Abstract
The swallowing reflex is centrally programmed by the lower brain stem, the so-called swallowing central pattern generator (CPG), and once the reflex is initiated, many muscles in the oral, pharyngeal, laryngeal, and esophageal regions are systematically activated. The mylohyoid (MH) muscle has been considered to be a “leading muscle” according to previous studies, but the functional role of the digastric (DIG) muscle in the swallowing reflex remains unclear. In the present study, therefore, the activities of single units of MH and DIG neurons were recorded extracellularly, and the functional involvement of these neurons in the swallowing reflex was investigated. The experiments were carried out on eight adult male Japanese white rabbits anesthetized with urethane. To identify DIG and MH neurons, the peripheral nerve (either DIG or MH) was stimulated to evoke action potentials of single motoneurons. Motoneurons were identified as such if they either (1) responded to antidromic nerve stimulation of DIG or MH in an all-or-none manner at threshold intensities and (2) followed stimulation frequencies of up to 0.5 kHz. As a result, all 11 MH neurons recorded were synchronously activated during the swallowing reflex, while there was no activity in any of the 7 DIG neurons recorded during the swallowing reflex. All neurons were anatomically localized ventromedially at the level of the caudal portion of the trigeminal motor nucleus, and there were no differences between the MH and DIG neuron sites. The present results strongly suggest that at least in the rabbit, DIG motoneurons are not tightly controlled by the swallowing CPG and, hence, the DIG muscle is less involved in the swallowing reflex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The swallowing reflex is centrally programmed by the lower brain stem, the so-called swallowing central pattern generator (CPG), and is evoked by mechanical, chemical, or electrical stimulation to the pharynx or related nerves [1]. Once the reflex is initiated, many muscles in the oral, pharyngeal, laryngeal, and esophageal regions are systematically activated. These muscles are innervated by trigeminal, facial, glossopharyngeal, vagus, hypoglossal, and cervical motoneurons, indicating that the swallowing CPG organizes and regulates their neuronal activities.
In a previous study, Doty and Bosma first described the pattern of related electromyographic (EMG) activity during the swallowing reflex [2]. They concluded that the overall firing patterns were highly constant among more than 20 muscles in the oral, pharyngeal, and laryngeal regions in the monkey, cat, and dog. They also proposed that the digastric (DIG) muscle was a swallow-related muscle in the monkey but not in the cat or dog. In EMG studies [3], however, electrical cross talk and/or leakage should be considered during evaluation of muscle activity. In our previous study, EMG activities of jaw muscles were recorded in the freely behaving rabbit during mastication [4]. Although the study was focused mainly on chewing behavior, EMG activity was also evaluated during swallowing. As a result, the mylohyoid (MH) muscle was considered to be a “leading muscle” as suggested by Doty and Bosma [2], and the DIG muscle showed small but tonic activity in this period. Because these activity patterns were observed on both the right and left sides regardless of chewing side, we suggested that the DIG muscle in the rabbit was controlled by the swallowing CPG. The activity pattern between the MH and DIG muscles was obviously different, although these muscles are both regarded as jaw-openers. In this regard, it should be noted that in the rabbit, the MH muscle attaches to the hyoid, while the DIG does not [5].
Thus, whether the swallowing CPG differentially controls the suprahyoid muscles such as the DIG, MH, and geniohyoid remains unknown. To elucidate this, single units of these neurons were extracellularly recorded and functional involvement of these neurons in the swallowing reflex was investigated.
Materials and Methods
The experiments were carried out on eight adult male Japanese white rabbits (weight range = 2.5–3.0 kg) in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1996). The experimental protocols were reviewed and approved by the Intramural Animal Care and Veterinary Science Committee of Niigata University. The animals were anesthetized with urethane (1.5 g/kg) administered intravenously through the marginal ear vein and supplemented if necessary. Throughout the experiment particular care was taken to confirm an adequate level of anesthesia such that noxious forelimb pinching evoked no response. Physiological saline was administered by intravenous infusion (10 ml/kg/h). Tracheal cannulation was performed. At all times, an electrocardiogram and rectal temperature were monitored, and the temperature was kept between 38 and 40°C with a feedback-controlled heating pad.
Figure 1 shows the experimental preparation. Pairs of stimulating electrodes made of urethane-coated silver wire 0.2 mm in diameter (Unique Medical Co., Ltd., Tokyo, Japan) were hooked around the DIG and MH nerves on the right side. For this purpose, these nerves were exposed just after separation from the MH nerve trunk, and a 1–5-mm interpolar distance was established. Pairs of urethane-coated copper wire electrodes 0.18 mm in diameter (Unique Medical Co., Ltd.) were inserted into the DIG muscle on the right side and into MH muscles on both the left and right sides for EMG recordings. To evoke the swallowing reflex, pairs of stimulating electrodes made of urethane-coated silver wire 0.2 mm in diameter (Unique Medical Co., Ltd.) were hooked around the internal branch of the superior laryngeal nerve (SLN), beside the larynx and just distal to its bifurcation into the internal and external branches. Swallowing was confirmed by the MH EMG burst as well as visually observed laryngeal elevation.
Illustration of the experimental setup. The digastric (DIGn) or mylohyoid (MHn) nerve was antidromically stimulated to record extracellular action potentials from single motoneurons in the trigeminal motor nucleus (NVmot). Swallowing reflex was evoked by either repetitive electrical stimulation of the superior laryngeal nerve (SLN) or mechanical stimulation of the posterior tongue and pharynx using a cotton swab. Swallowing reflex was monitored by the electromyographic (EMG) activities of the MH muscle. In the present study, we examined whether identified DIG or MH neurons were activated during the swallowing reflex
After the head had been fixed in a stereotaxic holder in which lambda was 1.5 mm lower than bregma, Epoxylite-coated tungsten monopolar electrodes (1 MΩ) (FHC, Inc., Bowdoin, ME, USA) were used for extracellular recording from single motoneurons on the right side. They were advanced into the brain stem toward areas in the trigeminal motoneuron pool (20° behind the vertical and P 3.0–4.2, L 1.5–2.5 mm to lambda) [6].
To identify DIG and MH neurons, the peripheral nerve (either DIG or MH) was antidromically stimulated. A stimulus current of twice the threshold was required to orthodromically activate DIG or MH EMG responses. In the present study, neurons were identified as motoneurons if they (1) responded to antidromic nerve stimulation of either the DIG or MH (single square pulse, 0.3-ms duration, <100 μA) in an all-or-none manner at threshold intensities, (2) the onset latency to the action potentials was consistent and <2 ms, and (3) they followed stimulation frequencies of up to 0.5 kHz [7, 8]. Reference electrodes were attached to neck muscles.
Once a motoneuron was identified, its spontaneous activity and stimulus latency and threshold for DIG or MH nerve stimulation were determined. Its possible involvement with the swallowing reflex was then determined by establishing patterns of its responses to the swallowing reflex evoked by either mechanical stimulation to the posterior tongue and pharynx using a cotton swab or electrical stimulation of the SLN (train pulses, 20 Hz, 0.3-ms duration, <100 μA).
At the termination of each experiment, a small electrolytic lesion was created by passing negative current (20 μA, 20 s) through recording electrodes. The animal was killed with an intravenous overdose of urethane, and 0.1 M phosphate buffer at pH 7.4 was then perfused through the left cardiac ventricle, followed by formalin fixative. Serial frozen coronal sections (30 μm thick) were stained with H&E. The recording sites in the brain were reconstructed according to the atlas of Meessen and Olszewski [9].
Results
Of all single motoneurons recorded, 11 MH and 7 DIG motoneurons were identified. Summarized data of the neurons are shown in Table 1. The mean value (mean ± SD) of the stimulus threshold was 71.2 ± 35.6 μA (n = 11) for MH neurons and 71.6 ± 22.4 μA (n = 7) for DIG neurons. The latency (mean ± SD) to the nerve stimulation was 1.6 ± 0.1 ms (n = 11) for MH neurons and 1.7 ± 0.3 ms (n = 7) for DIG neurons. None of these neurons had any spontaneous activity.
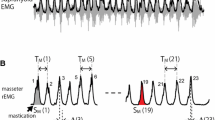
All MH neurons recorded were activated during the swallowing reflex (Fig. 2). The onset and offset of these activities were almost identical to those of the EMG burst in the left MH muscle, although the temporal relationship of burst patterns between single neurons and MH EMGs was not evaluated at this time. On the other hand, no activity of any DIG neurons was recorded during the swallowing reflex (Fig. 3).
Example of mylohyoid motoneuron. Left This neuron was antidromically activated by electrical stimulation of the mylohyoid nerve (MHn stim) (60 μA, 0.3-ms duration), while there were no responses to digastric nerve stimulation (DIGn stim). Five sweeps were superimposed for each nerve stimulation. The latency to the MHn stimulation was 1.6 ms. Right During swallowing, this neuron was synchronously activated with the MH EMG burst
Localization of the recorded motoneurons in the brain stem was then performed. All neurons were localized ventromedially at the level of the caudal portion of the trigeminal motor nucleus (Fig. 4). The recorded site in the brain stem was rostrocaudally extended to the area medial to the descending root of the facial nerve, and there were no differences between the MH and DIG neuron sites (Fig. 4).
Example of digastric motoneurons. These neurons were antidromically activated by electrical stimulation of the digastric nerve (DIGn stim) (91 μA, 0.3-ms duration for A and 57 μA, 0.3-ms duration for B), while there were small field potentials as a result of MH nerve stimulation (MHn stim). Five sweeps were superimposed for each nerve stimulation. The latencies to the DIGn stimulation were 1.5 and 1.7 ms for A and B, respectively. During swallowing, the two neurons showed no responses
Histological reconstruction of recorded motoneurons. Open and filled circles represent MH and DIG motoneurons, respectively. Left and right figures show the mid and caudal levels of the trigeminal motor nucleus (NVmot). All neurons were localized ventromedially at the level of the caudal portion of the trigeminal motor nucleus. NVmes the mesencephalic trigeminal nucleus, V the trigeminal tract, VII the facial nerve. Vertical and horizontal scale bars 1 mm
Discussion
The DIG and MH muscles are both known to be jaw-openers in all mammals, including rabbits [10]. Furthermore, they are regarded as hyoid and thyroid cartilage-elevators during swallowing [1–3]. However, all DIG motoneurons recorded in the present study were not active during swallowing, regardless of the lack of paralysis. The DIG muscle was less active during the solely evoked swallowing reflex in anesthetized [11, 12] and nonanesthetized [13] rabbits, although the authors did not focus on the differences. We previously investigated the effect of electrical stimulation of the SLN to evoke the swallowing reflex on the jaw-opening reflex responses in the DIG muscle. Swallowing movements (i.e., laryngeal elevation) were always identical to the MH burst but not to the DIG one [11], which was an advantage for measurement of the peak-to-peak amplitude of the reflex evoked in the DIG muscle during swallowing. There may be species-dependent differences in the activity patterns during swallowing [2, 3]. Numerous reports have examined the coordination of jaw, tongue, and hyoid muscle activity during chewing and swallowing [3, 14–16]. It should also be noted that EMG bursts in the DIG during swallowing have been reported during chewing or cortically evoked rhythmic jaw movements in anesthetized [17] and nonanesthetized [4, 18] rabbits. Very small but tonic bursts in the DIG were observed during swallowing, of which the timing was almost synchronous with that in the MH and thyrohyoid muscles. It is possible that the preceding or subsequent DIG activity during chewing behaviors overlapped during swallowing and/or sensory feedback actively modulated the DIG activity. Thus, it can be suggested that at least in the rabbit, DIG motoneurons were not tightly controlled by the swallowing CPG, and hence the DIG muscle is not involved in the swallowing reflex.
Another possibility should also be considered: functional heterogeneities exist not only among muscles, but among motor units within the same muscle, as previously suggested [3, 19–21]. In other words, it is possible that we did not record the activity of any DIG motoneurons that received inputs from the swallowing CPG in the present study. Thexton et al. [3] recorded EMG activities from 16 different muscles and two distinct sites in the same muscles during swallowing in the pig. They found a large difference in the DIG EMG burst patterns, not only among the animals but between the sites in the same muscle, including the DIG in one animal. In particular, overall variability in the same muscle was the largest, and the range of overlap of EMG signals from duplicate electrodes in the same muscle was small in the anterior belly of DIG muscle; this may reflect variable activity patterns of DIG motoneurons. As suggested by the authors, different patterns of within-muscle variation in EMGs reflected different organizational or morphological aspects of recorded muscles. Thus, the possibility that DIG motoneurons recorded in the present study did not receive inputs from the swallowing CPG, but received inputs from others, cannot be excluded.
If the DIG is not necessary for swallowing in rabbits, what is the explanation for the difference in contribution to swallow-related hyoid movements? The DIG is anatomically categorized as a suprahyoid muscle and consists of anterior and posterior bellies. The former is innervated by the trigeminal nerve, while the latter is innervated by the facial nerve. However, only the anterior belly of the DIG is present in rabbits, and its sources of innervation are richly located in the trigeminal nucleus, and less so in the facial nucleus [22]. Notably, the DIG muscle is not attached to the hyoid bone in rabbits [5]. During swallowing, many structures are involved in reflexively evoked sequential movements. It is apparent that the position and movement of the hyoid are critical to normal swallowing. As suggested by German et al. [23], the hyoid is a “floating bone,” and its position should be strictly maintained by a sling of muscles. Its superior and anterior movements are controlled by the antigravitational drive of related muscles, mainly the suprahyoid muscle group, including the DIG muscle. This may not be the case in rabbits; one reason is that, as mentioned above, the DIG muscle is not attached to the hyoid bone in rabbits. Another reason may be explained by a previous study [18] in which DIG tenotomy was performed and chewing behaviors and mylohyoid EMG patterns were compared between tenotomized and control rabbits. The authors found fewer changes in the patterns of jaw movements and EMG activity after tenotomy, suggesting that the DIG muscle is not critical to chewing or, possibly, swallowing behaviors in rabbits.
Interestingly, the locations of these neurons in the brain are known to be very close to each other [24], although their functional involvement in swallowing is quite different. Again, the DIG muscle, like the MH muscle, is regarded as a jaw-opener in all mammals in functions such as chewing [4, 25]. However, the precise activity patterns were a bit different between the two muscles in this study, even during chewing, in that the DIG activity peaked in the late part of the opening phase while the MH activity peaked in the early part. These results strongly suggest that input patterns from the masticatory CPG into both motoneurons were not consistent with each other. Thus, one can expect that in addition to chewing, central regulation of swallowing differs between the two jaw-openers.
References
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69.
Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60.
Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102:587–600.
Naganuma K, Inoue M, Yamamura K, Hanada K, Yamada Y. Tongue and jaw muscle activities during chewing and swallowing in freely behaving rabbits. Brain Res. 2001;915:185–94.
Takata H. A comparative anatomical study on suprahyoid muscles. Kyushu Dental Soc. 1989;43:899–913.
Inoue M, Nozawa-Inoue K, Donga R, Yamada Y. Convergence of selected inputs from sensory afferents to trigeminal premotor neurons with possible projections to masseter motoneurons in the rabbit. Brain Res. 2002;957:183–91.
Donga R, Lund JP, Veilleux D. An electrophysiological study of trigeminal commissural interneurons in the anaesthetized rabbit. Brain Res. 1990;515:351–4.
Landgren S, Olsson KA. Localization of evoked potentials in the digastric, masseteric, supra- and intertrigeminal subnuclei of the cat. Exp Brain Res. 1976;26:299–318.
Meessen H, Olszewski J. Cytoarchitektonischer Atlas des Rautenhirns des Kaninchens. Basel: Karger; 1949.
Weijs WA, Dantuma R. Functional anatomy of the masticatory apparatus in the rabbit. Netherlands J Zool. 1981;31:99–147.
Fukuhara T, Tsujimura T, Kajii Y, Yamamura K, Inoue M. Effects of electrical stimulation of the superior laryngeal nerve on the jaw-opening reflex. Brain Res. 2011;1391:44–53.
Sumi T. Modification of cortically evoked rhythmic jaw movements by reflex deglutition in rabbits. Jpn J Physiol. 1977;27:391–8.
Takagi M, Noda T, Yamada Y. Comparison of SLN-evoked swallows during rest and chewing in the freely behaving rabbit. Brain Res. 2002;956:74–80.
Lund JP. Mastication and its control by the brain stem. Crit Rev Oral Biol Med. 1991;2:33–64.
Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res. 1995;23:1–19.
Yamada Y, Yamamura K, Inoue M. Coordination of cranial motoneurons during mastication. Respir Physiol Neurobiol. 2005;147:177–89.
Amarasena J, Ootaki S, Yamamura K, Yamada Y. Effect of cortical masticatory area stimulation on swallowing in anesthetized rabbits. Brain Res. 2003;965:222–38.
Meng Y, Uchida K, Sato T, Yamamura K, Yamada Y. Difference in the burst patterns of digastric and mylohyoid activities during feeding in the freely behaving rabbit. Dysphagia. 1999;14:78–84.
Konow N, Thexton A, Crompton AW, German RZ. Regional differences in length change and electromyographic heterogeneity in sternohyoid muscle during infant mammalian swallowing. J Appl Physiol. 2010;109:439–48.
Umapathi T, Venketasubramanian N, Leck KJ, Tan CB, Lee WL, Tjia H. Tongue deviation in acute ischaemic stroke: a study of supranuclear twelfth cranial nerve palsy in 300 stroke patients. Cerebrovasc Dis. 2000;10:462–5.
van Lunteren E, Dick TE. Heterogeneity within geniohyoid motor unit subpopulations in firing patterns during breathing. Respir Physiol. 2001;124:23–33.
Baisden RH, Woodruff ML, Whittington DL, Benson AE. The motor innervation of the single-bellied digastric muscle in the rabbit: a retrograde horseradish peroxidase study. Neurosci Lett. 1985;56:129–36.
German RZ, Campbell-Malone R, Crompton AW, Ding P, Holman S, Konow N, Thexton AJ. The concept of hyoid posture. Dysphagia. 2011;26(2):97–8.
Matsuda K, Uemura M, Kume M, Matsushima R, Mizuno N. Topographical representation of masticatory muscles in the motor trigeminal nucleus in the rabbit: a HRP study. Neurosci Lett. 1978;8:1–4.
Inoue M, Ariyasinghe S, Yamamura K, Harasawa Y, Yamada Y. Extrinsic tongue and suprahyoid muscle activities during mastication in freely feeding rabbits. Brain Res. 2004;1021:173–82.
Acknowledgments
The authors thank Mr. Hidetoshi Hirano for technical assistance. This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#23659982 to M.I.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsujimura, T., Yamada, A., Nakamura, Y. et al. The Digastric Muscle is Less Involved in Pharyngeal Swallowing in Rabbits. Dysphagia 27, 271–276 (2012). https://doi.org/10.1007/s00455-011-9363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-011-9363-z