Abstract
Background
Gall bladder cancer (GBC) is the most common and aggressive malignancy of the biliary tract with extremely poor prognosis. Radical resection remains the only potential curative treatment for operable lesions. Although laparoscopic approach is now considered as standard of care for many gastrointestinal malignancies, surgical community is still reluctant to use this approach for GBC probably because of fear of tumor dissemination, inadequate lymphadenectomy and overall nihilistic approach. Aim of this study was to share our initial experience of laparoscopic radical cholecystectomy (LRC) for suspected early GBC.
Methods
From 2008 to 2013, 91 patients were evaluated for suspected GBC, of which, 14 patients had early disease and underwent LRC.
Results
Mean age of the cohort was 61.14 ± 4.20 years with male/female ratio of 1:1.33. Mean operating time was 212.9 ± 26.73 min with mean blood loss of 196.4 ± 63.44 ml. Mean hospital stay was 5.14 ± 0.86 days without any 30-day mortality. Bile leak occurred in two patients. Out of 14 patients, 12 had adenocarcinoma, one had xanthogranulomatous cholecystitis and another had adenomyomatosis of gall bladder as final pathology. Resected margins were free in all (>1 cm). Median number of lymph nodes resected was 8 (4–14). Pathological stage of disease was pT2N0 in eight, pT2N1 in three and pT3N0 in one patient. Median follow-up was 51 (14–70) months with 5-year survival 68.75 %.
Conclusions
Laparoscopic radical cholecystectomy with lymphadenectomy can be a viable alternative for management of early GBC in terms of technical feasibility and oncological clearance along with offering the conventional advantages of minimal access approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Gall bladder cancer (GBC) is the most common and aggressive malignancy of the biliary tract [1] with extremely poor prognosis, i.e., 5-year survival rate of 5 % with median survival of 3 months [2]. There is a significant geographic variation in occurrence of GBC with high prevalence in Indian subcontinent [3]. Radical resection remains the only potential curative treatment for operable lesions. Initial reports of radical surgery for GBC date back to twentieth century which advocated cholecystectomy with wedge resection of gall bladder fossa without regional lymphadenectomy [4, 5]. Because of poor outcomes with these initial procedures, Glenn et al. [6] in 1954 first described the “radical cholecystectomy” which included en bloc resection of gall bladder (GB), gall bladder fossa and node-bearing tissue in hepatoduodenal ligament. Many authors all over the world have shown improved survival in patients undergoing radical surgery for resectable GBC [7–10].
Past decade has witnessed major developments in technology for minimally invasive surgery and imaging. Along with this, better understanding of anatomy has resulted in exponential growth in laparoscopic surgery and now this approach is considered as standard of care for many gastrointestinal malignancies. Although laparoscopic cholecystectomy is one of the earliest procedures to develop, surgical community is still reluctant to use laparoscopic approach for GBC probably because of multiple reasons like fear of tumor dissemination during laparoscopy, difficulty in achieving adequate lymphadenectomy, complexities of laparoscopic liver resection and overall nihilistic approach toward this disease. Aim of this study is to share our initial experience of laparoscopic radical cholecystectomy (LRC) for suspected early gall bladder carcinoma.
Materials and methods
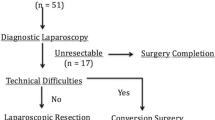
This is a retrospective analysis of patients who underwent LRC for suspected early gall bladder cancer at a tertiary care hospital from 2008 to 2013. A total of 91 patients were evaluated for suspected carcinoma of gall bladder, of which 57 (62.6 %) had metastatic or locally unresectable disease at the time of diagnosis or during staging laparoscopy. Laparoscopic approach has not been offered to another 20 patients in view of infiltration of bile duct, obvious involvement of liver or bulky nodal disease. Finally, 14 patients who had early disease underwent laparoscopic radical cholecystectomy and were included in this study.
Preoperatively all patients underwent routine blood investigations including complete blood counts, serum chemistry and liver function tests as well as tumor markers CEA and CA19-9. CECT abdomen has been done for staging of the disease. Patients with early GBC based on imaging and supported by diagnostic laparoscopy were offered LRC. All patients were operated under general anesthesia with endotracheal intubation. Prophylactic antibiotics were given at the time of induction.
Technique
Patient is placed supine with reverse Trendelenburg′s position keeping the legs apart. Surgeon stands between legs of the patient with camera surgeon standing on right side of the patient while monitor is kept at head end. Pneumoperitoneum is created with closed technique using Veress needle, and insufflation pressure is maintained at 14 mm Hg. We commonly use five ports (Fig. 1): camera port in supra-umbilical region (10 mm), 10-mm working port in left sub-costal space in mid-clavicular line, another working port in right sub-costal space in mid-clavicular line (5 mm), epigastric port (10 mm) and retraction port in right anterior axillary line just below left working port (5 mm). Additional ports are used when necessary.
First step after placing ports is to thoroughly inspect peritoneal cavity for signs of unresectable disease. Lymph node basins mainly looked for enlargement are inter-aortocaval and celiac group, approached through Kocherization of duodenum and opening of lesser sac, respectively. If found enlarged; the samples are sent for frozen analysis. Subsequently, the decision to defer the procedure or to opt for extended nodal clearance is taken in accordance with biopsy report. Once the decision is made to proceed, round and falciform ligaments are divided and used to retract the liver. We keep the handling of gall bladder to minimum to prevent accidental bile spillage.
After Kocherization, duodenum is retracted medially to expose the posterior aspect of head of pancreas and continued till exposing right lateral border of aorta. Figure 2 represents a schematic illustration showing lymph node stations to be included for removal. All fibro-fatty tissue along the posterior-superior aspect of pancreatic head till the right lateral aspect of vena cava, above the insertion of right renal vein is dissected and swept cranially. Lymphadenectomy progresses further by entering the lesser sac after opening gastrohepatic omentum. The origin of the celiac trunk is defined, and the tissues overlaying and along the common hepatic artery are excised, safeguarding the gastroduodenal artery (Fig. 4). All the tissues cleared from prior dissected areas are swept toward hepato-duodenal region; to be included in the final specimen, en bloc. Finally, the hepatoduodenal ligament is opened, portal structures are skeletonized circumferentially, and lymphadenectomy is completed after removing peri-portal, peri-choledochal and nodes along the hepatic artery proper till its bifurcation (Figs. 3, 5). All the resected tissue is then kept in plastic retrieval bag for removal. Cystic duct and artery are divided close to the origin after applying titanium clips. Entire fibro-fatty tissue is cleared from cystic triangle skeletonizing the portal branches and hepatic arteries and swept toward the cystic pedicle to be removed later along with gall bladder and liver bed. In cases with lesions involving neck of GB, transected margin of cystic duct is subjected for frozen examination.
Illustrative picture showing lymph node stations to be removed during radical cholecystectomy: 1—hilar, 2—cystic, 3—peri-choledochal, 4—nodes along hepatic artery, 5—periportal, 6—celiac, 7—common hepatic, 8—nodes around posterior-superior aspect of head of pancreas; GB gall bladder, D duodenum, P pancreas, V superior mesenteric vein, A superior mesenteric artery
We perform segmental resection involving segment 4B and 5 in all patients (Fig. 6). Resection plane is marked using harmonic hook or mono-polar diathermy after confirmation of anatomical landmarks using laparoscopic ultrasound. Initially, liver capsule is divided using harmonic scalpel (Ethicon endo-surgery, Cincinnati, USA), while deeper parenchymal division is performed using combination of Harmonic Scalpel, Ligasure (Valleylab, Boulder, CO) and bipolar diathermy according to surgeon’s preference. Any structures of >3 mm sizes are clipped. Proximal portion of middle hepatic vein encountered during parenchymal division is ligated with clips. Once resection is complete, specimen is placed in plastic retrieval bag along with previously resected lymphadenectomy tissue and removed via small supra-pubic incision. Surface hemostasis is achieved by applying argon plasma coagulation. Bile leak if any is controlled with suture ligation. Wide bore closed tube drain is kept in right sub-hepatic space.
Postoperatively all patients are kept in ICU for observation and shifted to room after 24 h. Oral liquid is started after return of bowel activity, and patients are discharged after removal of drain when output ceases.
Statistical analysis
Patient demographics, operative data and postoperative outcomes were analyzed. Continuous data were expressed as mean ± standard deviation (SD). Survival analysis was performed using the Kaplan–Meier method. Statistical software used was Prism 6 (GraphPad Software Inc., CA, USA).
Results
Mean age of the entire cohort was 61.14 ± 4.20 years with male to female ratio of 1:1.33. Most of the patients (71.42 %) presented with nonspecific symptoms such as upper abdominal discomfort, dyspepsia or were incidentally diagnosed. Significant abdominal pain was present in 4 (28.57 %). Significant weight loss was detected in 3 (21.42 %) while none of the patients having jaundice. Half of the patients had associated gall stones, while no one had positive family history. Patient demographics are shown in Table 1.
Mean operating time was 212.9 ± 26.73 min with mean blood loss of 196.4 ± 63.44 ml. None of the patient required blood transfusion in perioperative period. Pringle maneuver was not required in any of the patients. Procedure was completed laparoscopically in all without conversion. Patients were allowed liquids on first postoperative day (POD) and diet next day. Most patients had drains removed by 4th POD except one who had it for 2 weeks in view of bile leak. Mean hospital stay was 5.14 ± 0.86 days without any 30-day mortality.
Out of two patients who developed bile leak, first patient underwent endoscopic retrograde cholangiography (ERC) and stenting on 5th POD day due to persistent high bilious output. Second patient reported back to emergency services after 1 week of discharge with abdominal pain and fever. Ultrasound showed a biloma in right sub-hepatic space which was drained by placing pigtail catheter percuteniously with antibiotic cover. Both patients recovered without necessitating any surgical intervention. Lower respiratory tract infection occurred in two patients who recovered with conservative treatment. Operative data and postoperative outcomes are summarized in Table 2.
Of total 14 patients who underwent laparoscopic radical cholecystectomy, 12 had adenocarcinoma of gall bladder, one had xanthogranulomatous cholecystitis (XGC) and another had adenomyomatosis as final pathology. Pathological outcomes are shown in Table 3. Only patients with adenocarcinoma (n-12) were included for further pathological and survival analysis. Resected margins were free in all of them (>1 cm). Median number of lymph nodes resected was 8 (range 4–14). Pathological stage of disease was pT2N0 in 8, pT2N1 in 3 and pT3N0 in one patient. All patients were given gemcitabine/5-fluorouracil-based adjuvant chemotherapy.
Follow-up was based on regular hospital visits with telephonic interviews whenever needed. Median follow-up achieved was 51 months (range 14–70 months). Three patients died during follow-up period, of which two had node-positive disease and one with T3 lesion. Of three deaths, two had metastatic disease while in third, cause could not be determined. Overall, 5-year survival was 68.75 % (Fig. 7).
Discussion
Optimum surgical management of GBC remains controversial. Laparoscopic simple cholecystectomy alone is now widely accepted treatment for Tis and T1a GBC [11–13]. For incidentally detected GBC stage T1b, second open surgery with radical resection combined with lymphadenectomy and excision of port sites has been recommended [14]. Until recently, role of laparoscopy in preoperatively suspected GBC was restricted to staging purpose only. Recently, few reports have shown feasibility of laparoscopic radical resection for early gall bladder cancer [15–17].
First concern regarding use of laparoscopy among surgeons is the feasibility of adequate lymphadenectomy. Because of high propensity of lymphatic spread in patients with GBC, adequate lymphadenectomy is invaluable to improve survival after resection [18]. The extent of optimal lymphadenectomy in GBC is controversial, with eastern and western authors differing on their views regarding number of minimum nodes needed as well as extent of lymphatic clearance advised. In spite of differences, most authors will agree to a node retrieval count of six or more [9, 19, 20]. In our series, we could achieve a median resected lymph node count of eight (range 4–14). Better magnification in laparoscopy leading to clear anatomical delineation supported by our prior experience with advanced minimal access procedures [21] has contributed in performing adequate lymphadenectomy.
Intra-operative peritoneal dissemination of cancer cells with subsequent peritoneal metastasis and port site recurrence is another concern expressed with laparoscopic management of GBC [22–24]. It has been found that such complications were mainly due to inappropriate handling of gall bladder during laparoscopy, accidental perforation of gall bladder and CO2 pneumoperitoneum [14, 22, 25, 26]. We believe that this can be reduced by careful and minimal handling of gall bladder during surgery and use of plastic retrieval bag for removal of specimen once the resection is over, as also suggested by others [16, 27]. Gumbs et al. [15] and Takashi et al. [16] have not observed this complication in their respective series of laparoscopic radical cholecystectomy. We also believe that peritoneal dissemination and port site metastasis are related to disease stage as well as biological nature of tumor rather than approach used. Currently, routine port site resection is also not recommended for incidentally detected GBC following laparoscopic cholecystectomy [28, 29].
There is overall pessimistic approach toward this disease among the surgical community because of poor outcomes mainly contributed by presentation at advanced stage. In a review published in 1978 which included 5836 cases of GBC, authors have shown median survival of 5–8 months with 5-year survival rate of only 5 % [30]. In 1994, Cubertafond et al. [2] have shown median survival of only 3 months with 5-year survival rate of 5 % in their review of 724 cases of GBC. However, recent reports from both Asian and Western authors have shown improved 5-year survival (24.4–63 %) with radical resection strategies [7, 10, 31, 32]. In a recently published series of laparoscopic radical cholecystectomy, authors have reported 5-year survival rate of 100 and 83.3 % for T1b and T2 GBC, respectively [16]. In present series, overall 5-year survival rate was 68.75 % mainly in view of early disease.
In this series, out of total 14 patients, two came out to be benign conditions: xanthogranulomatous cholecystitis (XGC) and adenomyomatosis of gall bladder on final histology. Both these patients had uncertain preoperative diagnosis even after extensive evaluation and were considered as possible GBC. According to the literature, these two benign conditions of gall bladder can be frequently misdiagnosed as GBC. Xanthogranulomatous cholecystitis can mimic GBC because of similar clinical presentation, radiological findings and even intra-operative features with reported rate as high as 25 % [33, 34]. Reports also suggest high association of GBC with XGC and about 2–15 % of patients with XGC have coexisting GBC [33]. There are certain findings on imaging which can differentiate GBC from XGC such as presence of focal wall thickening, early wall enhancement, loss of continuous mucosal lining and local infiltration in former. However, on radiological imaging alone, differentiating GBC from XGC with a high degree of accuracy is not possible [33]. Simple cholecystectomy, which also carries risk of bile spillage in such setting, will significantly reduce survival [22]. In scenario of diagnostic uncertainty, radical en bloc resection is advisable, if expertise is available [33].
Another such benign condition is adenomyomatosis of gall bladder which is pathologically defined as an epithelial proliferation and hypertrophy of the GB muscularis with an outpouching of the mucosa into the thickened muscular layer (Rokitansky–Aschoff sinus) [35, 36]. Pathognomonic radiological findings of adenomyomatosis of gall bladder include intramural cyst within a thickened GB wall (Rokitansky–Aschoff sinus) and the presence of cholesterol crystals or stones deposited in the Rokitansky–Aschoff sinuses [37]. Although it is possible to differentiate adenomyomatosis from GBC in most cases based on imaging, there may be certain situations where distinction between the two is blurred, because of nonspecific findings of gall bladder thickening and enhancement [38].
With present series, we would like to share our initial experience regarding laparoscopic management of GBC. Apart from demonstrating the feasibility of the procedure by minimal access approach with excellent nodal clearance and comparable short-term outcomes, we could offer advantages of laparoscopy such as shorter hospital stay, less analgesic requirements and avoidance of wound-related complications. As authors, we agree that there are certain limitations of the present study such as small sample size and highly selected patients with early lesions, so the results need to be interpreted with caution and cannot be generalized. The procedure also needs considerable expertise in advanced laparoscopy.
Conclusion
Laparoscopic radical cholecystectomy with lymphadenectomy can be a viable alternative for management of early GBC in terms of technical feasibility and oncological clearance along with offering the conventional advantages of minimal access approach. Although the initial results are encouraging, authors advise a word of caution for its interpretation in wider perspective because of small number of highly selected patients.
References
Donohue JH, Stewart AK, Menck HR (1998) The National Cancer Data Base report on carcinoma of the gallbladder, 1989–1995. Cancer 83(12):2618–2628
Cubertafond P, Gainant A, Cucchiaro G (1994) Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg 219(3):275–280
Misra S, Chaturvedi A, Misra NC, Sharma ID (2003) Carcinoma of the gallbladder. Lancet Oncol 4(3):167–176
Sheinfeld W (1947) Cholecystectomy and partial hepatectomy for carcinoma of the gall bladder with local liver extension. Surgery 22(1):48–58
Glenn F, Hays DM (1953) Carcinoma of the extrahepatic biliary tract. Surg Clin North Am 479–492
Glenn F, Hays DM (1954) The scope of radical surgery in the treatment of malignant tumors of the extrahepatic biliary tract. Surg Gynecol Obstet 99(5):529–541
Dixon E, Vollmer CM Jr, Sahajpal A, Cattral M, Grant D, Doig C, Hemming A, Taylor B, Langer B, Greig P, Gallinger S (2005) An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 241(3):385–394
Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Furuse J, Saito H, Tsuyuguchi T, Yamamoto M, Kayahara M, Kimura F, Yoshitomi H, Nozawa S, Yoshida M, Wada K, Hirano S, Amano H, Miura F (2008) Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg 15(1):41–54
Ito H, Ito K, D’Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR (2011) Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 254(2):320–325
Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY (2012) Fourteen year surgical experience of gallbladder cancer: validity of curative resection affecting survival. Hepatogastroenterology 59(113):36–41
Jin K, Lan H, Zhu T, He K, Teng L (2011) Gallbladder carcinoma incidentally encountered during laparoscopic cholecystectomy: how to deal with it. Clin Transl Oncol 13(1):25–33
Misra MC, Guleria S (2006) Management of cancer gallbladder found as a surprise on a resected gallbladder specimen. J Surg Oncol 93(8):690–698
Toyonaga T, Chijiiwa K, Nakano K, Noshiro H, Yamaguchi K, Sada M, Terasaka R, Konomi K, Nishikata F, Tanaka M (2003) Completion radical surgery after cholecystectomy for accidentally undiagnosed gallbladder carcinoma. World J Surg 27(3):266–271
Varshney S, Butturini G, Gupta R (2002) Incidental carcinoma of the gallbladder. Eur J Surg Oncol 28(1):4–10
Gumbs AA, Hoffman JP (2010) Laparoscopic radical cholecystectomy and Roux-en-Y choledochojejunostomy for gallbladder cancer. Surg Endosc 24(7):1766–1768
Shirobe T, Maruyama S (2014) Laparoscopic radical cholecystectomy with lymph node dissection for gallbladder carcinoma. Surg Endosc. doi:10.1007/s00464-014-3932-9
Agarwal AK, Javed A, Kalayarasan R, Sakhuja P (2015) Minimally invasive versus the conventional open surgical approach of a radical cholecystectomy for gallbladder cancer: a retrospective comparative study. HPB (Oxford). doi:10.1111/hpb.12406
Shirai Y, Wakai T, Hatakeyama K (2007) Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am 16(1):221–232
Negi SS, Singh A, Chaudhary A (2011) Lymph nodal involvement as prognostic factor in gallbladder cancer: location, count or ratio? J Gastrointest Surg 15(6):1017–1025
Shirai Y, Sakata J, Wakai T, Ohashi T, Ajioka Y, Hatakeyama K (2012) Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol 10:87
Senthilnathan P, Srivatsan Gurumurthy S, Gul SI, Sabnis S, Natesan AV, Palanisamy NV, Praveen Raj P, Subbiah R, Ramakrishnan P, Palanivelu C (2015) Long term results of Laparoscopic Pancreaticoduodenectomy for Pancreatic and Periampullary Cancer- Experience Of 130 Cases from a Tertiary Care Center in South India. J Laparoendosc Adv Tech A. doi:10.1089/lap.2014.0502
Lee JM, Kim BW, Kim WH, Wang HJ, Kim MW (2011) Clinical implication of bile spillage in patients undergoing laparoscopic cholecystectomy for gallbladder cancer. Am Surg 77(6):697–701
Cavallaro A, Piccolo G, Panebianco V, Lo Menzo E, Berretta M, Zanghì A, Di Vita M, Cappellani A (2012) Incidental gallbladder cancer during laparoscopic cholecystectomy: managing an unexpected finding. World J Gastroenterol 18(30):4019–4027
Shirai Y, Yoshida K, Tsukada K, Muto T (1992) Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg 215(4):326–331
Evrard S, Falkenrodt A, Park A, Tassetti V, Mutter D, Marescaux J (1997) Influence of CO2 pneumoperitoneum on systemic and peritoneal cell-mediated immunity. World J Surg 21(4):353–356
Paolucci V (2001) Port-site recurrences after laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg 8(6):535–543
Drouard F, Delamarre J, Capron JP (1991) Cutaneous seeding of gallbladder cancer after laparoscopic cholecystectomy. N Engl J Med 325(18):1316–1319
Maker AV, Butte JM, Oxenberg J, Kuk D, Gonen M, Fong Y, Dematteo RP, D’Angelica MI, Allen PJ, Jarnagin WR (2012) Is port site resection necessary in the surgical management of gall bladder cancer ? Ann Surg Oncol 19(2):409–417
Fuks D, Regimbeau JM, Pessaux P, Bachellier P, Raventos A, Mantion G, Gigot JF, Chiche L, Pascal G, Azoulay D, Laurent A, Letoublon C, Boleslawski E, Rivoire M, Mabrut JY, Adham M, Le Treut YP, Delpero JR, Navarro F, Ayav A, Boudjema K, Nuzzo G, Scotte M, Farges O (2013) Is port site resection necessary in the surgical management of gall bladder cancer? J Visc Surg 150(4):277–284
Piehler JM, Crichlow RW (1978) Primary carcinoma of the gallbladder. Surg Gynecol Obstet 147(6):929–942
Shirai Y, Wakai T, Sakata J, Hatakeyama K (2012) Regional lymphadenectomy for gallbladder cancer: rational extent, technical details, and patient outcomes. World J Gastroenterol 18(22):2775–2783
Fong Y, Jarnagin W, Blumgart LH (2000) Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 232(4):557–569
Hale MD, Roberts KJ, Hodson J, Scott N, Sheridan M, Toogood GJ (2014) Xanthogranuolmatous cholecystitis: a European and global perspective. HPB (Oxford) 16(5):448–458
Yang T, Zhang B, Zhang J, Zhang Y, Jiang X, Wu M (2007) Surgical treatment of xanthogranulomatous cholecystitis: experience in 33 cases. Hepatobiliary Pancreat Dis Int 6:504–508
Colquhoun J (1961) Adenomyomatosis of the gall-bladder (intramural diverticulosis). Br J Radiol 34:101–112
Williams I, Slavin G, Cox A, Simpson P, de Lacey D (1986) Diverticular disease (adenomyomatosis) of the gallbladder: a radiological-pathological survey. Br J Radiol 59:29–34
Bang SH, Lee JY, Woo H, Joo I, Lee ES, Han JK, Choi BI (2014) Differentiating between Adenomyomatosis and Gallbladder Cancer: revisiting a comparative study of High-resolution ultrasound, multidetector CT, and MR imaging. Korean J Radiol 15(2):226–234
Pellino G, Sciaudone G, Candilio G, Perna G, Santoriello A, Canonico S, Selvaggi F (2013) Stepwise approach and surgery for gallbladder adenomyomatosis: a mini-review. Hepatobiliary Pancreat Dis Int. 12(2):136–142
Acknowledgments
Authors would like to give special thanks to Dr. Harish Kakkilaya of GEM hospital and research centre for his valuable contribution in preparing all illustrations for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Senthilnathan Palanisamy, Nikunj Patel, Sandeep Sabnis, Nalankilli Palanisamy, Anand Vijay, Praveenraj Palanivelu, Parthasarthi R and Palanivelu Chinnusamy have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Palanisamy, S., Patel, N., Sabnis, S. et al. Laparoscopic radical cholecystectomy for suspected early gall bladder carcinoma: thinking beyond convention. Surg Endosc 30, 2442–2448 (2016). https://doi.org/10.1007/s00464-015-4495-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4495-0