Abstract
Dysphagia is often associated with head and neck and upper gastrointestinal (GI) tract cancers. Evidence suggests that those with solid malignancies in other primary sites may also have swallowing difficulties. Timely and accurate identification of dysphagia is important given the impact it has on hydration, medical treatment, nutrition, prognosis, and quality of life. A systematic review was conducted to identify swallow screening, evaluation, and quality of life tools for those with solid malignancies outside the head and neck and upper GI tract. Ten electronic databases, one journal and two published conference proceedings were searched. Following deduplication, 7435 studies were examined for relevance. No tools were validated solely in this cancer population, though some included this group in larger cohorts. Comments are provided on the diagnostic properties and applicability of these tools. In the absence of appropriate diagnostic instruments, the exact prevalence of dysphagia and its impact on clinical and psychosocial well-being remain unknown. Accurate and adequate measurement of therapeutic intervention is also compromised. This review establishes the need for validated dysphagia evaluation tools for this clinical population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a multifaceted disease with a rising incidence. It is expected to consume greater healthcare resources internationally [1]. In 2015, cancer was the second-leading international cause of death after cardiovascular disease [2]. Cancer incidence was almost 18 million, while mortality was nearly 9 million. Head and neck and upper GI tract (esophagus, stomach, duodenum) cancers accounted for 18% of all new cancer cases and 19% of all cancer deaths. By contrast, solid malignancies located elsewhere accounted for 68% of all new cases and 68% of deaths [1].
Within oncology, dysphagia is typically associated with head and neck or upper GI tumors. Swallowing difficulties occur when malignancies lead to destruction of functional tissue or obstruction of bolus transit pathways. Anti-cancer treatments like radiotherapy and chemotherapy contribute towards difficulties. Dysphagia occurs when such treatments cause functional impairment in swallow structures, or with mucositis or xerostomia as treatment side effects [3,4,5,6]. Since swallowing difficulties are common in this clinical population, cohort-specific tools are available for dysphagia screening [7], evaluation [8], symptom assessment [9, 10], and quality of life measurement [11,12,13,14].
Less is known about dysphagia in other solid tumor cancers. Where prevalence estimates exist (Table 1), diagnosis is often established using general symptom checklists, non-expert raters, non-validated instruments, or patient self-report. Many tools listed in Table 1 do not distinguish oropharyngeal from esophageal dysphagia. One study [15] included a reference standard to identify dysphagia. In that study, the authors examined aspiration prevalence in 2000 patients of mixed primary sites referred for a videofluoroscopic swallowing study (VFSS). Within this group, 11/19 (58%) of those with brain tumors aspirated, 82% silently. The authors reported that those with brain tumors had more silent aspiration than any other clinical cohort, including stroke.
Swallowing difficulties are also prevalent in lung cancer [22, 31]. In a case series of eight individuals with lung cancer [34], malignancy was the precipitating cause of dysphagia, but the mechanisms varied. These included direct tumor compression of the esophagus, brain metastases, GI tract metastases, oropharyngeal and esophageal infections, and radiation-induced esophagitis. A recent review of swallowing difficulties in advanced lung cancer identified anti-cancer treatments, nerve compression, esophageal compression, and tracheoesophageal tumor invasion as potential causes of dysphagia [35]. The authors also noted that in lung cancer other common comorbidities may influence swallow function. These include chronic obstructive pulmonary disease, which may itself cause dysphagia through disruption of breath–swallow coordination [36].
In advanced cancer, dysphagia occurs in 12–70% (Table 1) regardless of primary tumor site [18, 19, 21, 23, 24, 30]. It is noted to be clinically important [37]. It occurs more frequently in the last week before death [24, 38] and increases in severity in the final 2 days of life [18, 24]. When swallowing difficulties occur in advanced cancer, they are an independent prognostic indicator of short-term mortality [23, 39,40,41,42,43].
The impact of dysphagia on quality of life in solid malignancies outside the head and neck and upper GI tract has not been deeply investigated. Some evidence suggests that dysphagia is important. Kenne Sarenmalm et al. [25] reported a study of women with breast cancer that those who had dysphagia described difficulty swallowing as ‘frequently to almost constantly,’ ‘severe to very severe,’ and ‘quite a bit to very much distressing.’ In another study involving mixed cancer sites [44], participants reported that dysphagia was highly distressing. A study by Roe et al. [45] examined eleven individuals with cancer outside the head, neck, and esophagus. Seven reported dysphagia, and one other had self-modified their diet. Two others had a prior history of dysphagia during their disease course. The swallowing quality of life (SWAL-QOL) [46] tool was used to evaluate dysphagia impact in these individuals. The authors noted significant impact of swallowing difficulties on quality of life.
Swallowing difficulties may have an important role in disease progression, recovery, and mortality. It is unknown whether dysphagia contributes towards malnutrition in those with cancer, though this is true in other populations [47,48,49]. Malnutrition in cancer is associated with higher morbidity [50]. Poorer intake is associated with reduced treatment and procedure compliance [50]. Early intervention for malnutrition improves patient outcomes [51]. In advanced cancer, infections are common and associated with shortened survival [52]. Infection is a serious complication in solid tumors and one of the leading causes of death, particularly in lung cancer [53]. The early and accurate identification and management of both dysphagia and aspiration pneumonia are therefore essential.
Given the potential impact dysphagia may have on those with cancer outside the head and neck and upper GI tract, a validated evaluation method is crucial. VFSS and fibreoptic endoscopic evaluation of swallowing (FEES) are reference standards, but may not always be appropriate or feasible for those with cancer. Patients may already have had radiation exposure, may be too unwell to travel to a radiology suite, or be too nauseous for an invasive transnasal endoscopic procedure. A comprehensive and validated bedside dysphagia evaluation tool is therefore necessary. It is also the first step in determining whether further evaluation with a reference standard is needed [54]. It is key that those with dysphagia are identified through rigorous screening and the impact of dysphagia on their quality of life is investigated.
Many tools exist to profile swallowing difficulties, but may not be suitable for those with cancers outside the head and neck and upper GI tract, as the etiology of dysphagia in this population may differ. For example, 18% of individuals with colorectal cancer treated with oxaliplatin-based chemotherapy have dysphagia due to paresthesia induced by cold temperatures [55]. The ingestion of a cold bolus may trigger swallowing difficulties, a feature unique to cancer. Tools validated in head and neck cancer may examine impairments particular to this form of malignancy. For instance, the Mann Assessment of Swallowing Ability—Cancer [8] measures mouth opening, since radiotherapy-induced trismus is a side effect of treatment [56].
Even when dysphagia etiology is similar to other conditions, variation in patient profile may necessitate different assessment tools. 65% of individuals with advanced glioblastoma have neurogenic dysphagia [57]. They may also have altered consciousness (95%), and be in functional decline [57]. Neurogenic dysphagia also occurs in up to 80% of those with stroke [58]. By contrast, only 35% of individuals with acute stroke have disordered consciousness [59] and may be medically stable or improving. Dysphagia evaluation would therefore differ between groups, since individuals with glioblastoma may be more unwell and less alert.
It is important for an evaluation tool to distinguish oropharyngeal from esophageal dysphagia, as management may involve different team members [60]. Strategies to address deficits in swallow may also vary by dysphagia etiology. An individual with neurogenic dysphagia caused by a brain tumor will require different management to an individual with a mediastinal tumor compressing the esophagus.
The aim of this review is to identify the diagnostic properties of validated tools for oropharyngeal dysphagia in solid tumors outside the head and neck and upper GI tract. Specifically, information is sought on tools designed for oropharyngeal dysphagia screening and evaluation, and those used to examine the impact of this symptom upon quality of life. For brevity, the term ‘dysphagia’ will refer here to ‘oropharyngeal dysphagia.’ The term ‘non-upper digestive cancer’ means any solid cancer excluding head and neck, esophageal, gastric, and duodenal malignant tumors.
Methods
The review was registered in the PROSPERO database (Ref: CRD42016038235) [61] after search terms were piloted. Title and abstract screening was through Covidence [62]; all subsequent review steps were manual. The PRISMA [63] framework guided the study report. QUADAS-2 [64] was selected for as the quality appraisal tool. RevMan 5 [65] was chosen for meta-analysis and forest plot generation.
Inclusion Criteria
Published studies were included if they had original data on the validation of any bedside tool designed to screen, assess, or evaluate quality of life impact of swallowing disorders in adults (≥ 18 years). Validation was defined as the use of common test performance metrics including sensitivity, specificity, positive/negative predictive value, positive/negative likelihood ratio, odds ratio, receiver operating characteristic curve, and reliability measurements. A bedside tool was any portable instrument that could reasonably be conducted at the patient’s bedside. This included paper-based evaluations, and techniques or devices as adjuncts to assessment, e.g., cervical auscultation, pulse oximetry monitoring. For screening and evaluation tools, only studies which compared these to a reference standard (VFSS or FEES) were included.
Exclusion Criteria
Studies not containing validation on adults with non-upper digestive cancers were excluded, as were non-solid tumors (e.g., leukemia, lymphoma). These criteria were confirmed through title, abstract, and full-text screening, rather than by using electronic search parameters to be as inclusive as possible. Articles about re-validation of an existing tool into another language were excluded. These require critical appraisal of translation, localization, and cultural adequacy and are beyond the scope of this review.
Where a broader study included those with a diagnosis of ‘cancer,’ authors were contacted to clarify whether this included the cohort of interest. If a study did not mention participant diagnosis or described a category of ‘other diagnoses,’ authors were not contacted and studies excluded. Any techniques considered invasive or involving radiological or sonographic imaging were not considered, as this review was designed to identify non-specialist instruments that could be used by any doctor, nurse, speech and language therapist, or other health professional. If conference abstracts reported the same data as original papers (i.e., same cohort population and numbers, study design, outcomes), the original paper was preferred and the abstract treated as a duplicate. Case reports, editorials, review articles (including systematic reviews), and studies of correlation were also excluded. Correlational studies were excluded on the basis that they would provide information only on statistical association but not diagnostic validation.
Search Strategy
A librarian was consulted to assist with search terms and database selection. A sample search strategy is in “Appendix.” Terms were thematically related to dysphagia AND diagnostic accuracy measures AND assessment. Terms related to cancer were not included. The list of potential cancers is extensive and the use of this term may have excluded studies where cancer was one of a subgroup of conditions upon which the assessment was validated. Specialist and generalist databases were searched from inception to the search date. This was originally conducted on May 6, 2016 and an updated search on August 2, 2017. The databases were CINAHL, Cochrane Library, Embase, ProQuest Dissertations & Theses A&I, ProQuest Nursing & Allied Health Database,Footnote 1 PsycINFO, PubMed, ScienceDirect, Scopus, and Web of Science. No language or other restrictions were used during searching. Deduplication of search results was conducted using EndNote X7 [66].
Handsearching was conducted on the journal Dysphagia. This was chosen as the most pertinent source of research related to oropharyngeal dysphagia. This included review of published abstracts from any conference proceedings or meetings indexed by this journal. Proceedings from the European Society for Swallowing Disorders (ESSD; 2011–2016) were searched, as were those from the Dysphagia Research Society (DRS; 1992–2016 except 2001, 2002 which were unavailable). It was originally planned to examine the meeting proceedings from the UK Swallow Research Group (UK-SRG), but these were not published and hard copies of all meeting abstracts were unavailable, so not included. The reference lists of relevant systematic reviews identified by electronic search were examined, as were reference lists from included studies.
Title and Abstract Screening and Full-Text Review
Screening and full-text review were blindly and independently conducted by two reviewers. Differences in decision were discussed until agreement was reached. If agreement could not be reached at any stage, a third reviewer adjudicated. Where full-text articles were identified as eligible for analysis but further information required, the corresponding authors were contacted electronically. In the event the corresponding author was unavailable, associate authors were contacted. When e-mails were undelivered, authors were contacted via online social networking. They were requested to acknowledge correspondence within 3 weeks of initial contact, or the study was excluded.
Data Extraction and Quality Appraisal
A data extraction sheet was developed by the first author. This was blindly and independently piloted on a single study by both the first and second authors. Following this, the authors discussed the adequacy of the data extraction sheet and changes made until agreement that it was comprehensive.
The standard signaling questions in the QUADAS-2 tool judged quality and risk of bias. This tool was independently and blindly piloted on one study by two reviewers to identify any differences in interpretation of signaling questions until these were resolved. Unanimous agreement was required for both data extraction and quality appraisal for all studies. Where agreement could not be reached, a final decision was made by a third reviewer.
Results
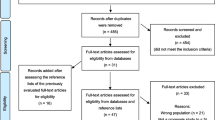
Figure 1 provides the PRISMA flow diagram of the total number of studies identified through electronic and manual searches, those excluded, and reasons. No studies were designed exclusively to evaluate dysphagia in non-upper digestive cancers. Four studies [67,68,69,70] included a subpopulation of this cohort. Three of these [67,68,69] provided a diagnosis of ‘brain tumor,’ which we regarded to mean ‘malignant’ and included for follow-up analysis. Ten studies [71,72,73,74,75,76,77,78,79,80] included a cancer population, but required further clarification whether this included non-upper digestive tumors. Additional information was requested from the authors of fourteen studies and all correspondence delivered successfully using the methods above.
No author response was received for any of the four studies [67,68,69,70] identified as including the cohort of interest. For the ten studies where further clarification was needed, no response was received for five. One author advised that their data could not be made available to external parties. The other four responded to clarify the status of their ‘cancer’ group as follows.
Two studies involved the use of the 3-ounce water swallow test [75, 76] and one an oral mechanism examination [80]. The author of these studies advised that they were highly likely to include the group under review, but data were not collected in a manner that this group could be separately extracted. The ‘cancer’ group in these studies contained head and neck cancer, esophageal cancer, and non-solid cancers.
The author of another study [77] confirmed that head and neck cancers were excluded from the ‘cancer’ group and the cohort of interest included in the overall dataset. Individual cancer sites were however not distinguished and grouped together. As a result, malignancies within the upper GI tract were grouped with those outside and data not separately extractable. The author noted that few (if any) individuals with upper GI cancers were likely to have been included.
Characteristics of Studies
Ultimately, no studies were identified as eligible for inclusion or meta-analysis. Nevertheless, eight studies contained some degree of validation in this clinical population. We decided to comment on these studies given the lack of validated studies in non-upper digestive cancers. These studies were grouped into those which included the cohort of interest in their published text [67,68,69,70] (Table 2) and those reported by the original authors to contain the cohort of interest [75,76,77, 80] (Table 3). The study by Ding and Logemann [70] (Table 2) contained interview questions about dysphagia and correlated these with VFSS findings. This study should therefore have been excluded, but sufficient data were available within the publication to reproduce and examine diagnostic test properties.
Of the studies listed in Tables 2 and 3, each was designed to screen or evaluate for swallowing difficulties. None measured the impact of dysphagia upon quality of life. One study [77] was retrospective, while all others were prospective. Where study setting was listed, most were in hospitals. Seven studies [67,68,69, 75,76,77, 80] examined the ability of the index test to predict aspiration, one study [70] investigated the ability of the index test to predict dysphagia, and another was designed to predict tolerance of oral diet [75]. There was disparity in test accuracy measures used. Only two studies reported index test reliability [76, 80]. All studies included validation in a heterogeneous list of primary diagnoses. Table 3 studies had more individuals with cancer than Table 2 (albeit with limited information on cancer site). No studies reported time taken to complete index testing, though the test used in one study [77] is reported elsewhere [81] to take 15–20 min for a moderately impaired individual.
Quality of Studies
Quality appraisal of studies that included the cohort of interest in published text is provided in Table 4. Table 5 contains appraisal of those where the original authors reported that the cohort of interest was included. All ratings are reported using the QUADAS-2 [64].
As noted, all but one study [77] used a prospective study design appropriate for a test of diagnostic accuracy. The reviewers had concerns about risk of bias in patient selection. This was often insufficiently detailed, with little information on how many participants were eligible versus recruited, whether a consecutive or random sampling strategy was used, and if there was clinical confirmation of dysphagia prior to testing. Whiting et al. [64] noted that inclusion of those with known disease may exaggerate diagnostic accuracy. Reliability was only described in two index tests [76, 80].
Since VFSS and FEES are subject to interpretation, studies were examined to determine whether sufficient information was provided about the expertise of those conducting the reference test and the framework used to judge abnormal results. Two studies addressed both these facets [69, 77], two [67, 68] did not. The interval between index test and reference standard was not always appropriate. In some, reference standard testing was conducted prior to index testing without sufficient detail about blinding of reference standard results [68, 75, 76]. Where index tests were before reference standard tests, the time interval between the two was not always clear [68, 70, 77]. Two tests used simultaneous reference and index tests. One [67] did not describe whether the reference test was rated blindly. The other [69] commented that a rater blinded to the index test results was used to judge the reference standard.
Discussion
This review was intended to identify validated tools that may be used to screen, evaluate, and measure quality of life impact of oropharyngeal dysphagia in adults with solid malignancies located outside the head and neck and upper GI tract. Despite how commonly these cancers occur and reportedly high prevalence rates of dysphagia, no tools exist that have been specifically validated in this population. This limits the ability to firmly establish a true prevalence. The absence of such tools may also cause an inability to distinguish dysphagia from other common symptoms such as anorexia, early satiety, and taste abnormalities. If unrecognized, dysphagia may contribute towards malnutrition, which can affect cancer treatment and prognosis.
Review Findings
Eight tools with at least partial validation on the cohort of interest were identified in this review. No data were available to examine the diagnostic properties of these applied to this cancer population alone. Nevertheless, there may be some value in several of the tools identified. The interview question described by Ding and Logemann [70] (‘Have you noticed any swallowing difficulty?’) demonstrates adequate diagnostic properties and was validated in a large, heterogeneous sample. Within that, 9/103 (8.7%) were those of interest, improving the relevance to this group. The interview question is short and concise, has high sensitivity, and seems useful for dysphagia screening. We identified few concerns regarding bias or applicability of this instrument. Nonetheless, the study was originally designed for statistical correlation to compare self-perception of dysphagia with reference test findings, rather than diagnostic accuracy. This was the only study to examine dysphagia as an endpoint. All other tests were designed to predict aspiration.
The 3-ounce water swallow test [75] was validated on a large population with various illnesses. Those with cancer made up 125/3000 (4%) of participants. Good sensitivity was found among all diagnostic categories (including ‘cancer’), but specificity was low. The test is sensitive for aspiration, but has a high false-positive rate. This makes the 3-ounce water swallow test a good tool for screening, but not diagnosis. It contains a potential bias in how it was validated (the results of the reference standard were available to examiners before the index test), but used clear criteria for test failure and in a second study had 100% reliability on examination [76].
The 3-ounce water swallow test [75] was also tested to see if it could identify individuals ‘unsafe for oral alimentation,’ and whether passing permits ‘specific diet recommendations to be made without further objective swallow assessment.’ This yielded high sensitivity and low specificity values. There are several concerns about the use of the test for these purposes. Firstly, the original authors acknowledged that clinical judgement and experience, individual patient factors, and objective information must be used to supplement such a decision. Secondly, the reference test (FEES) incorporated a purée and unmodified fluid consistency as a basis for judging whether an individual could tolerate any oral diet. The original authors compared water swallow test findings with diet recommendations. These included those who went on to have regular diet, soft diet, a chopped diet, purée diet, liquid diet, or nil by mouth. The reference test did not employ regular, soft, or chopped options; therefore, the ability of the test to provide specific diet recommendations is questionable. Finally, the authors provide diagnostic properties for the ability of the test to predict inability to tolerate oral diet, but the definition of ‘oral diet’ was not provided. It appears likely to include any oral diet in the study (i.e., regular, soft, chopped, purée, liquid). This would mean that an individual who passed the 3-ounce water swallow test may only have tolerated a liquid diet, inflating the test value.
The MASA [81] was originally validated a stroke population, but used by González-Fernández et al. [77] to predict aspiration in a mixed-disease population. In the MASA, the rater chooses an ‘ordinal risk rating’ based on their overall impression at the end of the test. This entails judging the risk of aspiration as ‘definite,’ ‘probable,’ ‘possible,’ or ‘unlikely.’ The authors found excellent diagnostic values associated with these categories, though predominantly where extremes were chosen. For example, an individual at ‘possible’ risk of aspiration had high sensitivity (94%), but low specificity (23%). A person with ‘definite’ risk of aspiration had low sensitivity (25%), but high specificity (93.6%). The value of the MASA in predicting aspiration or absence of aspiration is most applicable at these endpoints.
The oral motor mechanism evaluation described by Leder et al. [80] involves examining labial closure, lingual range of motion, and facial symmetry to determine whether deficits are associated with aspiration in a mixed-disease population. This was not designed to diagnose aspiration, but supplement examination. Nevertheless, the authors found that incomplete lingual range of motion or incomplete facial symmetry is associated with higher risk of aspiration. Of note, the examination of these mechanisms is also incorporated in the MASA [81]. The value of these features in non-upper digestive cancers is unclear. It would seem unlikely that these may be impaired, except perhaps in brain tumors.
The remaining assessment tools given in Table 2 were deemed to be inadequate. Pulse oximetry monitoring did not demonstrate adequate diagnostic properties according to the original studies [67, 69]. The combined water and food swallow test to predict aspiration described by Tohara et al. [69] has good sensitivity, though low specificity. This would afford it potential as a screening tool. On closer examination, there were concerns about potential validation bias. Most notably, the index test was completed before the reference test (VFSS) and the duration of time elapsed between the two was up to 1 week, so swallow function may have changed in the interim.
Potential Causes of Dysphagia
The pathophysiology of oropharyngeal dysphagia in solid malignancies outside the head and neck and upper GI tract is unknown, though several mechanisms may be responsible. Neurologically, primary malignant brain tumors and brain metastases can directly interfere with deglutition if lesions occur near swallow centers. When cognitive function is impaired, this may further disrupt the swallow [82, 83]. Spinal malignancies have been noted to cause oropharyngeal dysphagia due to interrupted nerve supply [84, 85] and erosion into the pharynx [86]. Peripherally, cancers of the thorax can compress the recurrent laryngeal nerve, causing difficulties with laryngeal airway protection [31].
Neuromuscular changes may also influence swallow mechanisms. Cachexia is a characteristic symptom of cancer and involves progress loss of skeletal muscle, leading to functional impairment [87]. Impaired swallow function due to cancer cachexia has not been examined, but sarcopenic loss of muscle mass in cancer is positively associated with increased dysphagia severity [88]. As such, a ‘cachectic dysphagia’ is hypothesized, with similar mechanisms to sarcopenic dysphagia [89]. Fatigue is also common in cancer [19, 24, 38]. In other conditions, it is associated with increased airway penetration, masticatory difficulties, and reduced swallow-related quality of life [90,91,92].
Chemotherapy and radiotherapy are known individually and in combination to produce mucositis in the esophagus and oropharynx [4, 6]. Mucositis can lead to difficulty swallowing, which is distressing for those affected [44]. Since chemotherapy is a widely used cancer treatment, exposed individuals may experience dysphagia. Radiotherapy can also induce esophageal and oropharyngeal dysphagia, even when these organs are not the therapeutic target. Radiotherapy to the thorax can cause esophagitis [4, 93]. This occurs due to the proximity of the esophagus to the radiation field and can lead to difficulty swallowing. Esophageal and oropharyngeal dysphagia have also been documented from radiotherapy for spinal malignancies [94,95,96].
Xerostomia is present in approximately 50% of individuals with cancer, regardless of primary site [97]. While usually associated with head and neck radiotherapy, it can be caused by chemotherapy [98], immunotherapy [98], and opioid prescription for pain management [99,100,101]. When present, it leads to difficulty swallowing [102]. Xerostomia-induced swallowing dysfunction has been noted to cause oral preparatory, oral, and pharyngeal stage difficulties [103, 104].
Complications and Limitations of Assessment
Many of those with cancer have symptoms that affect diet and intake. These include appetite changes [19, 105], taste changes [19, 24, 44, 106], smell changes [106], and nausea/vomiting [24, 38]. At face value, these appear different from difficulty swallowing, but may affect desire to eat and drink. If an individual with cancer is asked ‘Do you have difficulty swallowing?,’ they may respond affirmatively, since the desire to swallow is affected. Tools to profile dysphagia in this cohort should therefore seek to distinguish dysphagia from these common dietary symptoms.
Since quality of life instruments were not identified, measurement of this domain is absent. This is of particular concern, as those with cancer have identified issues that might be related to feeding as a research priority. The ‘impact on life, how to live with cancer’ was given as the top research priority by a group of individuals with cancer in one study [107]. While swallowing was not specifically mentioned, participants wanted to know how they could self-manage their cancer (particularly diet and lifestyle), improve their quality of life, and improve social function. Another study [108] examined the priorities of those with cancer in their last week of life, and those of family caregivers. Participants reported that ‘engaging in small everyday life activities’ was important. While neither text mentioned feeding, it is reasonable to assume that the ability to independently choose and consume meals, and to share mealtimes may be important.
The clinical importance of dysphagia in non-upper digestive cancers is to be determined. Malnutrition in cancer is important, with under-recognition, despite its prevalence and association with poorer survival [109]. Whether swallowing difficulties contribute towards poorer intake in cancer is unstudied, but a relationship may exist. This cannot be examined until adequate dysphagia evaluation tools have been developed. Tools to evaluate swallowing difficulties in cancer should distinguish oropharyngeal dysphagia from other nutritional symptoms that may affect intake like early satiety, lack of appetite, and taste and smell abnormalities.
For the cohort of interest to this review, the dearth of tools to examine dysphagia is a gap in practice. Dysphagia manifests differently depending on its underlying cause, and so the assessment and management needs of diverse clinical populations are often unique. This makes a one-size-fits-all assessment impractical when performing detailed swallow evaluation or generating a quality of life profile. There may be some value in using a generic screening tool, such as the interview question developed by Ding and Logemann [70], or the 3-ounce water swallow test [75]. These have good psychometric properties, though clinicians should consider that they were validated on a relatively small cancer population.
The lack of tools for use in this group will also affect the ability to adequately manage and treat swallowing difficulties. This may affect healthcare outcomes and quality of life of those with dysphagia even further.
Limitations of the Review
This review was deliberately narrow and concerned bedside oropharyngeal dysphagia screening and assessment. It excluded esophageal dysphagia, since this was already known to occur in the cohort under investigation. It also excluded studies that only used reference standards, or other tools that require specialist equipment or training. This was to ensure that identified tools could be used in any clinical setting by the healthcare team. No studies were found that met the criteria of this review, but other work of interest may have been conducted in this novel clinical population. An alternative methodology such as a scoping review may be useful in future to describe dysphagia measurement in this group.
It is possible that some studies were not identified due to choice of search terms, selection of databases, and targets for manual searches. Gray literature was not searched, as it was felt that this would be unlikely to include information on test validation. Broad terms such as ‘eating’ and ‘drinking’ were deliberately avoided in the search strategy. These increased the number of search results more than threefold, making their inclusion resource-intensive. The terms ‘dysphagi*’ and ‘swallow*,’ as well as the subject heading ‘Deglutition Disorders’ were included instead. It was deemed unlikely that any published dysphagia evaluation or quality of life tools would contain ‘eating’ and ‘drinking’ in their title or abstract, but not include the final search terms. It is nevertheless possible that some dysphagia screening tools were missed due to the exclusion of these broad search terms.
During full-text review, many studies did not list participant primary diagnosis, or had an ‘other’ diagnostic category. These studies were excluded from consideration. This may have caused the cohort of interest to be missed, but to contact original authors and verify primary diagnoses would have been too resource-intensive.
Conclusions
The prevalence of dysphagia in adults with solid malignancies outside the head and neck and upper GI tract is reported to be high. No oropharyngeal dysphagia screening, evaluation, or quality of life tools exist that have been specifically validated in this group. As such, the true prevalence needs to be established. Oropharyngeal dysphagia is likely underrecognized due to the lack of suitable screening and evaluation instruments. Custom tools should be developed for the cohort of interest to account for the potentially unique symptoms of dysphagia experienced by those with cancer, or existing tools should be re-validated in this population. This may lead to better identification and more timely management of dysphagia, and may potentially impact cancer prognosis.
Notes
This database was unavailable for the updated search and so was excluded from this update.
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48. https://doi.org/10.1001/jamaoncol.2016.5688.
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–544. https://doi.org/10.1016/s0140-6736(16)31012-1.
Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425–39. https://doi.org/10.1016/j.ijrobp.2004.05.050.
Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139(6):827–30. https://doi.org/10.5858/arpa.2014-0111-RS.
King SN, Dunlap NE, Tennant PA, Pitts T. Pathophysiology of radiation-induced dysphagia in head and neck cancer. Dysphagia. 2016;31(3):339–51. https://doi.org/10.1007/s00455-016-9710-1.
Heijnen BJ, Speyer R, Kertscher B, Cordier R, Koetsenruijter KW, Swan K, Bogaardt H. Dysphagia, speech, voice, and trismus following radiotherapy and/or chemotherapy in patients with head and neck carcinoma: review of the literature. Biomed Res Int. 2016;2016:6086894. https://doi.org/10.1155/2016/6086894.
Patterson JM, Hildreth A, McColl E, Carding PN, Hamilton D, Wilson JA. The clinical application of the 100 mL water swallow test in head and neck cancer. Oral Oncol. 2011;47(3):180–4. https://doi.org/10.1016/j.oraloncology.2010.11.020.
Carnaby GD, Crary MA. Development and validation of a cancer-specific swallowing assessment tool: mASA-C. Support Care Cancer. 2014;22(3):595–602. https://doi.org/10.1007/s00520-013-2011-4.
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, Leonard RJ. Validity and reliability of the eating assessment tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24.
Dwivedi RC, St Rose S, Roe JW, Khan AS, Pepper C, Nutting CM, Clarke PM, Kerawala CJ, Rhys-Evans PH, Harrington KJ, Kazi R. Validation of the Sydney Swallow Questionnaire (SSQ) in a cohort of head and neck cancer patients. Oral Oncol. 2010;46(4):e10–4. https://doi.org/10.1016/j.oraloncology.2010.02.004.
Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire—H&N35. J Clin Oncol. 1999;17(3):1008–19.
Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, Goepfert H. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson Dysphagia Inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–6.
Lagergren P, Fayers P, Conroy T, Stein HJ, Sezer O, Hardwick R, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43(14):2066–73. https://doi.org/10.1016/j.ejca.2007.07.005.
Rinkel RN, Verdonck-de Leeuw IM, Langendijk JA, van Reij EJ, Aaronson NK, Leemans CR. The psychometric and clinical validity of the SWAL-QOL questionnaire in evaluating swallowing problems experienced by patients with oral and oropharyngeal cancer. Oral Oncol. 2009;45(8):e67–71. https://doi.org/10.1016/j.oraloncology.2009.03.003.
Garon BR, Sierzant T, Ormiston C. Silent aspiration: results of 2,000 video fluoroscopic evaluations. J Neurosci Nurs. 2009;41(4):178–85.
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Coyle N, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3(3):183–9.
Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT. The Memorial Symptom Assessment Scale Short Form (MSAS-SF). Cancer. 2000;89(5):1162–71.
Chiu TY, Hu WY, Chen C-Y. Prevalence and severity of symptoms in terminal cancer patients: a study in Taiwan. Support Care Cancer. 2000;8(4):311–3. https://doi.org/10.1007/s005209900112.
Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender and performance status in 1,000 patients. Support Care Cancer. 2000;8(3):175–9. https://doi.org/10.1007/s005209900128.
Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. 2001;80(5):346–50.
Tranmer JE, Heyland D, Dudgeon D, Groll D, Squires-Graham M, Coulson K. Measuring the symptom experience of seriously ill cancer and noncancer hospitalized patients near the end of life with the Memorial Symptom Assessment Scale. J Pain Symptom Manag. 2003;25(5):420–9. https://doi.org/10.1016/s0885-3924(03)00074-5.
Gift AG, Jablonski A, Stommel M, William Given C. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004;31(2):203–12. https://doi.org/10.1188/04.onf.203-212.
Teunissen SC, de Graeff A, de Haes HC, Voest EE. Prognostic significance of symptoms of hospitalised advanced cancer patients. Eur J Cancer. 2006;42(15):2510–6. https://doi.org/10.1016/j.ejca.2006.05.025.
Tsai JS, Wu CH, Chiu TY, Hu WY, Chen CY. Symptom patterns of advanced cancer patients in a palliative care unit. Palliat Med. 2006;20(6):617–22.
Kenne Sarenmalm E, Ohlen J, Jonsson T, Gaston-Johansson F. Coping with recurrent breast cancer: predictors of distressing symptoms and health-related quality of life. J Pain Symptom Manag. 2007;34(1):24–39. https://doi.org/10.1016/j.jpainsymman.2006.10.017.
Molassiotis A, Wengstrom Y, Kearney N. Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manag. 2010;39(5):847–58. https://doi.org/10.1016/j.jpainsymman.2009.09.012.
Abu-Saad Huijer H, Abboud S, Doumit M. Symptom prevalence and management of cancer patients in Lebanon. J Pain Symptom Manag. 2012;44(3):386–99. https://doi.org/10.1016/j.jpainsymman.2011.10.019.
Stark L, Tofthagen C, Visovsky C, McMillan SC. The symptom experience of patients with cancer. J Hosp Palliat Nurs. 2012;14(1):61–70. https://doi.org/10.1097/NJH.0b013e318236de5c.
Ritchie C, Dunn LB, Paul SM, Cooper BA, Skerman H, Merriman JD, et al. Differences in the symptom experience of older oncology outpatients. J Pain Symptom Manag. 2014;47(4):697–709. https://doi.org/10.1016/j.jpainsymman.2013.05.017.
Mercadante S, Aielli F, Adile C, Ferrera P, Valle A, Fusco F, et al. Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Support Care Cancer. 2015;23(11):3249–55. https://doi.org/10.1007/s00520-015-2720-y.
Brady GC, Roe JWG, O’Brien M, Boaz A, Shaw C. An investigation of the prevalence of swallowing difficulties and impact on quality of life in patients with advanced lung cancer. Support Care Cancer. 2017. https://doi.org/10.1007/s00520-017-3858-6.
Portenoy RK, Thaler HT, Kornblith AB, McCarthy Lepore J, Friedlander-Klar H, Kiyasu E, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Can. 1994;30A(9):1326–36.
Uniform Data System for Medical Rehabilitation. The FIM system advantage. Uniform Data System for Medical Rehabilitation. 2016. https://www.udsmr.org/Documents/FIM/FIM_System_SNF.pdf. Accessed 03 Jan 2018.
Camidge DR. The causes of dysphagia in carcinoma of the lung. J R Soc Med. 2001;94(11):567–72.
Brady GC, Carding PN, Bhosle J, Roe JW. Contemporary management of voice and swallowing disorders in patients with advanced lung cancer. Curr Opin Otolaryngol Head Neck Surg. 2015;23(3):191–6. https://doi.org/10.1097/MOO.0000000000000155.
Gross RD, Atwood CW Jr, Ross SB, Olszewski JW, Eichhorn KA. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(7):559–65. https://doi.org/10.1164/rccm.200807-1139OC.
Kirkova J, Rybicki L, Walsh D, Aktas A, Davis MP, Karafa MT. The relationship between symptom prevalence and severity and cancer primary site in 796 patients with advanced cancer. Am J Hosp Palliat Care. 2011;28(5):350–5. https://doi.org/10.1177/1049909110391464.
Hui D, dos Santos R, Chisholm GB, Bruera E. Symptom expression in the last seven days of life among cancer patients admitted to acute palliative care units. J Pain Symptom Manag. 2015;50(4):488–94. https://doi.org/10.1016/j.jpainsymman.2014.09.003.
Bruera E, Miller MJ, Kuehn N, MacEachern T, Hanson J. Estimate of survival of patients admitted to a palliative care unit: a prospective study. J Pain Symptom Manag. 1992;7(2):82–6.
Chuang RB, Hu WY, Chiu TY, Chen CY. Prediction of survival in terminal cancer patients in Taiwan: constructing a prognostic scale. J Pain Symptom Manag. 2004;28(2):115–22. https://doi.org/10.1016/j.jpainsymman.2003.11.008.
Martin L, Watanabe S, Fainsinger R, Lau F, Ghosh S, Quan H, et al. Prognostic factors in patients with advanced cancer: use of the patient-generated subjective global assessment in survival prediction. J Clin Oncol. 2010;28(28):4376–83. https://doi.org/10.1200/JCO.2009.27.1916.
Trajkovic-Vidakovic M, de Graeff A, Voest EE, Teunissen SC. Symptoms tell it all: a systematic review of the value of symptom assessment to predict survival in advanced cancer patients. Crit Rev Oncol Hematol. 2012;84(1):130–48. https://doi.org/10.1016/j.critrevonc.2012.02.011.
Hui D, dos Santos R, Chisholm G, Bansal S, Silva TB, Kilgore K, et al. Clinical signs of impending death in cancer patients. Oncologist. 2014;19(6):681–7. https://doi.org/10.1634/theoncologist.2013-0457.
Cheng KK. Oral mucositis, dysfunction, and distress in patients undergoing cancer therapy. J Clin Nurs. 2007;16(11):2114–21. https://doi.org/10.1111/j.1365-2702.2006.01618.x.
Roe JW, Leslie P, Drinnan MJ. Oropharyngeal dysphagia: the experience of patients with non-head and neck cancers receiving specialist palliative care. Pall Med. 2007;21(7):567–74.
McHorney CA, Bricker DE, Kramer AE, Rosenbek JC, Robbins J, Chignell KA, Logemann JA, Clarke C. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia. 2000;15(3):115–21. https://doi.org/10.1007/s004550010012.
Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med. 2009;41(9):707–13. https://doi.org/10.2340/16501977-0415.
Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG, Navajas M, Palomera E, Clave P. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age Ageing. 2012;41(3):376–81. https://doi.org/10.1093/ageing/afs006.
Carrion S, Cabre M, Monteis R, Roca M, Palomera E, Serra-Prat M, Rofes L, Clave P. Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clin Nutr. 2015;34(3):436–42. https://doi.org/10.1016/j.clnu.2014.04.014.
van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S51–63. https://doi.org/10.1016/j.ejon.2005.09.007.
Davies M. Nutritional screening and assessment in cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S64–73. https://doi.org/10.1016/j.ejon.2005.09.005.
Thai V, Lau F, Wolch G, Yang J, Quan H, Fassbender K. Impact of infections on the survival of hospitalized advanced cancer patients. J Pain Symptom Manag. 2012;43(3):549–57. https://doi.org/10.1016/j.jpainsymman.2011.04.010.
Homsi J, Walsh D, Panta R, Lagman R, Nelson KA, Longworth DL. Infectious complications of advanced cancer. Support Care Cancer. 2000;8(6):487–92.
McAllister S, Kruger S, Doeltgen S, Tyler-Boltrek E. Implications of variability in clinical bedside swallowing assessment practices by speech language pathologists. Dysphagia. 2016;31(5):650–62. https://doi.org/10.1007/s00455-016-9724-8.
Lucchetta M, Lonardi S, Bergamo F, Alberti P, Velasco R, Argyriou AA, et al. Incidence of atypical acute nerve hyperexcitability symptoms in oxaliplatin-treated patients with colorectal cancer. Cancer Chemother Pharmacol. 2012;70(6):899–902. https://doi.org/10.1007/s00280-012-2006-8.
Loh SY, McLeod RWJ, Elhassan HA. Trismus following different treatment modalities for head and neck cancer: a systematic review of subjective measures. Eur Arch Otorhinolaryngol. 2017;274(7):2695–707. https://doi.org/10.1007/s00405-017-4519-6.
Thier K, Calabek B, Tinchon A, Grisold W, Oberndorfer S. The last 10 days of patients with glioblastoma: assessment of clinical signs and symptoms as well as treatment. Am J Hosp Palliat Care. 2016;33(10):985–8. https://doi.org/10.1177/1049909115609295.
Takizawa C, Gemmell E, Kenworthy J, Speyer R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia. 2016;31(3):434–41. https://doi.org/10.1007/s00455-016-9695-9.
Li J, Wang D, Tao W, Dong W, Zhang J, Yang J, Liu M. Early consciousness disorder in acute ischemic stroke: incidence, risk factors and outcome. BMC Neurol. 2016;16(1):140. https://doi.org/10.1186/s12883-016-0666-4.
Miles A, McFarlane M, Kainth P, Parmar P. Interdisciplinary management of dysphagia. Nurs Res Care. 2014;16(10):561–7. https://doi.org/10.12968/nrec.2014.16.10.561.
University of York. PROSPERO International prospective register of systematic reviews. University of York. 2017. https://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42016038235. Accessed 01 March 2018.
Veritas Health Innovation. Covidence systematic review software. Veritas Health Innovation. 2017. https://www.covidence.org/. Accessed 01 March 2018.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):1–6. https://doi.org/10.1371/journal.pmed.1000097.
Whiting P, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
The Cochrane Collaboration. Review Manager (RevMan) [Computer program], version 5.3 edn. Copenhagen: The Cochrane Collaboration, The Nordic Cochrane Centre; 2014.
Clarivate Analytics. EndNote X7. 7.1 edn. Philadelphia: Thomson Reuters; 2014.
Higo R, Tayama N, Watanabe T, Nito T. Pulse oximetry monitoring for the evaluation of swallowing function. Eur Arch Otorhinolaryngol. 2003;260(3):124–7. https://doi.org/10.1007/s00405-002-0527-1.
Tohara H, Saitoh E, Mays KA, Kuhlemeier K, Palmer JB. Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–34. https://doi.org/10.1007/s00455-002-0095-y.
Wang TG, Chang YC, Chen SY, Hsiao TY. Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–4. https://doi.org/10.1016/j.apmr.2004.10.021.
Ding R, Logemann JA. Patient self-perceptions of swallowing difficulties as compared to expert ratings of videofluorographic studies. Folia Phoniatr Logop. 2008;60(3):142–50. https://doi.org/10.1159/000120622.
McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, Bricker DE. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17(2):97–114. https://doi.org/10.1007/s00455-001-0109-1.
Shaw JL, Sharpe S, Dyson SE, Pownall S, Walters S, Saul C, et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–8. https://doi.org/10.1007/s00455-004-0008-3.
Ryu JS, Park SR, Choi KH. Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–7. https://doi.org/10.1097/01.phm.0000140798.97706.a5.
Miura H, Kariyasu M, Yamasaki K, Arai Y. Evaluation of chewing and swallowing disorders among frail community-dwelling elderly individuals. J Oral Rehabil. 2007;34(6):422–7. https://doi.org/10.1111/j.1365-2842.2007.01741.x.
Suiter DM, Leder SB. Clinical utility of the 3-ounce water swallow test. Dysphagia. 2008;23(3):244–50. https://doi.org/10.1007/s00455-007-9127-y.
Leder SB, Suiter DM, Green BG. Silent aspiration risk is volume-dependent. Dysphagia. 2011;26(3):304–9. https://doi.org/10.1007/s00455-010-9312-2.
González-Fernández M, Sein MT, Palmer JB. Clinical experience using the mann assessment of swallowing ability for identification of patients at risk for aspiration in a mixed-disease population. Am J Speech Lang Pathol. 2011;20(4):331. https://doi.org/10.1044/1058-0360(2011/10-0082).
Sato M, Tohara H, Iida T, Wada S, Inoue M, Ueda K. Simplified cough test for screening silent aspiration. Arch Phys Med Rehabil. 2012;93(11):1982–6. https://doi.org/10.1016/j.apmr.2012.05.016.
Silbergleit AK, Schultz L, Jacobson BH, Beardsley T, Johnson AF. The dysphagia handicap index: development and validation. Dysphagia. 2012;27(1):46–52. https://doi.org/10.1007/s00455-011-9336-2.
Leder SB, Suiter DM, Murray J, Rademaker AW. Can an oral mechanism examination contribute to the assessment of odds of aspiration? Dysphagia. 2013;28(3):370–4. https://doi.org/10.1007/s00455-012-9442-9.
Mann G. MASA: the mann assessment of swallowing ability. Clifton Park: Singular/Thomson Learning; 2002.
Winchester J, Winchester CG. Cognitive dysphagia and effectively managing the five systems. Perspect Gerontol. 2015;20(3):116–32. https://doi.org/10.1044/gero20.3.116.
Pu D, Murry T, Wong MCM, Yiu EML, Chan KMK. Indicators of dysphagia in aged care facilities. J Speech Lang Hear Res. 2017;60(9):2416–26. https://doi.org/10.1044/2017_JSLHR-S-17-0028.
Byun YH, Sohn S, Park SH, Chung CK. Cervical spine chondroma compressing spinal cord: a case report and literature review. Korean J Spine. 2015;12(4):275–8. https://doi.org/10.14245/kjs.2015.12.4.275.
Peker HO, Celik NC, Cikla U, Baskaya MK. Metastatic prostate adenocarcinoma to intradural foramen magnum. J Surg Case Rep. 2017;11:185. https://doi.org/10.1093/jscr/rjx185.
Baruah P, Randall CJ, Burgess A. Unusual presentation of cervical spine metastases to the ENT surgeon. J Laryngol Otol. 2013;127(1):92–5. https://doi.org/10.1017/S0022215112002708.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. https://doi.org/10.1016/s1470-2045(10)70218-7.
Wakabayashi H, Matsushima M, Uwano R, Watanabe N, Oritsu H, Shimizu Y. Skeletal muscle mass is associated with severe dysphagia in cancer patients. J Cachexia Sarcopenia Muscle. 2015;6(4):351–7. https://doi.org/10.1002/jcsm.12052.
Maeda K, Akagi J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int. 2016;16(4):515–21. https://doi.org/10.1111/ggi.12486.
Tabor L, Gaziano J, Watts S, Robison R, Plowman EK. Defining swallowing-related quality of life profiles in individuals with amyotrophic lateral sclerosis. Dysphagia. 2016;31(3):376–82. https://doi.org/10.1007/s00455-015-9686-2.
Youssof S, Romero-Clark C, Warner T, Plowman E. Dysphagia-related quality of life in oculopharyngeal muscular dystrophy: psychometric properties of the SWAL-QOL instrument. Muscle Nerve. 2017;56(1):28–35. https://doi.org/10.1002/mus.25441.
Gilheaney O, Zgaga L, Harpur I, Sheaf G, Kiefer L, Bechet S, Walshe M. The prevalence of oropharyngeal dysphagia in adults presenting with temporomandibular disorders associated with rheumatoid arthritis: a systematic review and meta-analysis. Dysphagia. 2017. https://doi.org/10.1007/s00455-017-9808-0.
Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, Cheung P, Chow E. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001–11. https://doi.org/10.1200/JCO.2007.15.3312.
Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7(2):151–60.
Wang XS, Rhines LD, Shiu AS, Yang JN, Selek U, Gning I, Liu P, Allen PK, Azeem SS, Brown PD, Sharp HJ, Weksberg DC, Cleeland CS, Chang EL. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1–2 trial. Lancet Oncol. 2012;13(4):395–402. https://doi.org/10.1016/s1470-2045(11)70384-9.
Sharma M, Bennett EE, Rahmathulla G, Chao ST, Koech HK, Gregory SN, Emch T, Magnelli A, Meola A, Suh JH, Angelov L. Impact of cervicothoracic region stereotactic spine radiosurgery on adjacent organs at risk. Neurosurg Focus. 2017;42(1):E14. https://doi.org/10.3171/2016.10.FOCUS16364.
Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC, Cella D, Reeve BB. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525–50. https://doi.org/10.1007/s00520-012-1688-0.
Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L, Lopes NN, Mello AL, Muniz LV, Murdoch-Kinch CA, Nair RG, Napenas JJ, Nogueira-Rodrigues A, Saunders D, Stirling B, von Bultzingslowen I, Weikel DS, Elting LS, Spijkervet FK, Brennan MT. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18(8):1039–60. https://doi.org/10.1007/s00520-010-0827-8.
Morita T, Tsunoda J, Inoue S, Chihara S. Contributing factors to physical symptoms in terminally-ill cancer patients. J Pain Symptom Manags. 1999;18(5):338–46.
Glare P, Walsh D, Sheehan D. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care. 2006;23(3):229–35.
Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32(Suppl 1):53–63. https://doi.org/10.2165/11630080-000000000-00000.
Raber-Durlacher JE, Brennan MT, Verdonck-de Leeuw IM, Gibson RJ, Eilers JG, Waltimo T, et al. Swallowing dysfunction in cancer patients. Support Care Cancer. 2012;20(3):433–43. https://doi.org/10.1007/s00520-011-1342-2.
Liedberg B, Öwall B. Masticatory ability in experimentally induced xerostomia. Dysphagia. 1991;6(4):211. https://doi.org/10.1007/BF02493529.
Rogus-Pulia NM, Logemann JA. Effects of reduced saliva production on swallowing in patients with Sjogren’s syndrome. Dysphagia. 2011;26(3):295–303. https://doi.org/10.1007/s00455-010-9311-3.
Davis MP, Walsh D, Lagman R, Yavuzsen T. Early satiety in cancer patients: a common and important but underrecognized symptom. Support Care Cancer. 2006;14(7):693–8. https://doi.org/10.1007/s00520-005-0015-4.
Spotten L, Corish C, Lorton C, Dhuibhir PU, O’Donoghue N, O’Connor B, et al. Subjective taste and smell changes in treatment-naive people with solid tumours. Support Care Cancer. 2016;24(7):3201–8. https://doi.org/10.1007/s00520-016-3133-2.
Corner J, Wright D, Hopkinson J, Gunaratnam Y, McDonald JW, Foster C. The research priorities of patients attending UK cancer treatment centres: findings from a modified nominal group study. Br J Cancer. 2007;96(6):875–81. https://doi.org/10.1038/sj.bjc.6603662.
Dobrina R, Vianello C, Tenze M, Palese A. Mutual needs and wishes of cancer patients and their family caregivers during the last week of life: a descriptive phenomenological study. J Holist Nurs. 2016;34(1):24–34. https://doi.org/10.1177/0898010115581936.
Aktas A, Walsh D, Galang M, O’Donoghue N, Rybicki L, Hullihen B, Schleckman E. Underrecognition of malnutrition in advanced cancer: the role of the dietitian and clinical practice variations. Am J Hosp Palliat Care. 2017;34(6):547–55. https://doi.org/10.1177/1049909116639969.
Acknowledgements
The authors gratefully acknowledge the support of Mr. Greg Sheaf (librarian, Trinity College Dublin) for his advice on database selection and search strategies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix: PubMed Search Strategy
Appendix: PubMed Search Strategy
“deglutition”[MeSH Terms] OR “deglutition disorders”[MeSH Terms] OR swallow[Title/Abstract] OR swallows[Title/Abstract] OR swallowing [Title/Abstract] OR deglutition[Title/Abstract] OR dysphagia[Title/Abstract] OR dysphagic[Title/Abstract]) AND (“validation studies as topic”[MeSH Terms] OR “validation studies”[Publication Type] OR “reproducibility of results”[MeSH Terms] OR “sensitivity and specificity”[MeSH Terms] OR psychometric[Title/Abstract] OR psychometrics[Title/Abstract] OR sensitivity[Title/Abstract] OR sensitivities[Title/Abstract] OR specificity[Title/Abstract] OR specificities[Title/Abstract] OR “predictive value”[Title/Abstract] OR “predictive values”[Title/Abstract] OR “diagnostic value”[Title/Abstract] OR “diagnostic values”[Title/Abstract] OR validate[Title/Abstract] OR validates[Title/Abstract] OR validated[Title/Abstract] OR validation[Title/Abstract] OR “prognostic value”[Title/Abstract] OR “prognostic values”[Title/Abstract] OR reliability[Title/Abstract] OR reliabilities[Title/Abstract]) AND (“patient outcome assessment”[MeSH Terms] OR “evaluation studies as topic”[MeSH Terms] OR “evaluation studies”[Publication Type] OR “outcome assessment (health care)”[MeSH Terms] OR “symptom assessment”[MeSH Terms] OR “diagnostic techniques and procedures”[MeSH Terms] OR “surveys and questionnaires”[MeSH Terms] OR “quality of life”[MeSH Terms] OR evaluation[Title/Abstract] OR evaluations[Title/Abstract] OR assessment[Title/Abstract] OR assessments[Title/Abstract] OR tool[Title/Abstract] OR tools[Title/Abstract] OR instrument[Title/Abstract] OR instruments[Title/Abstract] OR “quality of life”[Title/Abstract] OR QOL[Title/Abstract] OR scale[Title/Abstract] OR scales[Title/Abstract] OR screen[Title/Abstract] OR screens[Title/Abstract] OR measurement[Title/Abstract] OR measurements[Title/Abstract] OR questionnaire[Title/Abstract] OR questionnaires[Title/Abstract] OR survey[Title/Abstract] OR surveys[Title/Abstract] OR test[Title/Abstract] OR tests[Title/Abstract] OR exam[Title/Abstract] OR exams[Title/Abstract] OR examination[Title/Abstract] OR examinations[Title/Abstract] OR index[Title/Abstract] OR indexes[Title/Abstract] OR indices[Title/Abstract].
Rights and permissions
About this article
Cite this article
Kenny, C., Gilheaney, Ó., Walsh, D. et al. Oropharyngeal Dysphagia Evaluation Tools in Adults with Solid Malignancies Outside the Head and Neck and Upper GI Tract: A Systematic Review. Dysphagia 33, 303–320 (2018). https://doi.org/10.1007/s00455-018-9892-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-018-9892-9