Abstract
Background
Electrical stimulation of the phrenic nerve motor point of the diaphragm through laparoscopic implantation of a pacing system is an option for high spinal cord–injured patients with chronic respiratory insufficiency. This study assesses the operative learning curve for the initial series of patients.
Method
A series of six patients underwent laparoscopic placement of a diaphragm pacing system. The operative procedure was divided into the following four steps for analysis and rapid adjustment after each operation: exposure of the diaphragm, mapping of the phrenic nerve motor point, implantation of the pacing electrodes, and final routing of the wires to the external system.
Results
The first case required two operations, and the second case was unsuccessful because of a nonfunctioning phrenic nerve that led to a change in the preoperative screening criteria. The operative time decreased from 469 min for the first operation to 165 min for the sixth operation. The significant time decrease can be attributed to changes in the mapping and routing aspects of the operation. Key changes during this series that helped to reduce the operative time include abandonment of a software-dependent mapping technique, development of a grid algorithm for mapping, software improvement to increase the speed of stimulation and mapping, refinement of the mapping probe to maintain adequate suction on the diaphragm, shortening of the electrode lengths, and experience with the implantation of connections to the external electrodes. Presently, all five of the successfully implanted patients can be maintained on prolonged ventilatory support with the device.
Conclusion
Analysis of every step of this investigational procedure enabled us to make rapid changes in surgical protocol, leading to decreases in operative times and expectant improvements in patient safety and efficacy. In this series, analysis was the key to developing a low-risk cost-effective outpatient diaphragm pacing system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are an estimated 11,000 spinal cord injuries each year in the United States, with 4% resulting in long-term chronic respiratory insufficiency. These high tetraplegics (C1–C5) require positives-pressure mechanical ventilation. This treatment method has a great impact on their survival, the most common cause of death now being pneumonia. We have previously described a successful laparoscopic diaphragm pacing system that can support the ventilatory needs of these patients with a more physiologic negative-pressure ventilation [3, 5, 7]. This is now a viable option for these patients.
We have made significant improvements in the technique of placement of this system over a short period of time. In laparoscopic surgery, there has been significant interest in analyzing the learning curves for procedures; however, no absolute number of procedures to reach proficiency is ever clearly defined [2]. The goal of our project was to limit the learning curve of a truly investigational procedure using a novel technique in a difficult population of tetraplegics by continuous analysis of each procedure. In our protocol of implantation, the operation has always involved four key steps. In this paper, we analyze each of these steps with regard to time and describe what was done to decrese the time needed to accomplish the procedure and to increase patient safety and success.
Methods
At the University Hospitals of Cleveland, we received an FDA investigational device exemption and institutional review board approval (protocol # 029030) for laparoscopic implantation of a diaphragm pacing system. This study is based on the first six patients who were implanted. Inclusion criteria included tetraplegia with normal bilateral phrenic nerve function; freedom from active lung, cardiovascular, or brain disease, chest wall deformity, or obesity; and age >18 years. From the first patient until the last, all operations were videotaped and analyzed. For the initial patient, a formal review of all aspects of the procedure was undertaken by the entire team, which included the surgeon, pulmonologist, respiratory therapist, and biomedical engineers. A member of the biomedical engineering team was present in the operating room for all of the procedures.
The entire procedure used to implant the pacing electrodes for chronic ventilator dependence was divided into the following components: exposure, mapping of the diaphragm, implantation of the pacing wires, and final wire routing to the external location. Aspects of this procedure had been previously studied in canine experiments [4, 8, 9]. All changes in technique were categorized according to these four areas.
Exposure
The exposure time was defined as the time from the beginning of the procedure until the mapping probe was brought into the laparoscopic field. A total of four trocars were used: an umbilical Hasson trocar, two 12-mm lateral subcostal trocars, and a 5-mm epigastric port (Fig. 1) . The exposure time included the placement of the laparoscopic trocars and lyses of adhesions from previous operations, including the takedown of all previous gastrostomies.
Mapping
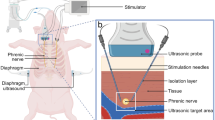
To achieve diaphragm activation the intramuscular electrodes need to be placed near the point, where the phrenic nerve enters into diaphragm-known as the phrenic nerve motor point. We have previously described this point in a cadaver study [6]. Because it cannot be visualized laparoscopically, we have developed a method to locate this point noninvasively [10]. The mapping procedure involved electrical stimulation of the diaphragm with a probe electrode (Synapse Biomedical, Oberlin, OH, USA) at several locations on each diaphragm in the presumed area of the phrenic nerve motor point (Fig. 2). For each test site, a quantitative assessment of the strength of the contraction was made by measuring changes in abdominal pressure. To measure abdominal pressure, a section of semi-rigid tubing extending from one of the surgical trocars was connected to a pressure transducer (Validyne Engineering Corp., Northridge, CA, USA). Larger increases in abdominal pressure meant we were closer to the motor point. Initially, a current distance model was used with mathematical analyses of data from several sites to predict the motor point for electrode placement. The mapping time was defined as the time from placement of the mapping probe into the operative field until the implantation device was brought into the field. We also analyzed the number of diaphragm locations mapped during each minute of this time period.
Implantation
Implantation time was defined as the time from the introduction of the electrode implant instrument into the operative field until all of the wires were brought out through the abdominal cavity. Figure 3 shows the laparoscopic implant instrument that is used to safely, dependably, and accurately place the electrodes in the diaphragm [1]. Two electrodes were placed in each diaphragm. This time period also included stimulation checks through the electrodes to confirm that there was adequate contraction of the diaphragm. We also analyzed how many times for each case an electrode was not placed correctly and therefor had to be removed. This is classified by the total wires used to successfully implant four electrodes in each patient.
Laparoscopic implant tool for electrode implantation Into the diaphragm. The electrode is carried in a hypodermic needle that is enclosed in the instrument for insertion into the abdominal cavity. The trigger controls the movement of the needle to enable the surgeon to control the angle of insertion into the tissue.
Wire routing
The wire routing period was defined as the time from implantation of all of the wires until the end of the procedure. This process involved connecting the implanted electrodes to the transcutaneous exiting wires. Because the connection is kept separate from the exit site, it is a less likely that an exit site infection will require removal of the stimulating electrode. The connection required the use of a spring connector, silicone sleeves, suture fixation, and glue. The exiting wires were then tunneled to a convenient chest wall exit site in a pattern that correlated to the location of the implanted electrodes. An additional indifferent (return) electrode is placed subcutaneously in the upper chest such that it exits with the other electrodes. The routing time period also included a double-check of the wires to confirm that there was no cardiac interaction and that contraction of the diaphragm was adequate.
Results
Table 1 summarizes the clinical data for the implanted patients in this initial series. All operations were done with only an overnight observational stay in the hospital. As shown in Table 2, we were able to maintain adequate tidal volume with the implanted electrodes in five of our six patients. The one failure was due to a false-positive phrenic nerve conduction study. Thereafter, we changed our preoperative screening protocol to include fluoroscopic evaluation of the diaphragm during the phrenic nerve conduction study. Table 3 shows a time analysis of the operation, with the addition of an analysis of the mapping sites and the number amount of wires implanted to result in four functioning electrodes . Because patient 1 required two operations to achieve placement of a functioning system, we added the amount of time needed for of each of the four areas to arrive at the total given in the tables. The adjustments made in each of the areas are outlined in the following paragraphs.
Exposure
This involves standard laparoscopic techniques, so there was no real change in the amount of time that was required. In two of the patients who had undergone exploratory laparotomy around the time of their initial trauma, the adhesions were lysed without difficulty or any increase in exposure time. All patients had previous enteral access, and these areas were taken down with laparoscopic linear cutters to decrease the risk of contamination of the implanted wires in cases where a small fistula tract was still present. Overall, no aspects needing improvement were identified in this area.
Mapping
This was the area requiring the greatest adjustment to the operative technique. The most important aspect is that in our first patient, we did not implant the electrodes correctly on the motor point of the right diaphragm. When we reviewed our data, we realized that our mathematical protocol for identifying the motor point was flawed in the assumption that you could be within 2 cm of the true motor point, as in our canine experiments. In humans, you had to be within 5 mm. With these findings, we simplified the mapping protocol by making modifications to the software and instruments. The surgeon could now map the entire diaphragm surface in the same time that it had taken to map three or four locations with the prior protocol. A grid pattern was overlaid on the diaphragm on one of the laparoscopic video monitors for a “home view” that could be reproduced (Fig. 4) . The response of each location was quantified according to the evoked abdominal pressure and visual observation of the diaphragm region recruited. Once this change was made, we were able to increase the area on the diaphragm being mapped from only 15 sites in our first patient up to 50 sites on each diaphragm in the subsequent patients. The speed of mapping also improved from requiring an average of 3 min to map a site (patient 1 had 0.35 sites per min mapped) to being able to map a site in <1 min (patients 5 and 6 had 1.25 sites mapped per min). The ability to do this rapidly enables the surgeon to repeatedly confirm the location for implantation.
Improvement of the mapping probe also helped to in increase the efficiency of mapping. In the first patient, the suction tubing would collapse, causing the probe to fall off the diaphragm intermittently during the process of mapping. The final probe electrode (type III) has a flared conducting metal contact held to the surface of the diaphragm muscle by vacuum tubing of sufficient durometer to prevent collapse and subsequent loss of contact.
Implantation
The implant instrument was designed to be similar to other laparoscopic instruments, so there was no significant improvement in this area after the first case. In the first patient, we needed to identify the best angle for insertion of the needle. To implant the electrode, the tip of the needle is inserted into the diaphragm 2 cm radially away from the predetermined implant site, perpendicular to the muscle fibers. This starting position places the stimulating tip of the electrode at the optimal placement site (the needle is 2.5 cm in length). When removing the needle of the implant instrument, it is also necessary to place countertraction on the muscle with a separate dissector so that the barb can be implanted into the muscle. The first case required 104 min, but thereafter there was no significant change in implantation time for patients 2–6 (50 to 48 min). In each case, there were from two to five electrodes that did not release into the muscle or did not adequately stimulate the motor point and were therefore removed. There was no significant improvement in this aspect over the series.
Wire routing
We have been able to achieve continuous and steady improvement in the time needed for this part of the procedure since the beginning of our serve. This part had the longest learning curve, because there are no procedures in general surgery comparable to the connection process. The connection site was also the site of a wound infection in one patient that was attributed to a coil of wire and a suture granuloma. For this one patient, we decided to externalize the connection site until an implantable device is available. To obviate such adverse events in the future, we have decreased the length of the implanted electrode by 20 cm so that there is less need to coil excess wire subcutaneously. We have also decreased the connecting suture length and now align the connecting sites parallel to each other and separate them subcutaneously which will also decrease the wound infection risk. We have also preloaded the silicone sleeve on the exiting wires to decrease operative time.
Discussion
In recent reports, we have shown that the laparoscopic placement of intramuscular electrodes can aid in the maintainence of full-time ventilatory support in tetraplegic patients. In the current study, we analyzed each step of the procedure to describe how operative improvements could be made rapidly to decrease the learning curve for implantation of an investigational device. By dividing the operations into four distinct steps, we were able to do a time analysis to show how these changes decreased the operative time.
The exposure and implantation stage of the operation showed no significant changes throughout the series because it involved standard laparoscopic techniques. When we designed the implant instrument, we wanted it to be able to be used by any surgeon without much practice. Our data have confirmed that this is possible.
Major improvements in time were achieved for the mapping stage and the routing of the wires. These improvements were possible because of the close working relationship between the biomedical design team and the operative surgeon. Between the initial patient’s first and second operation, we were able to design and build a new mapping probe that provided better suction and fixation to diaphragm. We also made smaller improvements to the device with subsequent operations. When we realized that our mathematical model of implantation had failed, we were able to come up with a completely different model that has enabled a more rapid location of the phrenic nerve motor point. The electrode connection site was the source of our only infection, and we have now modified it so that this should not be a problem in the future.
In conclusion, a close working relationship between the designing engineers and the surgeons can decrease the learning curve for investigational procedures and devices. In this series, we have been able to provide an alternative to mechanical ventilation for patients with injuries of the spinal cord. Five of five patients with an intact phrenic nerve have the capability to be free of the ventilator for extended periods of time.
References
H Aiyer TA Stellato RP Onders JT Mortimer (1999) ArticleTitleLaparoscopic implant instruments for the placement of intramuscular electrodes in the diaphragm IEEE Trans Rehab Eng 7 360–371
H Dagash M Choardhury A Pierro (2003) ArticleTitleWhen can I be proficient in laparoscopic surgery? A systemic review of the evidence J Pediat Surg 38 720–724 Occurrence Handle12720179
AF DiMarco RP Onders KE Kowalski ME Miller S Ferek JT Mortimer (2002) ArticleTitlePhrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes Am Resp Crit Care Med 166 1604–1606
ML Nochomovitz AF Dimarco JT Mortimer NS Cherniack (1983) ArticleTitleDiaphragm activation with intramuscular stimulation in dogs Am Rev Respir Dis 127 325–329 Occurrence Handle1:STN:280:BiyC2M%2FptFw%3D Occurrence Handle6830053
RP Onders AF DiMarco AR Ignagni JT Mortimer (2003) ArticleTitleLaparoscopic placement of diaphragm pacing systems in human subjects Lap SLS Rep 2 22–23
RP Onders H Aiyar JT Mortimer (2004) ArticleTitleCharacterization of the human diaphragm muscle with respect to the phrenic nerve motor points for diaphragmatic pacing Am Surg 70 241–247 Occurrence Handle15055848
RP Onders AF DiMarco AI Ignagni H Aiyer JT Mortimer (2004) ArticleTitleMapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series Surgery 136 819–826 Occurrence Handle15467667
DK Peterson ML Nochomotivz TA Stellato JT Mortimer (1994) ArticleTitleLong-term intramuscular electrical activation of phrenic nerve: efficacy as a Ventilatory prosthesis IEEE Trans Biomed Eng 41 1127–1135 Occurrence Handle1:STN:280:ByqC2MvosFI%3D Occurrence Handle7851914
DK Peterson ML Nochomotivz TA Stellate JT Mortimer (1994) ArticleTitleLong-term intramuscular electrical activation of phrenic nerve: safety and reliability IEEE Trans Biome Eng 41 1115–1126 Occurrence Handle1:STN:280:ByqC2MvosFE%3D
BD Schmit TA Stellato ME Miller JT Mortimer (1998) ArticleTitleLaparoscopic placement of electrodes for diaphragm pacing using stimulation to locate the phrenic nerve motor points IEEE Trans Rehab Eng 6 382–390 Occurrence Handle1:STN:280:DyaK1M%2FotFCrsQ%3D%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onders, R.P., DiMarco, A.F., Ignagni, A.R. et al. The Learning curve for investigational surgery: lessons learned from laparoscopic diaphragm pacing for chronic ventilator dependence. Surg Endosc 19, 633–637 (2005). https://doi.org/10.1007/s00464-004-8934-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-004-8934-6