnosis by resecting the lesion as a large piece. EMR is limited, however, in that it sometimes results in a multiple piecemeal resection. Multiple piecemeal resection is associated with a specimen burning effect that interferes with an accurate pathological diagnosis. Additionally, a local recurrence may occur, with a reported incidence of approximately 10 % [20]. In the present study, two cases of local recurrence were associated with incomplete resection. To overcome these problems, the ESD technique was developed to remove early gastric neoplasm. ESD makes it possible to perform complete resection regardless of size and location. A previous report showed that the EMR group had a significantly higher recurrence rate than the ESD group (18 vs. 3.7 %, respectively, p < 0.001) [26]. However, ESD requires advanced techniques; furthermore, there is a high frequency of complications associated with the procedure [11, 12]. To evaluate the efficacy of ESD for gastric LGD, we compared the risks and benefits of ESD. First, a diagnostic discrepancy rate was 20.1 % comparing pathologic results of EFB and ESD. Second, the complete en bloc resection rate was excellent with the rate of 95.4 %. Third, the serious complication rate was low. The perforation rate was 0.4 % and significant delayed bleeding was 2 %. All these complications were successfully treated with the endoscopic maneuver. Surgical treatment was not needed. This result seems to be acceptable.
There are several limitations in the present study. First, this retrospective study of gastric adenomas may involve selection bias. Second, we used only lesion diameter and macroscopic appearances of lesion for the analyses. If recent diagnostic technology such as IEE is used, more accurate preoperative diagnosis may be accomplished.
In summary, the therapeutic strategy for gastric LGD (category 3) is controversial. Some discrepancies can exist between EFB samples and resected specimens because EFB tissues may not be representative of the entire adenomatous lesion. This study's results show that there is a considerable risk of under-diagnosis of higher grade of lesions (category 4 or 5). A follow-up strategy might miss the chance for endoscopic therapy. Furthermore, repeated endoscopic examination with biopsies burdens the patient with physiological, psychological, and financial strains. Precautions should be taken in the management of patients with gastric LGD, especially when lesion size of more than 1 cm, surface redness and nodular surface are present. Endoscopic resection for diagnostic and therapeutic purposes can be considered. Although bleeding and perforation were found in a small number of the patients in the present study, all the complications were controlled by endoscopic and conservative management. Therefore, we suppose that ESD may be a therapeutic option for gastric LGD for risk factors such as a size of 1 cm or greater, surface redness or surface nodulariy. For lesions that have none of the risk factors, follow-up endoscopy may be recommended.
References
Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560.
Ming SC, Bajtai A, Correa P, et al. Gastric dysplasia. Significance and pathologic criteria. Cancer. 1984;54:1794–1801.
Goldstein NS, Lewin KJ. Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol. 1997;28:127–133.
Rugge M, Farinati F, Baffa R, et al. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology. 1994;107:1288–1296.
Park DI, Rhee PL, Kim JE, et al. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001;33:501–506.
Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131.
Park SY, Jeon SW, Jung MK, et al. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966–970.
Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390–396.
de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952.
Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378–381.
Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942.
Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77:23–28.
Takeuchi Y, Uedo N, Iishi H, et al. Endoscopic submucosal dissection with insulated-tip knife for large mucosal early gastric cancer: a feasibility study (with videos). Gastrointest Endosc. 2007;66:186–193.
Akasaka T, Nishida T, Tsutsui S, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by Osaka University ESD study group. Dig Endosc. 2011;23:73–77.
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112.
Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255.
Srivastava A, Lauwers GY. Gastric epithelial dysplasia: the Western perspective. Dig Liver Dis. 2008;40:641–649.
Kato M, Nishida T, Tsutsui S, et al. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group. J Gastroenterol. 2011;46:325–331.
Szaloki T, Toth V, Tiszlavicz L, Czako L. Flat gastric polyps: results of forceps biopsy, endoscopic mucosal resection, and long-term follow-up. Scand J Gastroenterol. 2006;41:1105–1109.
Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080–1084.
Tahara T, Shibata T, Nakamura M, et al. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246–253.
Yao K, Iwashita A, Tanabe H, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008;68:574–580.
Cho SJ, Choi IJ, Kim CG, et al. Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy. 2011;43:465–471.
Rugge M, Cassaro M, Di Mario F, et al. The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111–1116.
Park JC, Lee SK, Seo JH, et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24:2842–2849.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A091047).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, C.W., Kim, H.W., Shin, D.H. et al. The Risk Factors for Discrepancy After Endoscopic Submucosal Dissection of Gastric Category 3 Lesion (Low Grade Dysplasia). Dig Dis Sci 59, 421–427 (2014). https://doi.org/10.1007/s10620-013-2874-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2874-8