Abstract
Background
The learning curve of laparoscopic Roux-en-Y gastric bypass (LRYGB) surgery has been well investigated. The learning curve is defined by complications and/or by duration of surgery (DOS). Previous studies report an inverse relationship between patient outcome and patient volume. In this study, we investigate whether the learning curve of preceding bariatric surgeons is of additional influence for surgeons who start to perform LRYGB in the same centre.
Materials and Methods
We retrospectively analysed the records of all 713 consecutive primary LRYGB patients operated in our centre from December 2007 until July 2012. Surgeon 1 and 3 had previous laparoscopic bariatric experience whilst Surgeon 2 and 4 had not. We stratified the data between the four surgeons with different levels of experience and in a chronology of 50 cases.
Results
Sixty-seven (9.4 %) complications occurred in the study period. Surgeon 1 had more complications occurring within the first 50 cases than Surgeon 4 (10 versus 1, p < 0.05). There was no difference in complication rate between groups of 50 consecutive cases. None of the patients died. DOS decreased for every consecutive surgeon, irrespective of their experience. The learning curve defined by DOS was steepest for Surgeon 1, followed by Surgeon 2, 3 and 4.
Conclusion
In this study, we show that the learning curve of the preceding surgeon positively influences the learning curve of latter surgeons, irrespective of their experience. Therefore, the ‘preceding surgeon factor’ should be taken in account in addition to volume requirements when starting new bariatric facilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of morbid obesity (body mass index (BMI) ≥40 kg/m2) is increasing still. In the United States, self-reported data from the Behavioural Risk Factor Surveillance revealed a 70 % increase between 2000 and 2010 in the prevalence of morbid obesity, and an even steeper increase in the prevalence of super morbid obesity (BMI > 50 kg/m2) [1].
To date, bariatric surgery has proven itself to be the only sustainable solution to reduce weight and accompanying comorbidities in this patient group [2]. The increasing demand for bariatric surgery is a heavy burden on health care facilities and emphasizes the need for the training of new bariatric surgeons and institutes.
Laparoscopic bariatric procedures in morbidly obese patients, especially Roux-en-Y gastric bypass (LRYGB), require advanced laparoscopic skills. Several authors stress that a 6-year residency in general surgery is too short to master these advanced laparoscopic skills [3–5]. The relationship between skills and the time (or patient volume) to master these skills is condensed in the concept learning curve, often defined by complications and/or duration of surgery (DOS) (See Figs. 1 and 2).
A learning curve could give ethical discomfort because every patient deserves the same complication risk, irrespective of the treating surgeon [6]. Many studies have investigated the learning curve and showed that patient volume is inversely related to outcome (in either complications or DOS). Unfortunately, most of these studies involved series of consecutive patients treated by expert bariatric surgeons who already passed their learning curve. In this study, we investigate whether the learning curve of a preceding bariatric surgeon is of influence on surgeons who start to perform LRYGB within the same centre.
Materials and Methods
We retrospectively reviewed all consecutive patients that underwent a primary LRYGB procedure at our bariatric facility between the start of our bariatric service in December 2007 until July 2012. Patients that required a secondary intervention during the LRYGB (cholecystectomy, ventral hernia repair, etc.) were excluded from the analysis. A total of 713 patients were enrolled, and their demographic data, comorbidities, operating surgeon, short-term complications and DOS were entered into a database. In order to compare patients groups, the data was segregated into 5 categories of 50 cases based on chronology.
Comorbidities were calculated with the modified Charlson comorbidity index. This score is calculated by assigning each of the 19 predefined clinical conditions with a value from 1 to 6 [7]. Additionally, the obesity surgery mortality risk score (OS-MRS) was used. This scoring system assesses the change of mortality but has also proven itself in assessing complications [8, 9]. The OS-MRS assigns 1 point for 5 predefined conditions (male gender, age ≥ 45 years, hypertension, known risk factors for pulmonary embolism and a BMI ≥ 50). Scores from 0 to 1 are classified as ‘A’ with a mortality risk of 0.3 %, scores from 2 to 3 formed class ‘B’ with a mortality risk of 1.9 % (a 5-fold increase compares with class A). Scores from 4 to 5 formed class ‘C’ with a mortality risk of 7.6 % (a 12-fold increase in comparison to class A) [8]. Short-term complications were defined as any complication with Clavien-Dindo classification of Grade II or higher [10, 11], occurring within 30 days of surgery. Complications were divided based on aetiology. Surgical complications included leakage, stricture or stenosis, the formation of an intra-abdominal abscess, bleeding and wound infections. The DOS was defined as the time in minutes from the first incision to the final intra-cutaneous stitch.
Previous Experience of the Institute and Surgeons
There was no previous institutional experience in bariatric surgery before December 2007. Patient volume increased from 3 LRYGB patients in 2007 to 600 LRYGB patients in 2012. Surgeon 1 (S1) developed the programme and had extensive laparoscopic experience. S1 had performed approximately 1000 laparoscopic procedures elsewhere (appendix, gallbladder, colon, fundoplications, etc.), attended several laparoscopic bariatric courses and performed the first two laparoscopic bariatric procedures in this centre under proctorship of an experienced laparoscopic bariatric surgeon. Surgeon 2 (S2) joined the team as a surgeon in April 2009 after completing his training as a general surgeon in the same institute. S2 had performed a number of laparoscopic procedures (appendix, gallbladder, inguinal hernia and colorectal surgery) but had no previous experience in laparoscopic bariatric surgery. Surgeon 3 (S3) started working in our facility in April 2010. S3 also had extensive laparoscopic experience. He had performed about 2500 laparoscopic procedures (appendix, gallbladder, colon, inguinal hernia, adrenalectomy and nephrectomy) and had laparoscopic bariatric experience (about 100 gastric bands and 10 LRYGB’s). Surgeon 4 (S4) started working as a surgeon at our facility in August 2010, after completing his training as a general surgeon in the same institute. Like S2, he had laparoscopic experience but no previous laparoscopic bariatric experience. S2, 3 and 4 were all proctored by S1 during their first procedures. Patient selection was applied: each surgeon refrained from operating on older patients, males, patients with a BMI > 50 kg/m2, and patients with OS-MRS class C, until they gained sufficient experience.
Patient Optimization
Patients were encouraged to lose some weight prior to their surgery, but no weight loss or diet aimed at liver mass reduction was mandatory. Prior to surgery, all patients received subcutaneous thromboprophylaxis with a low-molecular-weight heparin (LMWH). Patients were not allowed to eat solids after midnight prior to the day of surgery. Liquids (water) were allowed up to 2 h prior to surgery.
Surgical Technique
A 30–50 ml gastric pouch is created with the use of two to three 60-mm linear staplers (Endo GIA, Covidien and Dublin, Ireland). The proximal jejunum is brought to the upper abdomen in an antecolic/antegastric fashion. The gastrojejunostomy is stapled to the posterior pouch with a 30-mm linear stapler device. The remaining anterior defect is closed with an absorbable unidirectional barbed 3-0 V-Loc™ suture (Covidien, Dublin, Ireland). Distally of the gastrojejunostomy, the alimentary limb is measured at 150 cm. The gastrojejunostomy is created using two linear staplers. The jejunum between the gastrojejunostomy and the jejuno-jejunostomy is divided with a 60-m linear stapler. The gastrojejunal anastomosis is tested for leakage with methylene blue through the orogastric tube. In case of a leak, the anastomosis is over-sewn with a V-Loc™ suture and the test is repeated. The mesenteric defects and Petersen’s space were left open. There is no routine placement of drains. The orogastric tube is removed at the end of surgery.
During the surgery, the surgeon is aided by surgical residents (from 1st to 6th year in their residency) who hold the instruments or, depending on their level of residency, participate in dissection and suturing. A medical student operates the camera, and a dedicated surgical nurse hands the surgeon the instruments.
There were some modifications in our surgical technique of which the majority took place in 2010. The diathermic scalpel was replaced by a harmonic scalpel. The transition from a fully hand-sewn gastrojejunostomy to partly linear stapling technique took place. The jejuno-jejunostomy, which was partially hand-sewn before this time, became fully stapled. The V-Loc unidirectional barbed wire is introduced in July 2010 (after 120 procedures). Previously, a 2.0 absorbable suture was used to create the gastrojejunostomy. The mesenteric defects and Petersen’s space were closed with an unabsorbable suture. Later, the mesenteric defect and Petersen’s space were left open. Although there were modifications in surgical technique, it is important to emphasize that there was no difference in technique between the surgeons at our centre.
The positioning of the patients shifted from supine horizontal to supine anti-Trendelenburg because of the improved respiratory conditions and improved surgical view. Incorporated in our fast track programme since January 2011 was a shift from a ‘high propofol, low remifentanil’ to ‘low propofol, high remifentanil’ anaesthesia. This shift in regimen causes quicker emergence from anaesthesia [12]. The relationship between the surgeon and the anaesthesiologist has intensified. The anaesthesiologist plays an active role in testing the gastrojejunostomy, and the surgeon provides him or her with feedback about muscle relaxation and intra-abdominal pressure.
Statistical Analysis
Descriptive statistics are reported as mean with standard deviation (SD). Not normal distributed data is reported as median and range. For comparison between categorical variables, the Chi square or Fishers’ exact test with Bonferroni correction was used where appropriate. For comparison between continuous data, the Students t test was used in parametric data. The Mann Whitney U test was used when neither normality nor homogeneity of variance could be assumed. Linear and non-linear regression was used for the comparison of different slopes. A two-sided p value of <0.05 was considered statistically significant. All statistical analyses were performed in SPSS version 20 for windows (IBM Corporation, New York, USA). The graphs were created with GraphPad Prism version 6.0 for Mac (GraphPad Software Inc, La Jolla, California, USA).
Results
Patient Characteristics
S1 performed the most LRYGB’s (n = 239) compared to S2 (n = 186), S3 (n = 200) and S4 (n = 88). The mean age of patients operated by S1 was slightly higher than S2, S3 and S4 (43 ± 10 versus 42 ± 11, 41 ± 9.8 and 41 ± 9.8, p < 0.05, respectively). S4 performed LRYGB in lesser males than S1 and S2 (6.8 % versus 23.4 % and 39 %, p < 0.05, respectively). The median BMI of patients operated by S1 was higher than S2, S3 and S4 (44 kg/m2 versus 43 kg/m2, 42 kg/m2 and 42 kg/m2, p < 0.05, respectively). S1 operated less OS-MRS class A patients and more OS-MRS class B patients in comparison with S3 and S4 (57/41 % versus 71/29 % and 76/24 %, p < 0.05, respectively). There were no differences in modified Charlson comorbidity indexes between surgeons (See Table 1).
Short-Term Complications
A total of 67 patients developed complications (9.4 %) over the study period. There was a significant difference in complication rate between the first 50 cases of S1 and S4 (10 versus 1, p < 0.05). There was no difference in complication rate between groups of 50 cases (see Fig. 3a). Seventy-four (6.6 %) complications were of surgical aetiology. Sixteen (6.7 %) surgical complications occurred in the patients operated by S1, followed by 14 (7.5 %) in patients operated by S2, 14 (7 %) in patients operated by S3 and 3 (3.4 %) in patients operated by S4, but these differences did not reach statistical significance (see Fig. 3b). Except for S3, most complications occurred in the first 50 consecutive patients, but the difference between consecutive groups of 50 cases did not reach statistical significance (see Fig. 3a). None of the patients died during the course of this study.
Duration of Surgery
Total DOS was significantly longer for S2 than S4 (74 min versus 69 min, p < 0.05). All surgeons had the longest median DOS in their first 50 consecutive cases. The first 50 cases of S1 took longer than those of S2 (169 min versus 104 min, p < 0.001), S3 (169 min versus 96, p < 0.01) and S4 (169 min versus 74, p < 0.001). In addition, S2’s first 50 cases took longer than those of S3 (104 min versus 96, p < 0.05) and S4 (104 min versus 74, p < 0.001). The first 50 cases of S3 took significant longer than those of S4 (96 min versus 74 min, p < 0.01) (see Fig. 4b). For every individual surgeon, the DOS decreased significantly per 50 cases within the first 150 cases. After the first 150 procedures, the DOS seemed to plateau for S1, S2 and S3 (see Fig. 4a).
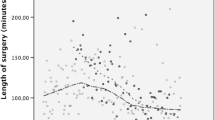
Figure 1 presents the different learning curves (defined by DOS and case number) for our surgeons. The first procedure performed by S1 took 270 min, which is longer than the first procedures of S2, S3 and S4 (262 min, 135 min and 125 min, respectively). This is depicted in the y intercepts of the curves. The learning curve of S1 is much steeper than those of S2 and S3. Whereas for S4, the curve is almost flat. Each of the slopes differs significantly from one another (p < 0.001). The learning curve of S4 is not significantly different from zero (p = 0.0584) (see Fig. 1). Figure 2 depicts the scatterplot of each individual patient’s DOS over time. This figure clearly shows that every subsequent surgeon ‘steps in’ at the level established by their predecessor (see Fig. 2).
Discussion
Laparoscopic Roux-en-Y gastric bypass surgery is a complex procedure. Previous studies mention a long learning curve of 50–100 procedures before mastering this procedure. In several studies, adverse outcomes were more frequent with surgeons who were still within their learning curve compared with surgeons who already surpassed their learning curve [13–17]. In our opinion, patient outcome should be similar for all patients, irrespective of the experience and background of their treating surgeon. In the present study, we examine how different surgeons with different backgrounds, but working in the same centre, affect each other’s learning curve. We show that the learning curve of the preceding surgeon positively influence the learning curve of the latter surgeon, irrespective of their experience.
Defined in complications, our study shows a steep learning curve for S1 in the first 50 cases, which differs significantly from the learning curve of S4. Interestingly enough, this difference is only depicted in overall short-term complications. When looking only at complications of surgical aetiology, no learning curve seems apparent. This might reflect the learning of a centre (in detecting and handling symptoms of complications) rather than the learning curve of an individual surgeon.
Patient volume has been the main determent in many studies reporting the learning curve of LRYGB. The learning curve of LRYGB is between 50 and 100 cases, depending on the experience of the surgeon [4, 18, 19]. The relation between volume (of either centre or individual surgeon) and complication rate is also well investigated and, generally, is a negative one: the lower the patient volume, the higher the rate of complication [13–17].
In several studies, training has shown to flatten the learning curve. A meta-analysis investigated the learning curve of surgeons with and without training in laparoscopic bariatric surgery and found a difference in complication rate of 18.1 % (without training) versus 7.7 % (with training) [20]. S1 had extensive laparoscopic surgical experience gained elsewhere and attended several bariatric courses before commencing the bariatric programme in our hospital. But still, there was no institutional experience with bariatric surgery at that time. When S4 started working at our facility in 2010, institutional experience was readily available. This finding is an addition to those of several other authors. Provided that less experienced surgeons operate in a centre with extensive bariatric experience, under proctorship of an experienced bariatric surgeon and with applied patient selection, their outcomes are comparable to those of more experienced colleagues [3, 5, 21, 22].
Defined in DOS, the learning curve is clearer. In the first 50 cases, we see a sharp decrease in DOS for every subsequent surgeon. For every individual surgeon, we observed a decrease in DOS within the first 150 cases. In previous studies, the emphasis is put on surgical experience as primary explanation for a decrease in surgical time [23]. The decrease in DOS after every 50 patients is indeed well explained by an increase in experience, but the differences between the surgeons are not. S1 and S3 had extensive laparoscopic experience whilst S2 and S4 did not. S2 and S4 probably benefited directly from the experience already gained by S1 and S3. Furthermore, institutional experience grew parallel to individual experience over the course of time. We propose that ‘centre-bound factors’ like preceding surgeon and institutional experience should be taken in account as much as individual gained experience when reporting a learning curve.
Like any study with a retrospective nature, this study has several limitations. First and foremost, the learning curve defined by both complication rate and DOS are in part dependent on factors that could not be accounted for in this study. As described in the methods section, changes in surgical technique were made during the course of the study. These technical modifications certainly confounded DOS as outcome measure. The first procedures of S1 differ in a lot of ways to the first procedures of S4, and these improvements magnify differences between surgeons. Still, it must be said that all surgeons used the same technique at the same time. Therefore, we expected to find a longer DOS with inexperienced surgeon in comparison to their more experienced colleagues. To our surprise, we did not find this at all. Every novel surgeon (experienced or inexperienced) seemed to ‘step in’ at the same DOS of a more experienced colleague. This is clearly visible from Fig. 2.
Another limitation to this study is the applied patient selection (on sex, BMI, age and OS-MRS score) might bias outcomes in favour of surgeons still within their learning curve (like S4). In this case, the limitations of this study are also its strengths. The modification of the surgical technique and applied patient selection might blur the individual contribution to the learning curve but are probably also the cause of a low complication rate and DOS for inexperienced surgeons.
Currently, minimal volume standards are set through the establishment of Centre of Excellence programmes, demanding a facility volume of at least 80 qualifying bariatric procedures per year and an individual surgeon volume of at least 125 qualifying bariatric procedures in his/her lifetime, with at least 50 cases performed in the last 12 months [24]. We strongly support the implementation of these programmes but also comprehend that these requirements will inhibit the establishment of new bariatric centres in a time that obesity is endemic. We advise starting bariatric facilities to account the ‘centre-bound’ factors in addition to the ‘patient volume factor’. Individual surgeons should gain experience performing laparoscopic bariatric procedures in centres with an established programme, a matured (peri)surgical care path, a high patient volume and to apply patient selection in their first procedures.
References
Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int. J. Obes. (Lond). [Internet]. Nature Publishing Group; 2012 [cited 2013 Jan 22];1–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22986681.
Sjöstrom L, Lindroos AK, Petlonen M, et al. New Engl J Med. 2004;351:2683–93.
Gonzalez R, Nelson LG, Murr MM. Does establishing a bariatric surgery fellowship training program influence operative outcomes? Surg Endosc [Internet]. 2006;21:109–14. doi:10.1007/s00464-005-0860-8.
Suter M, Giusti V, Héraief E, et al. Laparoscopic Roux-en-Y gastric bypass: initial 2-year experience. Arch Surg [Internet]. 2003;17:603–9. http://www.ncbi.nlm.nih.gov/pubmed/12582767.
Breaux JA, Kennedy CI, Richardson WS. Advanced laparoscopic skills decrease the learning curve for laparoscopic Roux-en-Y gastric bypass. Surg Endosc [Internet]. 2007;21:985–8. http://www.ncbi.nlm.nih.gov/pubmed/17623252.
Buchwald H, Scopinaro N. Retiring the learning curve. Obes Surg [Internet]. 2009;19:541–2. doi:10.1007/s11695-009-9833-7.
Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9.
DeMaria EJ, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg [Internet]. 2007;246:578–84. http://www.ncbi.nlm.nih.gov/pubmed/17893494.
Sarela AI, Dexter SPL, McMahon MJ. Use of the obesity surgery mortality risk score to predict complications of laparoscopic bariatric surgery. Obes Surg [Internet]. 2011;21:1698–703. doi:10.1007/s11695-011-0379-0.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg [Internet]. 2009;250:187–96. http://www.ncbi.nlm.nih.gov/pubmed/19638912.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg [Internet]. 2004;240:205–13. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000658-200408000-00003.
White PF, Kehlet H, Neal JM, et al. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg [Internet]. 2007;104:1380–96. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000539-200706000-00013.
Courcoulas A, Schuchert M, Gatti G, et al. The relationship of surgeon and hospital volume to outcome after gastric bypass surgery in Pennsylvania: a 3-year summary. Surgery [Internet]. 2003;134:613–21. http://linkinghub.elsevier.com/retrieve/pii/S0039606003003064.
Nguyen NT, Paya M, Stevens CM, et al. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg [Internet]. 2004;240:184–92. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00153307-200401220-00020.
Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg [Internet]. 2004;199:543–51. http://www.ncbi.nlm.nih.gov/pubmed/15454136.
Flum DR, Salem L, Elrod JAB, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA [Internet]. 2005;294:1903–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16234496.
Murr MM, Martin T, Haines K, et al. A state-wide review of contemporary outcomes of gastric bypass in Florida: does provider volume impact outcomes? Ann Surg [Internet]. 2007;245:699–706. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1877059&tool=pmcentrez&rendertype=abstract.
Schauer P, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc [Internet]. 2003;17:212–5. http://www.ncbi.nlm.nih.gov/pubmed/12457218.
Shikora SA, Kim JJ, Tarnoff ME, et al. Laparoscopic Roux-en-Y gastric bypass. Arch Surg. 2005;140:362–7.
Sánchez-Santos R, Estévez S, Tomé C, et al. Training programs influence in the learning curve of laparoscopic gastric bypass for morbid obesity: a systematic review. Obes Surg [Internet]. 2012;22:34–41. doi:10.1007/s11695-011-0398-x. Springer-Verlag.
Søvik TT, Aasheim ET, Kristinsson J, et al. Establishing laparoscopic Roux-en-Y gastric bypass: perioperative outcome and characteristics of the learning curve. Obes Surg [Internet]. 2008;19:158–65. doi:10.1007/s11695-008-9584-x.
Iordens GIT, Klaassen RA, van Lieshout EMM, et al. How to train surgical residents to perform laparoscopic Roux-en-Y gastric bypass safely. World J Surg [Internet]. 2012;36:2003–10. http://www.ncbi.nlm.nih.gov/pubmed/22576184.
Ballantyne GH, Ewing D, Capella RF, et al. The learning curve measured by operating times for laparoscopic and open gastric bypass: roles of surgeon’s experience, institutional experience, body mass index and fellowship training. Obes Surg. 2005;15:172–82.
COEMBS Designation Requirements [Internet]. Available from: http://www.surgicalreview.org/wp-content/uploads/COEMBS-Requirements_Rev-02-2013.pdf.
Acknowledgments
The authors would like to acknowledge Dr. D. Moes, Dr. B. van den Heuvel and Dr. I. Kappers for their contribution in providing data and feedback for this paper.
Grant Information
None of the authors have received grants or any other kind of financial support to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Informed Consent
Prior to surgery, an informed consent form is filled in by all patients that receive surgery in the Slotervaart Hospital. All patients agreed to the usage of their data in an anonymous form.
Statement of Human and Animal Rights
Due to the retrospective nature of this study, formal consent is not required and has been waived by the hospital’s Medical Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geubbels, N., de Brauw, L.M., Acherman, Y.I.Z. et al. The Preceding Surgeon Factor in Bariatric Surgery: a Positive Influence on the Learning Curve of Subsequent Surgeons. OBES SURG 25, 1417–1424 (2015). https://doi.org/10.1007/s11695-014-1538-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1538-x