Abstract
Background
In the era of minimally invasive surgery, laparoscopy has a great role to play in the management of pseudocyst of pancreas. We present our surgical experience over the past 12 years (May 1994 to April 2006) in the management of pancreatic pseudocysts.
Materials and Methods
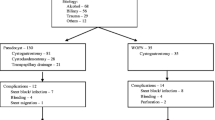
The total number of cases was 108, with 76 male and 32 female patients. Age ranged from 18 to 70 years. Duration of symptoms ranged from 45 days to 7 months. Fifty-nine patients presented with pain abdomen. Sixty-one patients had co-morbid illness. Ten patients had abdominal mass on clinical examination. Predisposing factors were gallstones in 58 cases, alcohol in 20 cases, trauma in eight cases and post-pancreatectomy in one case. In 21 cases there are no predisposing factors.
Results
All the cases were successfully operated without any significant intraoperative complication. Laparoscopic cystogastrostomy was done in 90 cases (83.4%), laparoscopic cystojejunostomy in eight cases (7.4%), open cystogastrostomy in two cases (1.8%), and laparoscopic external drainage in eight cases (7.4%). Laparoscopic cholecystectomy was done in 47 cases along with the drainage procedure. The mean operating time was 95 minutes. Mean blood loss was 69 ml. Mean hospital stay was 5.6 days. Percutaneous tube drain to assist decompression of the cyst was kept in all the laparoscopic cystojejunostomy (LCJ) group. Two patients were re-operated for bleeding and gastric outlet obstruction. We had no mortality in the postoperative period. With mean follow up of 54 months (range 3–145 months); only one patient who underwent laparoscopic cystogastrostomy (LCG) earlier in this series had recurrence due to inadequate stoma size. This patient later underwent OCG
Conclusion
Laparoscopy has a significant role to play in the surgical management of pseudocysts with excellent outcome. It offers all the benefits of minimally invasive surgery to the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Pancreatic pseudocysts (PP) accounts for over 75% of the cystic lesions of the pancreas. These cysts are treated by percutaneous, endoscopic or surgical approaches. Reports of therapeutic laparoscopy of the pancreas have been few. Laparoscopic surgery of the pancreas remains in its infancy, and its role in pancreatic disease is still unclear. Although several laparoscopic pancreatic procedures have been described, debate continues over which procedures can be safely and adequately performed and which clearly benefit the patient when performed laparoscopically. Minimally invasive surgery is now increasingly used in the management of pseudocyst pancreas. We present our surgical experience over the past 12 years in the management of patients with pancreatic pseudocysts admitted for surgery.
Materials and Methods
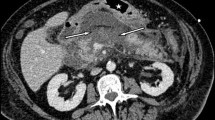
We analyze and present our retrospective data of patients admitted for surgery for PP. A total of 108 patients were treated for PP during the past 12 years (May 1994 to April 2006). The indications for surgical intervention were: (1) symptomatic cysts. (2) rapidly enlarging cysts, (3) cyst diameter of more than 6 cm, six weeks after the development of the cyst and presence of pancreatic necrosis, and (4) development of complications such as rupture, infection and obstruction of the bile duct, duodenum and other adjacent organs. All patients were evaluated by complete hemogram, fasting blood sugar, renal profile, liver function tests, coagulation profile, serum amylase and lipase, ultrasonography (USG) and dual-phase computerized tomography (CT) with a specific pancreatic imaging protocol. Magnetic resonance cholangiopancreatography (MRCP) was used selectively in patients who presented with jaundice and altered liver function tests. Upper GI flexible endoscopy was done in patients who presented with features of gastric outlet obstruction. Pre-surgical anesthetic fitness was obtained in all cases. Antibiotic prophylaxis was started with third-generation cephalosporin and metronidazole. All patients were operated under general anesthesia. For retrogastric cysts, patients were positioned in modified semi-lithotomy position, with the operating surgeon standing between the legs of the patient, the camera surgeon on the right side of the patient and the assistant surgeon standing on the left side of the patient. The monitor was placed at the head end of the patient. However, if the cyst presented through the left paracolic gutter, the patient was placed in the supine position with 15° right lateral tilt and the operating surgeon stood on the right side of the patient with the camera surgeon on the left hand side of the surgeon. The assistant surgeon, if required, stood on the left hand side of the camera surgeon.
Laparoscopic cystogastrostomy (LCG)
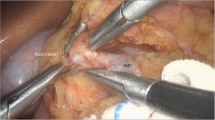
The camera port (10 mm) was placed in the midline, 3 cm above the umbilicus. The right-hand working port (5 mm) was placed in the left hypochondrium of the patient and the left-hand working port (5 mm) was placed in the right paramedian position, both these ports being cranial in relation to the camera port. An additional fourth port (5 mm), if required, was placed in the subxiphoid area for the liver retraction (32 cases). The operation involves anterior gastrotomy at the summit of the cyst as the first step (Figure 1). The harmonic scalpel (Ethicon Endosurgery, Cincinnati, USA) is an excellent tool for bloodless incision in the anterior gastric wall. In six cases with a very large cyst, we aspirated the fluid partially from the pseudocyst under laparoscopic visualization, by using percutaneous transgastric puncture with a Veress needle, before anterior gastrotomy. This step gave better exposure for the insertion of the other working ports and for performing anterior gastrotomy. After anterior gastrotomy, multiple interrupted everting stitches with silk were made from the edges of the gastrotomy to the anterior gastric wall about 2 cm away from the gastrotomy wound. This maneuver aided in keeping the gastrotomy wound open, especially after decompression of the cyst. Diagnostic aspiration was done under direct vision using a spinal needle attached to a 10 ml syringe (Figure 2). A stay stitch was placed at the summit of the bulge incorporating the posterior gastric wall with the anterior cyst wall (Figure 3). A 4 cm stoma was created using the harmonic scalpel between the cyst and the stomach, which was made easier by lifting of the stay suture on the pseudocyst (Figure 4). Hemostatic sutures were placed with either continuous or interrupted absorbable sutures (polyglactin 2 0) between the posterior gastric wall and the anterior wall of the cyst. The cyst cavity was examined using the 30° telescopes and all the necrotic material was debrided using a large fenestrated bowel grasper (Figure 5). The cyst cavity was irrigated thoroughly and the nasogastric tube was placed within the cyst. Intracorporeal sutures with 2–0 polyglactin were used to close the anterior gastrotomy (Figure 6). The peritoneal cavity was lavaged and a drainage tube placed.
Laparoscopic cystojejunostomy (LCJ)
In cases where the pseudocyst presented through the gastrocolic omentum or through the paracolic gutter (left paracolic approach) at the left iliac fossa, we performed LCJ for dependent drainage (Figure 7A–D). We used four ports depending on the site of the pseudocyst, with one port as 12 mm for the insertion of the Endo GIA stapler (Ethicon Endosurgery, Cincinnati, USA). To approach the cyst projecting through the gastrocolic omentum, the same was widely opened. A Roux-en-Y jejunal loop was created using the Endo-GIA stapler. After debridement, wide (4cm), hand-sewn pseudocystojejunal anastomosis was performed with continuous polyglactin sutures. A drainage tube was kept in the left paracolic gutter.
Laparoscopic external drainage (LED)
We performed LED in those patients having severe abdominal pain and vomiting due to gastric outlet obstruction, jaundice due to bile duct compression in whom pseudocystojejunostomy/gastrostomy was not feasible either because the pseudocysts had a thin wall (wall thickness <5 mm on imaging) or the pseudocyst wall was not adherent to the posterior wall of the stomach. LED also managed partial rupture and infection. The position of the patient and the team setup was similar to that of LCG. Thorough peritoneal lavage was given and an 18 or 20 F size drainage tube was introduced into the pseudocyst for decompression and external drainage.
Open cystogastrostomy (OCG)
The technique adopted was similar to that of LCG, done using upper midline incision.
Postoperative protocol and follow-up
Post-operatively all the patients were observed in an intensive care unit (ICU) for 24 hours. Nasogastric tube was removed on the first postoperative day and oral diet was started after the bowels moved. During postoperative follow-up, the patients were evaluated by clinical examination and ultrasound abdomen after 15 days, one month, three months, six months and yearly thereafter.
Results
The patient characteristics are summarized in Table 1. Ten cases were asymptomatic and were detected during routine USG for other complaints. Fifty-two patients (48.2%) had mass in the abdomen on clinical examination (41 in the epigastric region and 11 in the left hypochondrium). Predisposing factors were gallstones in 58 cases (54%), alcohol in 20 cases (18.5%), trauma in eight cases (7.5%) and previous distal pancreatectomy for serous cystadenoma of the tail of the pancreas in one case. In 21 cases (19%), there were no detectable predisposing factors.
Two patients (1.9%) had multiple pseudocysts and one (0.9%) patient had recurrence following open cystogastrostomy. Four patients (3.0%) presented with partial rupture of the pseudocyst. Two patients had infected pseudocyst and underwent external drainage. The operative characteristics are summarized in Table 2. An extra port was placed in the sub-xiphoid region in 32 cases where LCG was being performed. Of the eight patients in whom LCJ was performed, the pseudocyst was approached through the gastrocolic omentum in four cases and through the left paracolic gutter in the remaining four cases. Laparoscopic cholecystectomy (LC) was done along with pseudocyst surgery in 47 cases.
All the cases were successfully operated without any significant intraoperative complications. For the laparoscopic approach, there were no conversions. The mean post-operative hospital stay was 5.6 days (range 3–22 days). The mean ICU stay was 1.2 days (range 1–6 days). Morbidity is described in Table 3. Patients managed with LED and LCJ with a decompression tube into the cyst had a prolonged hospital stay (means of 14 and 12 days, respectively). Two patients in this group developed intra-abdominal infection, which required prolonged parenteral antibiotics. One patient in the LCJ group developed infection in the residual cavity with prolonged purulent drainage that lasted for 28 days. Three patients in the LED group had prolonged drainage for more than two weeks. One patient who underwent LED earlier for cyst rupture developed gastric outlet obstruction and infection of the residual cyst, managed by laparatomy with necrosectomy and drainage on the 11th post-operative day. Another patient in the LCG group developed upper GI bleed, managed by laparatomy to control the bleeding site at the cystogastrostomy stoma. This patient had re-bleeding, which was managed by selective angiographic embolization. We had one more post-operative bleeding in the LCG group, manifested by falling hemoglobin, managed conservatively by three units of blood transfusion. We had no mortality in the postoperative period. We randomly followed up the cases for obliteration of the pseudocyst by gastroscope in 22 cases following LCG and found that the cavity was obliterated by seven days. With a mean follow up of 54 months (range 3–145 months); only one patient who underwent LCG earlier in this series had recurrence due to inadequate stoma size. This patient later underwent OCG.
Discussion
Pancreatic pseudocyst is a collection of amylase-rich pancreatic juice enclosed by a wall of fibrous granulation tissue typically related to an antecedent episode of pancreatic inflammation [8]. In relation to an episode of acute pancreatitis, fluid collection cannot be called a pseudocyst until at least four weeks have elapsed from the onset of the episode [2, 8]. A generally observed surgical precept is that a pseudocyst that persists beyond 4–6 weeks after an episode of pancreatitis is likely both to lead to symptoms and fail to resolve in the absence of intervention, although there is literature to suggest that some pseudocysts may be safely followed up [29, 31]. Ammori listed both large (>6 cms.) and persistent (>6 weeks) as indications for internal drainage of the pseudocyst [1]. We consider the presence of necrotic debris in large persistent pseudocysts to constitute an indication for surgery. Other indications of surgery are presence of infection, rupture, biliary obstruction and gastric outlet obstruction. The treatment options available are endoscopic drainage, percutaneous drainage and surgical drainage or surgical excision of the cyst. Factors determining the route and the time of intervention are: (a) location of the cyst, (b) maturity of the cyst wall when the patient presents with symptoms, (c) presence or absence of complications, and (d) availability of local expertise and experience.
Endoscopic drainage, especially in the presence of endoscopic ultrasound (EUS), is an important option in the management of pseudocysts. This is especially true for cysts indenting the stomach or duodenum and in the absence of necrotic tissue. Cysts can be drained into the duodenum by placing a stent through the papilla across the strictured pancreatic duct or into the pseudocyst itself if there is a cyst at the level of head of pancreas communicating with the main pancreatic duct [6]. Cysts can also be drained into the duodenum by placing a stent into the cyst by EUS-guided transmural puncture or into the stomach by placing transmural stent [4]. Endoscopic drainage is associated with failure to drain (15.4%), morbidity (13.3%) and recurrence (10.7%) when pseudocysts that complicate acute necrotizing pancreatitis are approached [5, 24]. Endoscopic transmural drainage is also not possible in the following conditions: (1) the stomach or duodenal wall do not share a common wall with the pseudocyst, and (2) the distance between the pseudocyst and the stomach wall is >1 cm on preoperative evaluation [3, 10, 12, 13, 26, 28]. The services of a highly skilled endoscopist are required. In selected patients, success rates of 65–100% have been reported [19, 26]. We had to perform debridement of necrotic tissue in all our patients. In the presence of necrotic tissue, endoscopic drainage has been besieged with complications such as stent blockage and inadequate drainage with subsequent infection [16, 26].
We have used intracorporeal suturing to create the cystogastrostomy and the cystojejunostomy as well as to close the anterior gastrotomy. This does require advanced laparoscopic suturing skills. If required, this steps can be facilitated using laparoscopic staplers, however, at added expenses.
We used laparoscopic external drainage mainly for infected pseudocysts and obstructing pseudocysts with immature wall. It was also used in situations where definitive internal drainage could not be done, such as partly leaking cysts. In one series, percutaneous drainage was successful in 42% of patients and patients treated by percutaneous drainage had a higher mortality rate (16% vs. 0%), a higher incidence of complications (64% vs. 27%), and a longer hospital stay (45 ± 5 days vs. 18 ± 2 days) than patients treated by surgery [17]. In a review of published reports of percutaneous approaches with continuous catheter drainage, recurrence ranged from 0% to 22%, with failure of drainage approaching 33% in some studies [15]. The treatment of pancreatic pseudocysts has traditionally been surgical [7, 9, 11, 14, 18, 20, 21, 25, 28]. The laparoscopic approach is now being commonly used for pancreatic resections and pseudocyst management. A recent literature review showed that laparoscopic cystogastrostomy or cystojejunostomy achieves adequate internal drainage, facilitates concomitant debridement of necrotic tissue within the pseudocysts and achieves good result with minimal morbidity [5]. With the availability of advanced imaging systems and cameras, better hemostatic equipments and excellent suturing skills, most pseudocysts can be approached and managed by laparoscopic approach. We performed LCG as described earlier. Ultrasonic shears used for dissection were found to be an excellent device for hemostatic dissection, thereby keeping the operative field dry with minimal blood loss. When pseudocysts are located in close contact with the posterior wall of the stomach, they can also be drained by intragastric surgical techniques. In this technique, under standard laparoscopic observation, three intragastric ports are placed through the abdominal and anterior gastric walls, establishing working channels for a telescope and hand instruments. The posterior wall of the stomach and the cyst wall are incised, a sufficient drainage orifice is made and the cyst contents are thoroughly debrided. The intragastric ports are removed and defects in the gastric wall are closed with sutures placed via the standard laparoscopic approach [21]. We have never used this approach. We feel that the laparoscopic anterior gastrotomy approach gives wide access, is technically easy and does not require specialized balloon trocars in contrast to the transgastric approach. We were able to achieve wider stoma and place it in the most dependent portion of the pseudocyst. In cases where the most dependent portion of the pseudocyst was caudal to the stomach, we used the jejunum to perform the internal drainage. In our experience the LED and LCJ approaches are associated with prolonged hospital stay, prolonged drainage through the drain and a higher infection rate. Open surgery also has a definite role to play in the management of PP. Recurrent cysts following open surgery, patients who have multiple cysts and patients who have undergone multiple prior surgeries require OCG if endoscopic or percutaneous approaches are not possible. These are all relative contraindications for LCG.
Two of our patients required re-operation, one for gastric outlet obstruction and infection of the residual pseudocyst after LED and another for anastomotic site hemorrhage after LCG. In both the cases, the subsequent laparotomy was devoid of technical difficulties as there were virtually not adhesions due to the prior laparoscopic surgery. This is also an advantage of the laparoscopic approach.
With reasonably good long-term follow-up, only one of our patients during our early experience had recurrence following LCG. In our view, the laparoscopic approach to the management of pancreatic pseudocyst is the procedure of choice in patients who are fit for general anesthesia, if the surgeon is experienced in doing this procedure. LCG or LCJ is associated with the highest success rate, similar to that of open surgery, unlike the endoscopic or percutaneous approaches. Minimally invasive treatment of PP produces good results and avoids difficulties linked with percutaneous drainage or endoscopic internal procedures [22].
Conclusion
Surgery has got an important role to play in the management of pseudocyst pancreas, especially in patients presenting with cysts in the infracolic and paracolic compartment, cysts in the tail of the pancreas, cysts complicated by rupture or obstruction (which are not uncommon), cysts containing large amount of debris, suspicion of neoplastic cyst and in the absence of expertise for endoscopic treatment. Laparoscopy plays a major role in the surgical management of pseudocysts, with excellent long-term outcomes. The laparoscopic approach also facilitates debridement of the necrotic material. It is safe, feasible, effective, requires a short hospital stay and enables early resumption of diet.
References
Ammori BJ (2003) Pancreatic surgery in the laparoscopic era. J Pancreas 4: 187–192
Baron TH, Harewood GC, Morgan DE, Yates MR (2002) Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc 56: 7–17
Beckingham IJ, Krige JE, Bornman PC, Terblanche J (1997) Endoscopic management of pancreatic pseudocysts. Br J Surg 84: 1638–1645
Beckingham IJ, Krige JE, Bornman PC, Terblanche J (1999) Long term outcome of endoscopic drainage of pancreatic pseudocysts. Am J Gastroenterol 94: 71–74
Bhattacharya D, Ammori BJ (2003) Minimally invasive approaches to the management of pancreatic pseudocysts: review of the literature. Surg Laparosc Endosc Percutan Tech 13: 141–148
Binmoeller KF, Seifert H, Walter A, Soehendra N (1995) Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 42: 219–224
Bodker A, Kjaergaard J, Schmidt A, Tilma A (1981) Pancreatic pseudocysts. A follow-up study. Ann Surg 194: 80–84
Bradley EL, 3rd (1993) A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 128: 586–590
Bumpers HL EL B (1998) Treatment of pancreatic pseudocysts. In Howard J, Idezuki Y, Ihse IPrinz R (eds) Surgical Disease of the Pancreas. Williams & Wilkins Baltimore 423–432
Cooperman AM (2001) An overview of pancreatic pseudocysts: the emperor’s new clothes revisited. Surg Clin N Am 81: 391–397; xii
Cooperman AM (2001) Surgical treatment of pancreatic pseudocysts. Surg Clin N Am 81: 411–419; xii
Cremer M, Deviere J, Baize M, Matos C (1990) New device for endoscopic cystoenterostomy. Endoscopy 22: 76–77
Gerolami R, Giovannini M, Laugier R (1997) Endoscopic drainage of pancreatic pseudocysts guided by endosonography. Endoscopy 29: 106–8
Grace PA, Williamson RC (1993) Modern management of pancreatic pseudocysts. Br J Surg 80: 573–581
Gumaste VV, Pitchumoni CS (1996) Pancreatic pseudocyst. Gastroenterologist 4: 33–43
Hariri M, Slivka A, Carr-Locke DL, et al. (1994) Pseudocyst drainage predisposes to infection when pancreatic necrosis is unrecognized. Am J Gastroenterol 89:1781–1784
Heider R, Meyer AA, Galanko JA, Behrns KE (1999) Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg 229: 781–787; discussion 787–9
Kiviluoto T, Kivisaari L, Kivilaakso E, Lempinen M (1989) Pseudocysts in chronic pancreatitis. Surgical results in 102 consecutive patients. Arch Surg 124: 240–243
Lawson JM, Biallie J, (1995) Endoscopic therapy for pancreatic pseudocysts. Gastrointest Clin N Am 5: 181–193
Moran B, Rew DA, Johnson CD (1994) Pancreatic pseudocyst should be treated by surgical drainage. Ann R Coll Surg Engl 76: 54–58
Mori T, Abe N, Sugiyama M, Atomi Y, Way LW (2000) Laparoscopic pancreatic cystgastrostomy. J Hepatobiliary Pancreat Surg 7: 28–34
Park AE, Heniford BT (2002) Therapeutic laparoscopy of the pancreas. Ann Surg 236: 149–158
Pedrazzoli S, Sperti C, Pasquali C (2001) Pancreatic head resection for noninflammatory benign lesions of the head of the pancreas. Pancreas 23: 309–315
Rosso E, Alexakis N, Ghaneh P, Lombard M, Smart HL, Evans J, Neoptolemos JP (2003) Pancreatic pseudocyst in chronic pancreatitis: endoscopic and surgical treatment. Dig Surg 20: 397–406
Sahel J, Bastid C, Pellat B, Schurgers P, Sarles H (1987) Endoscopic cystoduodenostomy of cysts of chronic calcifying pancreatitis: a report of 20 cases. Pancreas 2: 447–453
Smits ME, Rauws EA, Tytgat GN, Huibregtse K (1995) The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastsrointest Endosc 42: 202–207
Usatoff V, Brancatisano R, Williamson RC (2000) Operative treatment of pseudocysts in patients with chronic pancreatitis. Br J Surg 87: 1494–1499
Vidyarthi G, Steinberg SE (2001) Endoscopic management of pancreatic pseudocysts. Surg Clin N Am 81: 405–410, xii
Vitas GJ, Sarr MG (1992) Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery 111: 123–130
Warshaw AL, Rattner DW (1985) Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann Surg 202: 720–724
Yeo CJ, Bastidas JA, Lynch-Nyhan A, Fishman EK, Zinner MJ, Cameron JL (1990) The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 170: 411–417
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palanivelu, C., Senthilkumar, K., Madhankumar, M.V. et al. Management of pancreatic pseudocyst in the era of laparoscopic surgery – Experience from a tertiary centre. Surg Endosc 21, 2262–2267 (2007). https://doi.org/10.1007/s00464-007-9365-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9365-y