Abstract
Background
A learning curve (LC) is a graphic display of the number of consecutive procedures performed necessary to reach competence and is defined by complications and duration of surgery (DOS). There is little evidence on the LC of surgical residents in bariatric surgery. Aim of the study is to evaluate whether the laparoscopic Roux-en-Y gastric bypass (LRYGB) can be safely performed by surgical residents, to evaluate the LC of surgical residents for LRYGB and to assess whether surgical residents fit in the LC of the bariatric center which has been established by their proctors.
Methods
Records of all 3389 consecutive primary LRYGB patients, operated between December 2007 and January 2016 in a bariatric center-of-excellence in Amsterdam, were reviewed. Differences in DOS were assessed by means of a linear regression model. Differences in complications (classified as Clavien-Dindo ≥ 2) were evaluated with the χ 2 or the Fisher exact test. Cases were clustered in groups of 70 for comparison and reported for residents with ≥70 cases as primary surgeon.
Results
Four surgeons (S1-4) and three residents (R1-3) performed 2690 (88.2%) and 361 (11.8%) of 3051 LRYGBs, respectively. Median (IQR) DOS was 52.0 (42.0–65.0) min for S1-4 versus 53.0 (46.0–63.0) min for R1-3 (p = 0.52). The LC of R1-3 in their first 70 cases (n = 210) differs significantly from the individual (n = 70) LCs of surgeon 1, 2, and 3, with remarkably shorter DOS for the residents (adjusted p < 0.0001; p < 0.001 and p = 0.0002, respectively) and the same amount of surgical complications 5.1% (137/2690) for S1-4 versus 3.0% (11/361) for R1-3 (p = 0.089).
Conclusion
Laparoscopic Roux-en-Y gastric bypass can be safely performed by surgical residents under supervision of experienced bariatric surgeons. Surgical residents benefit from the experience of their proctors and they fit faultlessly in the LC of the surgical team, as set out by their proctors in a large bariatric center-of-excellence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The laparoscopic Roux-en-Y gastric bypass (LRYGB) is considered a complex surgical procedure [1, 2]. Expertise in general laparoscopic surgery is mainly attained during surgical residency whereas experience in bariatric surgery is acquired as a senior surgeon initiating a bariatric program, as a senior surgeon participating in an existing bariatric center, in a bariatric fellowship, or during surgical residency. In the latter, the experience consists mostly of assistance during surgery which can gradually proceed towards mastery of the procedure and a supervised performance of the entire surgery [3,4,5]. On the contrary, the goal of a senior or a fellow-surgeon is to practice bariatric surgery entirely without being supervised by a proctor [6, 7]. A learning curve (LC) can grant insight in the process of initial experience towards mastery of a procedure as it is a graphic display of the number of consecutive cases performed to reach proficiency, which is often a predefined expert-derived benchmark. The concept of a LC is based on the premise that people become better at their task with repetition and a LC can be defined by duration of surgery (DOS) and by complications [8,9,10]. Available evidence shows that the LC plateaus between 50 and 100 cases in senior surgeons pioneering in bariatric surgery [11,12,13]. The LC of the preceding surgeon (i.e., the proctor) positively influences the LC of consecutive senior surgeons, irrespective of their experience [12, 14]. LRYGB is considered safe performed by fellow-surgeons and DOS shortens after introduction of a bariatric fellowship [6, 7, 15]. Literature on the LC of residents in this surgery is limited. It is unknown to what extent surgical residents benefit from their proctor’s experience and the LC of surgical residents has not been defined yet. Notwithstanding suggestions that senior resident participation in LRYGB is safe, there is only one report of surgical residents in the role of operating surgeon [4, 5]. Iordens et al. demonstrated that LRYGB can be introduced safely during training in senior residents, based on 83 surgeries completed independently by five senior residents with a median DOS of 129 min [3]. Notably, none of these residents surpassed the number of 50–100 procedures, in conjunction with the LC as defined for senior surgeons. To warrant safety and effectiveness in bariatric surgery, guidelines for (initiating) a bariatric surgery program have been adopted by the American Society of Bariatric Surgeons (ASMBS) and the Society of American Gastrointestinal Endoscopic Surgeons (SAGES) and consequently Bariatric centers-of-excellence (COE) have been established [16,17,18,19]. As a result of the large exposure to bariatric surgery these centers of expertise seem highly conductive not only to train fellow-surgeons but surgical residents as well in bariatric surgery. The surgical residency in the Netherlands consists of a 4-year training in general surgery and 2 years of specialization within a field of interest. At the department of surgery in Medical Center (MC) Slotervaart, a bariatric COE, the 4 years of training in general surgery are offered. Consequently surgical residents are not only granted the opportunity to become competent in general surgery, but in the art of bariatric surgery as well. As of now it is unclear whether the resident is competent enough to master the procedure during or directly after surgical residency. It must be stressed that it is of the utmost importance that patients should not be offered less than optimal results regardless of the status and the training level of their operating surgeon [20]. Aim of this study is to evaluate whether bariatric surgery can be safely performed by junior and senior surgical residents in a bariatric COE under supervision of their proctors, to characterize the LC of surgical residents and to assess whether surgical residents fit in the LC of the center which has been established by their proctors in our bariatric COE.
Materials and methods
Study population and design
The Institutional Review Board approved this study. All consecutive patients who met the criteria for bariatric surgery by the International Federation of Surgery for Obesity (IFSO) [21] and who had a primary LRYGB at MC Slotervaart from onset of the bariatric program in 2007 until January 2016 were eligible for inclusion in the present study. Patients that underwent a concomitant intervention, revision surgery and/or variations on LRYGB and patients operated on by a fellow-surgeon or by a surgical resident who performed less than 70 procedures as operating surgeon, were excluded.
All patients were screened preoperatively by a bariatric surgeon, an endocrinologist, a dietician and a psychologist and were operated after obtaining written informed consent. Surgeries were numbered chronologically as consecutive case number of the total amount LRYGBs performed in MC Slotervaart and they were numbered chronologically as case per operating surgeon. Consequently, cases were clustered into groups of 70 to allow for comparison between patient groups.
Medical charts were reviewed on demographics, anthropometrics, medical history, comorbidities scored according to the modified Charlson Comorbidity index, operating and assisting surgeon, obesity surgery mortality risk score (OS-MRS), American Association of Anesthesiologists classification (ASA-score), duration of surgery and intra-operative events and conversion to open surgery [22, 23]. Hospital stay, emergency room visits, readmissions, laboratory testing, radiologic evaluation within 30 days of surgery were scored to evaluate short term morbidity and mortality.
Study end-points
The primary end-point was the occurrence of a complication within 30 days of surgery, which was registered in accordance with the Clavien-Dindo classification by three independent researchers (A.R., D.M., and N.G.) [24]. Any complication scored as Clavien-Dindo grade II or higher was reported in the present study. It was scored whether the etiology of the complication was surgical or non-surgical. Surgical complications included anastomotic leaks, formation of intra-abdominal abscesses, strictures or stenosis, intra-abdominal or intra-luminal hemorrhage, wound infection, and direct post-operative nausea and vomiting. Pulmonary embolization, the need for anti-hypertensive or anti-diabetic medication, antibiotics indicated by pneumonia or urinary tract infection were designated as non-surgical complications. Secondary end-points were DOS, calculated as time from incision to dressing of the wound, identification and correction for factors increasing surgical complexity, and the outcome of LRYGB by residents against the benchmarked level of complications and DOS after the procedure had gone through the LC in MC Slotervaart.
Surgical technique
All LRYGB were standardized, with division of the stomach into a 30–50 ml gastric pouch and a gastric remnant with the use of two or three 60-millimeters (mm) linear staplers (Endo GIA Tri-staple™, Medtronic). The proximal jejunum is brought up antecolic and antegastric and a side-to-side gastrojejunostomy (GJ) is created with a 30 mm linear stapler, which is over sewn with an absorbable 3–0 V-loc™ suture (Medtronic). The alimentary limb is measured 150 cm and the biliary limb at approximately 50 cm. A side-to-side jejunojejunostomy (JJ) is created with a 45 mm stapler and consequently, the defect is closed with a 60 mm stapler (Endo GIA Tri-staple™, Medtronic). The jejunum between the GJ and the JJ is divided with a 60 mm stapler. A methylene blue test is performed to test the GJ anastomosis. Since July 2012 the Y- and the Petersen window are closed with staples. All patients were urged to lose weight before the operation to improve surgical risk. In January 2011 a fast track program was implemented at our institution. This protocol applied to all patients that were planned for a primary laparoscopic gastric bypass [25]. All patients received subcutaneous thromboprophylaxis with a low-molecular-weight-heparin (LMWH).
The surgical residency and previous experience of the institute
Medical Center Slotervaart is a public hospital and it is one of the 45 Dutch hospitals certified to offer the first 4 years of the surgical residency in which the focus is on general laparoscopic surgery. All these surgical programs are monitored every 4–8 years on their aptness to harbor the residency, which encompasses close individual evaluation of the local Head of the surgical residency and his deputy upon their personal ability and qualification in these positions. There are no special requirements for the other surgeons in the light of the surgical residency. All surgeons at our department are accredited laparoscopic surgeons and some have extra training in leadership and education. A few surgeons take part as a lecturer in the national educational program of surgical residents. The residents attend a basic suturing and laparoscopic course in the first 2 years of the residency, followed by an advanced suturing course in year three and four in addition to the abovementioned and obliged nationwide educational program for surgical residents. The bariatric program at MC Slotervaart was started in 2007. Patient volumes increased from three in 2007 to 1112 in 2015, which includes various primary and revision procedures. In 2013, MC Slotervaart has been accredited as Bariatric and Metabolic Surgery Center-of-Excellence by the European Accreditation Council for Bariatric Surgery [16]. The surgical experience of surgeons and residents is depicted in Tables 1 and 2.
Patient selection and supervision
Male patients with OS-MRS class C and a body mass index (BMI) above 50 kg/m2 were not operated on by surgeons and residents without enough experience, as judged by the proctor. The proctor’s supervision consisted of attendance during the full-length of surgery with verbal directions and, in most surgeries, the surgeon acted as assistant-surgeon and/or held the camera. A surgery was designated as performed by the resident after completion of all fundamental steps in the LRYGB; i.e., creation of the gastric pouch followed by the GJ, the JJ and closure of the mesenteric defects.
Statistical analysis
Differences in categorical variables between groups were evaluated with the χ 2 or the Fisher exact test, as appropriate; continuous data were compared with the Student t test. Differences in learning curves between groups (surgeons, residents) were assessed by means of a linear regression model with an interaction term between number of cases with LRYGB and groups (surgeons or residents). We adjusted for potential confounders by means of multivariable models. Logistic regression analyses were performed to identify predictors of complications (classified as Clavien-Dindo ≥ 2). The analyses were performed using the generalized estimating equation method to account for correlations within patients operated on by the same surgeon/resident. The exchangeable correlation structure was used for these models. Skewed data were log-transformed for the analyses. Two-sided p values <0.05 were considered statistically significant. The statistical analyses were performed with SPSS software (version 19.0, Chicago, Illinois, USA).
Results
Patient characteristics
From December 2007 until January 2016 a total of 3389 patients underwent a primary LRYGB, performed by four surgeons, one fellow-surgeon, and nine residents. Of these, we excluded 338 patients, who were operated on by a fellow-surgeon or by a surgical resident with less than 70 cases as operating surgeon. Our study population comprised of the remaining 3051 patients, of whom 2690 were operated on by four surgeons (S1-4) and 361 by three residents (R1-3).
Baseline demographic and clinical characteristics of patients operated on by surgeons or residents are summarized in Table 3, both for all patients and for the sum of the first 70 cases of the surgeons (n = 280) and residents (n = 210) separately.
Duration of surgery
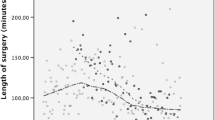
Median and interquartile range (IQR) of DOS of all cases by S1-S4 was 53 (46–63) min and 52 (42.0–65.0) min for the cases by R1-3 (p = 0.52). When the DOS of the first 70 cases of S1-4 (n = 280) and R1-3 (n = 210) are compared, median surgery time is significantly shorter for residents (91 (70–129) min versus 57 (50–67) min, respectively; p < 0.001). Figures 1A, B depict operating times of all cases and the first 70 cases, respectively, for the surgeons and residents individually. Figures 2A, B show the LCs of the surgeons and residents for the first 70 cases individually (n = 70) and as a group (surgeons n = 280, versus residents n = 210). Figure 2B shows that the LC of the group of residents is less steep than the LC of the surgeons after correction for gender, BMI, age, and abdominal surgery in the medical history (adjusted p = 0.021). The LC of R1-3 in their first 70 cases (n = 210) differs significantly from the individual (n = 70) LCs of surgeon 1, 2, and 3 (adjusted p < 0.0001, p < 0.001 and p = 0.0002, respectively) and is the same as the LC of surgeon 4 (adjusted p = 0.24). Figure 3 represents the LC of the procedure in MC Slotervaart as it displays the first 70 cases of S1-4 and R1-3 individually plotted against the case number of all LRYGBs performed from onset of the program. Residents continue the learning curve of the procedure at the bariatric COE flawlessly, which has been set out by their proctors.
A Median duration of surgery in minutes per primary surgeon, all cases legenda: *p < 0.05; **p < 0.001, NS not significant; depicts differences between S1 versus S2, S3, S4, R1, R2, R3, respectively. B Median duration of surgery per primary surgeon, first 70 cases. Legenda: S4 versus R1, R2, R3, respectively: *p < 0.05; **p < 0.001
Complications within 30 days of surgery
The number and type of complications of surgeons and residents are shown in Tables 4 and 5. Overall, no significant difference in the occurrence of complications was found between the two groups (p = 0.095). Five surgeries were converted to open surgery (0.2%), all in patients operated by S1-4 versus no conversions in the surgeries performed by R1-3. Pneumonia occurred significantly more in patients operated on by surgeons for all cases and the sum of their first 70 cases (p = 0.018 and p = 0.040 respectively). The rate of complications (classified as Clavien-Dindo ≥ 2) by resident 1-3 in their first 70 cases (n = 210) differed significantly from the individual complication rates (n = 70) of surgeon 1, 2 and 3 (adjusted p < 0.001, p < 0.001 and p = 0.0002, respectively) when corrected for gender, BMI, age, and abdominal surgery in the medical history; there was no difference between S4 and R1-3 (adjusted p = 0.22). Overall, S1-4 had 137 (5.1%) surgical complications (CD ≥ 2) versus 11 (3.1%) for R1-3 (unadjusted p = 0.089).
Discussion
With the report of 361 LRYGBs performed by three residents in the present study, there is convincing evidence on the outcome of this type of bariatric surgery by surgical residents in a high-volume bariatric center. This is the first study which analyzed a series of LRYGBs by residents, exceeding the reported LC of senior surgeons (50–100 cases) [3]. Overall, there was a slight difference in complication rate of non-surgical etiology with a Clavien-Dindo grade II or higher between groups (data not shown). Most likely, this difference is due to patient selection. After selection of the first 70 cases and adjusted for patient complexity, more complications with a Clavien-Dindo grade II or higher were seen in the first cases by surgeon 1, 2, and 3 (n = 70) when compared with the first 70 cases of the three residents (n = 210). The residents were also significantly faster in the first 70 cases than their proctors had been in their 70 cases. Nevertheless this discrepancy disappeared when all cases in the study were taken into consideration. The disparity in complication rate and duration of surgery in the first 70 cases of S1-3 and the residents can be partly explained by the evolution of the procedure in our bariatric center, which is reflected in the first 70 cases of these surgeons. Arguably, surgeon 4 stepped in right after S1-3 had gone through the LC of the procedure and therefore no difference is seen between surgeon 4 and the residents. This is illustrated in Fig. 3, in which the first 70 cases of surgeon 4 are performed whilst the LC of the procedure has started to flatten. Another explanation for the differences seen is the feedback, excellent assistance and/or camera holding offered by the surgeons during the full-length of the LRYGB, facilitating safe and effective surgery. However, the observation that residents perform well on these two outcome measures within their first 70 cases might as well indicate that residents benefit from their proctors’ experience, which has thus far only been shown to be true for senior surgeons in the process of mastery of the LRYGB [14]. Subgroup analysis has been performed to evaluate the impact of the low number of male patients on the complication rate and the interpretation of the results. No differences in complications in male patients have been found in the whole cohort and in the first 70 cases (data not shown). To conclude that resident performance of LRYGB is in accordance with the level of care in the bariatric center (i.e., the benchmark), the position of the residents within the LC of the bariatric center was assessed. As mentioned, the LC of the procedure of MC Slotervaart had been set out by S1 and was continued by the consecutive surgeons. In Fig. 3 the transition of this surgeon-initiated LC towards resident contribution is depicted. It is shown that the LC is flawlessly continued by surgical residents, hinting that residents perform according to the benchmarked level in the bariatric COE.
Coming forth from these observations it would be desirable to define the LC of surgical residents. When the LC of surgical residents would solely be based on DOS, the LC of surgical residents would not differ from the LC of senior surgeons and would lie within the range of 50–100 cases. However, the number and severity of complications should be weighed when defining the LC of surgical residents. The report of three residents in this series might be too low to generalize these data to all surgical residents and large series of multiple residents would be required to further define and express the LC of surgical residents as an exact number. Also, the moment a resident is deemed competent to perform a LRYGB independently differs amongst residents as it depends on the skill set and progression of each trainee. Based upon the results of this study with data of three residents, it is impossible to define a certain interval in which the requisite skill level is achieved. Therefore, the level of performance of each resident should be judged by a proctor in order to define the appropriate moment of first independent LRYGB performance. Our study shows that LRYGB is safe and effective when performed by surgical residents under strict supervision of experts in a high-volume bariatric COE. Another intriguing finding is that the experience of two junior residents and one senior resident is reflected in this study, making the case not to reserve this type of surgery for senior residents as a rule. This is in line with the report of Birkmeyer et al. that surgical skill is not per se related to years in bariatric practice, the completion of fellowships, but strongly related to procedure volume, which is achieved by the residents in the current study in the early years of their surgical residency [2].
The major strength of the present study is the high number of LRYGBs performed by surgical residents and senior surgeons. Laparoscopic Roux-en-Y gastric bypasses performed by six other surgical residents were excluded from analysis, given the relatively low personal case number, compromising analysis up to, and including the pre-set LC of senior surgeons between 50 and 100 cases. Surgeries by one fellow-surgeon were excluded as his surgical skills and experience was considered to be in between the level of senior surgeons and the residents. The exclusion of these residents and fellow-surgeon could have induced selection bias. However, analysis of these data show no adverse outcome in this group (data not shown). Another strength is that S1-4 started their bariatric experience at MC Slotervaart and all four contributed to the LC of the procedure. This granted the opportunity for comparison of the true LCs of surgeons and residents.
Important limitations are embedded within the retrospective nature of the study. The number of GJs and JJs performed by assisting residents upon the first solo-LRYGB performance was not kept track of. Additionally, the requisite level of surgical skill upon this moment was subject to the proctor’s opinion and it is reckoned that there might have been some variation between the residents at the time of their first independent performance of a LRYGB. The number of assisted procedures is therefore the surrogate measure by which prior bariatric experience was defined, although it does not precisely reflect surgical skill at onset of the first self-reliant procedure. Furthermore, influence upon the LC of R1-3 might have been the ratio in which each senior surgeon contributed to the resident’s procedures in the role of proctor, monitoring progress, providing feedback, and assistance. Vice versa, the ratio in which R1-R3 assisted in the surgeries performed by S1-S4 differs and might influence individual results of S1-S4. Also, it is unclear what the influence is of the number of LRYGBs assisted before the first autonomous LRYGB was performed. In the current study, the ability of a surgeon and surgical resident to perform a LRYGB was defined by complications as a gauge of patient outcome and quality of care, combined with DOS as an indicator of process efficiency.
In conclusion, LRYGB can be performed safely by surgical residents under supervision of experienced bariatric surgeons. Surgical residents benefit from the experience of their proctors and they fit faultlessly in the LC of the surgical team, as set out by their proctors in a large bariatric COE.
References
Schauer PR, Ikramuddin S (2001) Laparoscopic surgery for morbid obesity. Surg Clin North Am 81:1145–1179
Birkmeyer JD, Finks JF, O’Reilly A, Oerline M, Carlin AM, Nunn AR, Dimick J, Banerjee M, Birkmeyer NJO (2013) Surgical skill and complication rates after bariatric surgery. N Engl J Med 369:1434–1442. doi:10.1056/NEJMsa1300625
Iordens GIT, Klaassen RA, Van Lieshout EMM, Cleffken BI, Van Der Harst E (2012) How to train surgical residents to perform laparoscopic roux-en-Y gastric bypass safely. World J Surg 36:2003–2010. doi:10.1007/s00268-012-1620-2
Rovito PF, Kreitz K, Harrison TD, Miller MT, Shimer R (2005) Laparoscopic Roux-en-Y gastric bypass and the role of the surgical resident. Am J Surg 189:33–37. doi:10.1016/j.amjsurg.2004.06.041
Fanous M, Carlin A (2012) Surgical resident participation in laparoscopic Roux-en-Y bypass: is it safe? Surgery (United States) 152:21–25. doi:10.1016/j.surg.2012.02.014
Gonzalez R, Nelson LG, Murr MM (2007) Does establishing a bariatric surgery fellowship training program influence operative outcomes? Surg Endosc Other Interv Tech 21:109–114. doi:10.1007/s00464-005-0860-8
Kothari SN, Boyd WC, Larson CA, Gustafson HL, Lambert PJ, Mathiason MA (2005) Training of a minimally invasive bariatric surgeon: are laparoscopic fellowships the answer? Obes Surg 15:323–329. doi:10.1381/0960892053576640
Zacharoulis D, Sioka E, Papamargaritis D, Lazoura O, Rountas C, Zachari E, Tzovaras G (2012) Influence of the learning curve on safety and efficiency of laparoscopic sleeve gastrectomy. Obes Surg 22:411–415. doi:10.1007/s11695-011-0436-8
Khan N, Abboudi H, Khan MS, Dasgupta P, Ahmed K (2014) Measuring the surgical “learning curve”: methods, variables and competency. BJU Int 113:504–508. doi:10.1111/bju.12197
Corey R (1978) and Manufacturing of learning curves limitations of learning curves. Hosp Heal Serv Adm
Nguyen NT, Rivers R, Wolfe BM (2003) Factors associated with operative outcomes in laparoscopic gastric bypass. J Am Coll Surg 197:548–555. doi:10.1016/S1072-7515(03)00648-3
Ballantyne GH, Ewing D, Capella RF, Capella JF, Davis D, Schmidt HJ, Wasielewski A, Davies RJ (2005) The learning curve measured by operating times for laparoscopic and open gastric bypass: roles of surgeon’s experience, institutional experience, body mass index and fellowship training. Obes Surg 15:172–182. doi:10.1381/0960892053268507
Oliak D, Ballantyne GH, Weber P, Wasielewski A, Davies RJ, Schmidt HJ (2003) Laparoscopic Roux-en-Y gastric bypass defining the learning curve. Surg Endosc 17:405–408. doi:10.1007/s00464-002-8820-z
Geubbels N, de Brauw LM, Acherman YIZ, van de Laar AWJM, Wouters MWJM, Bruin SC (2015) The preceding surgeon factor in bariatric surgery: a positive influence on the learning curve of subsequent surgeons. Obes Surg 25:1417–1424. doi:10.1007/s11695-014-1538-x
Oliak D, Owens M, Schmidt HJ (2004) Impact of fellowship training on the learning curve for laparoscopic gastric bypass. Obes Surg 14:197–200. doi:10.1381/096089204322857555
Melissas J (2008) IFSO guidelines for safety, quality, and excellence in bariatric surgery. Obes Surg 18:497–500. doi:10.1007/s11695-007-9375-9
ASBS bariatric Training Committee (2006) American society for bariatric surgery’s guidelines for granting privileges in bariatric surgery. Surg Obes Relat Dis 2:65–67. doi:10.1016/j.soard.2005.10.012
Clements R, Saber A, Teixeiro J, Provost D, Fanelli R, Richardson W (2011) Guidelines for institutions granting bariatric privileges for the use of laparoscopic techniques. Surg Endosc Other Interv Tech 25:671–676. doi:10.1007/s00464-010-1375-5
De Luca M, Himpens J, Weiner R, Angrisani L (2015) IFSO statement: credentials for bariatric surgeons 2015. Obes Surg 25:394–396. doi:10.1007/s11695-014-1553-y
Buchwald H, Scopinaro N (2009) Retiring the learning curve. Obes Surg 19:541–542. doi:10.1007/s11695-009-9833-7
Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres A, Weiner R, Yashkov Y, Frühbeck G (2014) Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 24:42–55. doi:10.1007/s11695-013-1079-8
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251. doi:10.1016/0895-4356(94)90129-5
DeMaria EJ, Murr M, Byrne TK, Blackstone R, Grant JP, Budak A, Wolfe L (2007) Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg 246:578–582. doi:10.1097/SLA.0b013e318157206e
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213. doi:10.1097/01.sla.0000133083.54934.ae
Geubbels N, Bruin SC, Acherman YIZ, VandeLaar AWJM, Hoen MB, De Brauw LM (2014) Fast track care for gastric bypass patients decreases length of stay without increasing complications in an unselected patient cohort. Obes Surg 24:390–396. doi:10.1007/s11695-013-1133-6
Acknowledgements
The authors would like to acknowledge A. Amarti, Dr. J.K. de Bakker and Dr. G.F. Giannakopoulos for their contributions to this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
A. van Rijswijk, D.E. Moes, N. Geubbels, B.A. Hutten, Y.I.Z. Acherman, A.W. van de Laar, M. de Brauw, S.C. Bruin declared that they have no conflicts of interest or financial ties to disclose.
Ethical approval
The Medical Ethics Committee has waived ethical approval of this study.
Informed consent
All patients within the used database have agreed upon, and signed for, the anonymous use of their data for scientific use before they underwent bariatric surgery at MC Slotervaart.
Additional information
Anne-Sophie van Rijswijk and Daan E. Moes contributed equally to the paper and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
van Rijswijk, AS., Moes, D.E., Geubbels, N. et al. Can a laparoscopic Roux-en-Y gastric bypass be safely performed by surgical residents in a bariatric center-of-excellence? The learning curve of surgical residents in bariatric surgery. Surg Endosc 32, 1012–1020 (2018). https://doi.org/10.1007/s00464-017-5779-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5779-3