Abstract
Background
The proportion of elderly patients who undergo surgery has rapidly increased. However, clinical indicators that predict outcomes are limited. Frailty is thought to estimate physiological reserves, although its use has not been evaluated in laparoscopic surgical patients. This study aimed to evaluate the significance of preoperative modified frailty index (PMFI) in octogenarians undergoing a laparoscopic gastrectomy.
Methods
We reviewed prospectively collected data from 119 patients with gastric cancer (GC) aged 80 years or older who underwent a radical laparoscopic gastrectomy (RLG) between January 2007 and December 2012. Three baseline frailty traits were measured using routine preoperative laboratory data: albumin < 3.4 g/dL, haematocrit < 35%, and creatinine > 2 mg/dL. Patients were categorized by the number of positive traits as follows: low preoperative modified frailty index (LPMFI): 0–2 traits and high preoperative modified frailty index (HPMFI): 3 traits. We compared patient characteristics, operative outcomes, pathological results, morbidity, and survival.
Results
A total of 43 (36.1%) patients were considered HPMFI, and 76 (63.9%) patients were considered LPMFI. HPMFI was associated with an increased risk of postoperative complications (HPMFI group: odds ratio 2.506; 95% CI, 1.113–5.643, P = 0.027). With a median follow-up of 39.0 months, the 3-year overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS) rates for the entire cohort were 47.9, 34.3, and 51.7%, respectively. Significant differences were observed in OS (HPMFI group, 37.2%; LPMFI group, 53.9%; P = 0.038) and RFS (HPMFI group, 23.3%; LPMFI group, 40.5%; P = 0.012) between the groups, but no difference was found for CSS (HPMFI group, 43.5%; LPMFI group, 56.4%; P = 0.078).
Conclusions
HPMFI based on an easily calculable preoperative measure may be useful for predicting postoperative complications and have a negative impact on 3-year OS and RFS after an RLG in octogenarians. Therefore, HPMFI can serve as a low-cost, simple screen for high-risk individuals who might suffer more than expected during the postoperative period after an RLG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the global increase in the ageing population and rapid developments in medicine, the proportion of octogenarians undergoing surgery is expected to increase each year [1, 2]. However, because octogenarian patients often have a variety of comorbidities, their abilities to endure both surgery and anaesthesia are reduced, potentially leading to a number of postoperative complications or death [3, 4]. Therefore, it is necessary to carefully evaluate the physiological condition of octogenarian patients to accurately evaluate the level of operative risk and predict postoperative outcomes.
Frailty is a geriatric syndrome thought to reflect an age-related, multi-system deterioration in function characterized by reduced stamina reserves. Frailty is associated with increased hospitalizations, disability, death, and other adverse outcomes [5]. Recent studies have found that preoperative frailty (PF) is a better predictor of postoperative complications than age [6, 7]. Furthermore, many scholars, including Ma L, have reported that PF is closely associated with postoperative long-term outcomes for octogenarian patients [8, 9]. Aged patients with gastric cancer (GC), for example, have higher morbidity and mortality after a laparotomy. Therefore, surgical procedures must be carefully chosen with emphasis on both safety and efficacy [10, 11]. A radical laparoscopic gastrectomy (RLG) has been associated with several benefits, such as a shorter hospital stay, earlier mobilization, fewer pulmonary complications, and earlier functional recovery [12, 13]. In recent years, an increasing number of aged patients with GC have been treated with a laparoscopic gastrectomy and have achieved good results [14, 15]. However, PF has not been commonly used to predict the prognosis of octogenarians with GC after gastrectomy, especially laparoscopic gastrectomy. The problems faced in the frailty literature of using other tools are cumbersome. It is necessary to use simple tools that can be applied to routine practice. The aim of this study is to use the preoperative modified frailty index to assess the risk of surgery in patients with laparoscopic gastrectomy.
Materials and methods
Materials
We reviewed prospectively collected data of aged patients who were diagnosed with primary GC and who underwent an RLG by the same group of surgeons at Fujian Medical University Union Hospital between January 2007 and December 2012. The inclusion criteria were as follows: (1) an age than 80 years, (2) a diagnosis of primary GC based on a pathology report, without evidence of distant metastasis, (3) an R0 resection, and (4) no preoperative chemoradiotherapy. The exclusion criteria included (1) the presence of other malignancies, (2) a preoperative or intraoperative examination showing distant metastasis, (3) T4b tumours, (4) lack of a pathologically confirmed diagnosis, and (5) conversion to laparotomy. A total of 119 patients were included (Fig. 1). All patients voluntarily selected laparoscopic surgery and signed an informed consent form. Lymph node dissection and postoperative pathological examinations were conducted in accordance with the 14th edition of Japan’s Stomach Cancer Treatment Statute [16]. Staging was determined according to the 7th edition of the International Union Against Cancer (UICC) TNM classification [17]. After surgery, 91 (76.47%) patients with stage II or higher received postoperative adjuvant chemotherapy with 5-FU-based regimens: 31 (72.09%) in the HPMFI group and 60 (78.95%) in the LPMFI group.
Preoperative care pathway and surgical procedure
After confirming patients with gastric cancer by gastroscopic pathology, preoperative blood routine, urine routine, stool routine, biochemical whole set, abdominal enhanced CT, chest CT, Ultrasonic cardiogram, pulmonary function, blood type cross examination were performed. For patients without obvious surgical contraindications, laparoscopic gastrectomy was performed.
Laparoscopic curative gastrectomies with radical LNs dissection were performed following the Japanese Gastric Cancer Treatment Guidelines. Patients were placed in the supine position, with legs apart and 20°–30° head-up tilt. The surgeon stood on the left of the patient, the assistant surgeon stood on patient’s right, and the video laparoscope operator stood between the patient’s legs. Five trocars were used; one 10-mm trocar for the laparoscope was inserted below the umbilicus. One 12-mm trocar was inserted in the left pre-axillary line 2 cm below the costal margin as a major hand port. A 5-mm trocar was placed at the contralateral site for traction and exposure of the liver. A 5-mm trocar was inserted as an accessory port in the left and right mid-clavicular line 2 cm above the level of the umbilicus. The operative technique for the LG procedures has previously been described in detail [18]. After the laparoscopic operation, a small laparotomy incision was made under the xyphoid (5–7 cm). Distal gastrectomy with Billroth I, Billroth II or total gastrectomy with Roux-en-Y anastomosis was extra corporeally performed using the hand-sewn method. The specimen was pulled out of the peritoneal cavity through the small laparotomy incision.
Follow-up
Trained personnel from the outpatient clinic were responsible for visiting patients, posting letters, and conducting phone calls to follow up with the patients after surgery. The last follow-up evaluation was conducted in June 2017. Most routine follow-up appointments included a physical examination, laboratory tests (including measurement of CA19-9, CA72-4, and CEA levels), chest radiography, and abdominopelvic ultrasonography or computed tomography, as well as an annual endoscopic examination. Overall survival (OS) was defined from the day of surgery until death or until the final follow-up date of June 2017, whichever occurred first. Disease-free survival was defined from the date of surgery to recurrence or death. Tumour recurrence was confirmed by radiologic or pathologic identification of local recurrence or distant metastasis. Cancer-specific survival (CCS) was measured from the date of surgery to death from cancer.
Definitions
Three baseline frailty traits were measured using preoperative laboratory data [19]: albumin < 3.4 g/dL, haematocrit < 35%, and creatinine > 2 mg/dL. Patients were categorized by the number of positive traits as follows: HPMFI (n = 43): 3 traits and LPMFI (n = 76): 0–2 traits [19]. The definition of each complication was based on the literature [20]. Complications were classified according to the modified version of the Clavien–Dindo classification system reported by Dindo et al. [21].
Statistical analysis
All of the statistical analyses were conducted with SPSS, version 18.0 for Windows (SPSS Inc, Chicago, IL, United States). Continuous data are expressed as means ± SD. Categorical variables were analysed with the χ2 test or Fisher’s exact test, while continuous variables were analysed with Student’s t test. Variables with a value of P < 0.05 in the univariate analysis were subsequently included in a multivariate binary logistic regression model. Variables that remained significant in the multivariate analysis were considered independent risk factors. Kaplan–Meier curves were used to estimate OS time, and univariate comparisons were performed between groups using the log-rank test. P values < 0.05 were considered statistically significant.
Results
Clinicopathological characteristics
The characteristics of the patients (43 HPMFI vs. 76 LPMFI) are listed in Table 1. The mean age was 82.0 ± 2.4 years. A total of 36.1% (43/119) of patients were diagnosed with HPMFI. The albumin level of the HPMFI group was significantly lower than that of the LPMFI group. The serum creatinine level, the proportion of haematocrit < 35%, and the anaesthesia risk score (ASA score) were significantly higher in the HPMFI group. No significant differences were observed between the two groups in terms of age, gender, tumour size, tumour site, tumour differentiation, excision extension, body mass index (BMI), and tumour stage (Table 1).
Perioperative outcomes
Compared to the LPMFI group, the operative time was longer (174.4 vs. 154.5 min, P = 0.037) and n significant differences were observed between the two groups in terms of intraoperative blood loss, number of lymph node dissections, time to first flatus, need for a liquid diet, and duration of postoperative hospital stay (Table 2).
Postoperative complications
Table 3 details the incidence of postoperative complications. Complication was higher (55.8% vs. 30.3%, P = 0.006) in the HPMFI group. Systemic complications (P = 0.049) and Grade II complications (P = 0.041) were more frequent in HPMFI group. However, there was no significant difference in local complications between the two groups (P = 0.706).
Risk factors influencing complications after RLG for postoperative complications
The univariate analysis showed that HPMFI, a BMI ≥ 25 kg/m2, and an operative time ≥ 240 min were associated with an increased risk of postoperative complications. The multivariate analysis revealed that HPMFI [odds ratio (OR) 2.506, P = 0.027], BMI ≥ 25 kg/m2 (OR 1.829, P = 0.047), and operative time ≥ 240 min (OR 2.862, P = 0.012) were independent risk factors for postoperative complications (Table 4).
Long-term outcomes
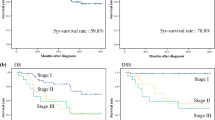
The median follow-up period was 37 (range 2–68) months. The 3-year OS rate, RFS rate, and CSS rate were 47.9, 34.3, and 51.7%, respectively (Fig. 2). The 3-year OS rate (37.2% vs. 53.9%, P = 0.038) and the 3-year RFS rate (23.3% vs. 40.5%, P = 0.012) were lower in the HPMFI group than in the LPMFI group. No significant difference was found between the two groups for the CSS rate (43.5% vs. 56.4%, P = 0.078) (Fig. 3).
Discussion
With the rapidly growing ageing population in society, the proportion of older patients with GC who undergo surgery is expected to increase [22]. However, both morbidity and mortality are high in aged patients with GC [5,6,7,8]. Some researchers have used the ASA score and preoperative comorbidities indexes to predict the postoperative efficacy of treatment of elderly patients with GC, but the predictive value is limited due to the failure to include the reserve function [23]. Recently, increasing evidence has indicated that PF is a better predictor of short- and long-term outcomes for elderly patients who undergo surgery [24, 25]. Some studies have reported that PF is associated with increased in-hospital mortality, serious complications, and prolonged hospitalization for older patients who undergo GC surgery [26, 27]. With the development of minimally invasive techniques, older patients are increasingly choosing to undergo minimally invasive surgery [28, 29]. However, few studies have evaluated the relationship between PF and the short- and long-term outcomes of octogenarians with GC after a laparoscopic gastrectomy. Therefore, in this study, we assessed these relationships in aged patients with GC undergoing a laparoscopic gastrectomy.
Presently, there is no standardized method of measuring physiologic reserves in older surgical patients. A retrospective study found that frailty can be assessed using the Groningen Frailty Indicator (GFI) [27]. Martin et al. evaluated frailty based on a validated scoring system that characterizes frailty as an age-associated decline in five domains: shrinking, weakness, exhaustion, low physical activity, and slowed walking speed [25]. The Edmonton frail scale is a simple brief, user friendly screening tool for frailty in older patients. It measures cognitive impairment, balance/mobility, mood, functional independence, medication use, social support, nutrition, health attitudes, continence, burden of medical illness, and quality of life. It has been shown to be a valid measure of frailty and a reliable tool that can be completed by people without special training in geriatric medicine [30,31,32]. Compared with the Edmonton frail scale, our modified frailty index which used routine preoperative laboratory data (i.e. albumin < 3.4 g/dL, haematocrit < 35%, and creatinine > 2 mg/dL) may be easier to obtain, simpler and more suitable for clinical application [19].
Revenig et al. assessed 80 patients who underwent minimally invasive surgery and found that the incidence of 30-day complications was higher in patients with PF than in non-frail patients [33]. Obeid et al. also found that elderly patients with PF have a significantly higher risk of grade IV–V complications (OR 14.4, P = 0.001) after laparoscopic colon surgery [34]. Similar to previously reported results, the incidence of postoperative complications in our study was 55.8% in the HPMFI group, which was significantly higher than that in the LPMFI group (30.3%, P = 0.006). PF is clearly associated with prolonged hospitalization in elderly patients [25]. We also found that hospitalization was slightly longer in the HPMFI group, although the difference was not significant. Makary et al. found that PF is an independent risk factor for postoperative complications and increases the risk of morbidity by 2.54 times compared to patients without PF [25]. This latter study also found that HPMFI measurement significantly improves the ability to predict postoperative complications. In our study, the univariate and multivariate analysis showed that HPMFI was an independent risk factor for postoperative complications; moreover, we found that HPMFI have better predictive power to identify patients at risk compared to classical parameters such as ASA classification.
PF not only can significantly affect postoperative complications in elderly patients, but it can also affect the long-term prognosis. Fried et al. found that the 3- and 7-year mortality rates for the PF group were 6 and 3 times higher than those of the non-PF group, respectively. Furthermore, PF is an independent predictor of death [28]. Green et al. evaluated 244 patients who underwent aortic valve replacement. The 1-year mortality rate in the PF group was clearly higher than that in the non-PF group (32.7% vs. 15.9%, P = 0.004) [35]. In our study, the 3-year OS and RFS rates of the HPMFI group were significantly lower than those of the LPMFI group, although no significant difference was observed between the two groups regarding the 3-year CSS rate. We believe that because immune function, nutritional status, and preoperative comorbidities were poorer in the HPMFI group than in the LPMFI group, the former are more prone to death, and therefore the RFS rate is low. It is well known that CSS is closely related to the pathological staging of the tumour, tumour size, and differentiation. In our study, the tumour pathological characteristics of the two groups were similar, resulting in a similar CSS rate. Migita et al. [36] found that the nutrition index (PNI) is closely related to the long-term prognosis of patients with GC who have undergone a gastrectomy. Wagner et al. [37] showed that PF predicts 1-year mortality for older patients who have undergone surgery, although the effect on the long-term prognosis was not reported. To our knowledge, no report has described the effect of the HPMFI on the long-term prognosis of elderly patients who have undergone an RLG.
Some shortcomings are present study in the current. First, this study is a single-centre retrospective study. Second, the patients included in this study lived in the Eastern hemisphere, and therefore, the results may not apply to individuals in the Western hemisphere.
In conclusion, this study is the first to report that HPMFI, which can be quickly determined by easily acquired preoperative blood indicators, can significantly affect the incidence of postoperative complications and the OS and RFS rates for octogenarian patients undergoing an RLG. These findings may provide a reference for surgeons to design individualized treatment strategies for this group of elderly patients. However, large sample, multicentre, randomized controlled trials are required to confirm these results.
References
Shin A, Kim J, Park S (2011) Gastric cancer epidemiology in Korea. J Gastric Cancer 11:135–140
Korc-Grodzicki B, Downey RJ, Shahrokni A, Kingham TP, Patel SG, Audisio RA (2014) Surgical considerations in older adults with cancer. J Clin Oncol 32:2647–2653
Takeshita H, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E (2013) Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg 37:2891–2898
Fujiwara Y, Tsujie M, Hara J, Kato H, Kitani K, Isono S, Takeyama H, Yukawa M, Inoue M, Kanaizumi H (2014) Comparison of gastric cancer surgery between patients aged> 80 years and < 79 years: complications and multivariate analysis of prognostic factors. Hepato-Gastroenterol 61:1785–1793
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381:752–762
Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, Sharp TJ, Moss M (2009) Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 250:449–455
Oresanya LB, Lyons WL, Finlayson E (2014) Preoperative assessment of the older patient: a narrative review. JAMA 311:2110–2120
Ma L, Zhang L, Tang Z, Sun F, Diao L, Wang J, Zhao X, Ge G (2016) Use of the frailty index in evaluating the prognosis of older people in Beijing: a cohort study with an 8-year follow-up. Arch Gerontol Geriatr 64:172–177
Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, Chemaitilly W, Smith WA, Wilson CL, Sklar CA, Shelton K, Srivastava DK, Ali S, Robison LL, Hudson MM (2013) Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude lifetime cohort study. J Clin Oncol 31:4496–4503
Jeong O, Park YK, Ryu SY, Kim YJ (2010) Effect of age on surgical outcomes of extended gastrectomy with D2 lymph node dissection in gastric carcinoma: prospective cohort study. Ann Surg Oncol 17:1589–1596
Hayashi T, Yoshikawa T, Aoyama T, Ogata T, Cho H, Tsuburaya A (2012) Severity of complications after gastrectomy in elderly patients with gastric cancer. World J Surg 36:2139–2145
Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y (2002) A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 131(1 Suppl):S306–S311
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241:232–237
Kim MG, Kim HS, Kim BS, Kwon SJ (2013) The impact of old age on surgical outcomes of totally laparoscopic gastrectomy for gastric cancer. Surg Endosc 27:3990–3997
Yasuda K, Sonoda K, Shiroshita H, Inomata M, Shiraishi N, Kitano S (2004) Laparoscopically assisted distal gastrectomy for early gastric cancer in the elderly. Br J Surg 91:1061–1065
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Sobin LH, Gospodarowicz MK, Wittekind C (2010) International Union Against Cancer (UICC) TNM classification of Malignanttumours, 7th edn. Wiley, New York
Huang CM, Zheng CH (2015) Laparoscopic gastrectomy for gastric cancer. Springer, Berlin
Lu J et al (2017) The preoperative frailty versus inflammation-based prognostic score: which is better as an objective predictor for gastric cancer patients 80 years and older? Ann Surg Oncol 24(3):754–762
Huang C-M, Tu R-H, Lin J-X (2015) A scoring system to predict the risk of postoperative complications after laparoscopic gastrectomy for gastric cancer based on a large-scale retrospective study. Medicine 94(17):e812
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Polanczyk CA, Marcantonio E, Goldman L, Rohde LE, Orav J, Mangione CM, Lee TH (2001) Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med 134:637–643
Koo CY, Hyder JA, Wanderer JP, Eikermann M, Ramachandran SK (2015) A meta-analysis of the predictive accuracy of postoperative mortality using the American Society of Anesthesiologists’ physical status classification system. World J Surg 39:88–103
Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Moss M (2013) Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 206:544–550
Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP (2010) Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 210:901–908
Tegels JJ, de Maat MF, Hulsewé KW, Hoofwijk AG, Stoot JH (2014) Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointest Surg 18:439–445 (discussion: 445–446)
Tegels JJ, de Maat MF, Hulsewé KW, Hoofwijk AG, Stoot JH (2014) Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointest Surg 18:439–446
Mochiki E, Ohno T, Kamiyama Y, Aihara R, Nakabayashi T, Asao T, Kuwano H (2005) Laparoscopy-assisted gastrectomy for early gastric cancer in young and elderly patients. World J Surg 29:1585–1591
Barbosa J, Carneiro S, Preto J, Sousa H, Pinho A, Maia C (2013) Laparoscopic gastrectomy after the age of eighty: still a good choice? J Cancer Ther 4:27–30
Keenan LG et al (2017) Assessment of older patients with cancer: Edmonton Frail Scale (EFS) as a predictor of adverse outcomes in older patients undergoing radiotherapy. J Geriatr Oncol 8(3):206–210
Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K (2006) Validity and reliability of the Edmonton Frail Scale. Age Ageing 35(5):526–529
Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC (2012) The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist 17(11):1439–1449
Revenig LM, Canter DJ, Master VA, Maithel SK, Kooby DA, Pattaras JG, Tai C, Ogan K (2014) A prospective study examining the association between preoperative frailty and postoperative complications in patients undergoing minimally invasive surgery. J Endourol 28:476–480
Obeid NM, Azuh O, Reddy S, Webb S, Reickert C, Velanovich V, Horst HM, Rubinfeld I (2012) Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg 72:878–883
Green P, Arnold SV, Cohen DJ, Kirtane AJ, Kodali SK, Brown DL, Rihal CS, Xu K, Lei Y, Hawkey MC, Kim RJ, Alu MC, Leon MB, Mack MJ (2015) Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol 116:264–269
Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y (2013) The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol 20:2647–2654
Wagner D, Büttner S, Kim Y, Gani F, Xu L, Margonis GA, Amini N, Kamel IR, Pawlik TM (2016) Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. Br J Surg 103:e83–e92
Acknowledgements
The authors are thankful to Fujian Medical University Union Hospital for their management of our gastric cancer patient database. All the authors are grateful for the statistics related consultation provided by the Public Health School of Fujian Medical University.
Funding
National key clinical specialty discipline construction program of China (No. [2012]649). Scientific and technological innovation joint capital projects of Fujian Province, China (No.2016Y9031). Minimally invasive medical centre of Fujian Province (2011708#). The youth research project of Fujian Provincial Health and Family Planning Commission (2014-1-48). QIHANG funds of Fujian Medical University (No. 2016QH025). Fujian nature and health joint fund project (2015J01464).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Jun Lu, Chao-Hui Zheng, and Chang-Ming Huang designed the study; Hua-Long Zheng, Ping Li, Jian-Wei Xie, Jia-bin Wang, Jian-Xian Lin, Qi-Yue Chen, Long-long Cao, Mi Lin, and Ru-Hong Tu collected the data. All the authors participated in interpreting the data, drafting the article, critically revising the paper for content, and providing final approval of the version submitted for publication. All the authors have seen, approved, and are completely familiar with the contents of the manuscript. All the authors are responsible for the accuracy of the manuscript, including the statistical calculations. Jun Lu, Chao-Hui Zheng, Chang-Ming Huang, Hua-Long Zheng, Ping Li, Jian-Wei Xie, Jia-bin Wang, Jian-Xian Lin, Qi-Yue, Chen, Long-long Cao, Mi Lin, and Ru-Hong Tu have no conflicts of interest or financial ties to disclose.
Additional information
Jun Lu and Hua-Long Zheng contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Lu, J., Zheng, HL., Li, P. et al. High preoperative modified frailty index has a negative impact on short- and long-term outcomes of octogenarians with gastric cancer after laparoscopic gastrectomy. Surg Endosc 32, 2193–2200 (2018). https://doi.org/10.1007/s00464-018-6085-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6085-4