Abstract
Synechocystis sp. has remarkable mixotrophic capabilities, as well as an efficient exploitation of nitrogen and phosphorus, that may be applied in wastewater treatment based on cyanobacteria. To better investigate the exploitation of algal mixotrophy in bioremediation, this species was used in axenic respirometric tests to ascertain the effect of high light and non limiting CO2 supply on the overall regulation of mixotrophy, resulting in an inhibition of the exploitation of organic carbon. The same species was then cultured in real, unsterilized effluent obtained from the acidogenic fermentation of sludge, which contains a high concentration of nutrients (approximately 600, 90 and 6000 mg L−1 of N, P and COD, respectively) and it is often inhibiting for many microalgal species. On the contrary, Synechocystis sp., showed a remarkable growth and a removal up to 96% of phosphorus, 66% of nitrogen and of 68% of COD in such a complex waste stream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays the production of biomass by microorganisms, specifically microalgae and cyanobacteria, have several advantages over traditional crops, such as no requirement for arable land, capacity to produce a biomass rich in protein, carbohydrate and lipids, their high growth rate and their capacity to synthetize high value products servicing a number of markets (foods, fuels, nutraceuticals and plastics). Moreover, the cultivation of microalgae in wastewater offers the unique opportunity to simultaneously achieve both nutrient removal and production of a high-value algal biomass [1]. Besides the techno-economic feasibility of using wastewater for high-value products production, the recovery of nutrients such as nitrogen and phosphorus is potentially applicable in the agricultural sector. For this reason, phycoremediation using waste streams is a key element within a circular economy concept. Other advantages of cultivating microalgae with bacteria in wastewater have already been reported by several authors. In fact, their ability to supply oxygen to bacteria reduces the energy demand for aeration, that actually accounts for more than 50% of the traditional wastewater treatment processes [2]. In addition, the microalgal capability of shifting their metabolism to mixotrophy was reported as an interesting feature that may actually increase the nutrient removal from wastewater [3].
Cyanobacteria are prokaryotes able to survive in a wide range of habitats, including those defined as extreme, such as frozen lakes, hot springs and salt flats [4]. These species are promising candidates for environmental applications due to their ability to tolerate high levels of pollution, to degrade highly persistent organic contaminants and to remove heavy metals [5]. Also Vijayakumar [6] already reported the exploitation of cyanobacteria in wastewater treatment, due to their capability to use nitrates, ammonia and phosphate and to remove metal ions such as chromium, cobalt, copper and zinc. Moreover, the production of cyanobacterial biomass is potentially more efficient than any terrestrial crop and can be used as a source for high-value products. Indeed under proper cultivation conditions, some of these microorganisms showed their ability to produce polyhydroxyalkanoates (PHA) and other high-value compounds, such as cyanophycin. Cyanobacteria are the only prokaryotes that accumulate PHA by oxygenic photosynthesis [7, 8]. Balaji et al. [9] reported that the occurrence of poly-β-hydroxybutyrate (PHB) has been demonstrated for several cyanobacterial species, including Synechococcus sp. and Synechocystis sp.
In particular Synechocystis sp. PCC 6803 has shown versatile carbon metabolisms, growing under photoautotrophic, mixotrophic and heterotrophic conditions. Sheng et al. [10] highlighted that this cyanobacteria has excellent potential for large-scale biomass production due to its fast growth rate, naturally high lipid content and ability to be genetically transformed. Moreover, Synechocystis metabolism has unique responses to environmental stresses (e.g., cold-stress, hyperosmotic stress and salt stress) [10]. In an unfavourable environment, alternate pathways for the acquisition of carbon and nitrogen and for the reduction of photosynthesis efficiencies are activated [11].
In this work, a number of respirometric test were carried out in axenic condition, to better ascertain the effect of different light intensity and CO2 supply on the performance of Synechocystis sp. PCC 6803. Respirometric tests are emerging as a new quick method to ascertain the effect of operating variables on microalgal growth in shorter time of measure than conventional growth curves. Results obtained were then applied and confirmed by cultivating Synechocystis sp. PCC 6803 within real, unsterilized wastewater with a high concentration of biodegradable carbon, so to evaluate its mixotrophic capabilities in such a complex environment. This effluent, in fact, is obtained from acidogenic fermentation of primary sedimentation sludge, which leads to the release of volatile fatty acids (VFA) in the medium and involves also the release of huge amount of nutrients (ammonium and phosphate) in the liquid phase (approximately 600, 90 and 6000 mg L−1 of N, P and COD, respectively). Such a rich waste stream was found to be inhibiting for many microalgae, while Synechocystis sp. showed remarkable capabilities of growth and nutrient removal.
Materials and methods
Microorganism and experimental apparatus
The species used in this work was Synechocystis sp. PCC 6803 (from Pasteur Culture collection of Cyanobacteria, France). Maintenance and propagation of axenic culture were performed at ambient temperature, under continuous agitation, in freshwater media (BG11) [12], supplemented with HEPES 10 mM pH 8 to avoid acidification due to CO2 bubbling, and to maintain the pH in the optimal range between 7 and 8. Experiments were carried out in batch systems. Reactors used for both growth curves and preinocula were Quickfit® Drechsel Bottles with a volume of 250 mL and a diameter of 5 cm. In some of the experiments, a CO2-air (5% v/v) mixture was fed to the reactor by continuously bubbling it at the bottom of the bottle, for the overall duration of the growth curve. The total gas flow rate was 1 L h−1 for each bottle. Additionally, a magnetic stirrer was used to prevent any deposition of biomass, thus ensuring a good mixing within the reactor, in particular in the case of real wastewater which also has a higher turbidity. Thus, the mixing can be helpful to cyclically expose cells to the irradiated surface. The reactor temperature was maintained constant in a thermostated incubator (Frigomeccanica Andreaus, Padova) at 28 °C, unless as specifically reported in the corresponding section. Artificial light was provided by a LED system, a warm white panel lamp, and it was measured with a photoradiometer (HD 2101.1 from Delta OHM) which quantifies the photosynthetically active radiation (PAR). All the experiments were performed in at least two/three independent biological replicates. The rationale of the experiments are summarized in Table 1.
Analytical methods
To verify the growth of the microorganisms, the value of the optical density (OD750) was checked every day (measured at 750 nm, 1 cm optical path length, by a double beam UV–visible UV 500 spectrophotometer from Spectronic Unicam, UK). The sample was properly diluted so that the measurement would be within the range [0.1–1] of absorbance. In the case of wastewater, this last was used as blank. The value of pigments, chlorophyll a and carotenoids, were measured daily and they were used as an index of growth to account for the growth of the cyanobacterial species only in real wastewater, where a complex bacterial community is present. Accordingly, a qualitative comparison between chlorophyll content and OD750 may help to evaluate the growth of cyanobacteria and the overall biomass (cyanobacteria-heterotrophic bacteria consortium), respectively. Their extraction and quantification was made using N,N-dimethylformamide (DMF). 500 μL of sample was taken daily and centrifuged at 9960 rcf for 10 min. After the supernatant was removed, 1 mL of solvent was added. This was done in the dark because once taken into solution, pigments are photosensitive. Samples were then stored in a freezer for at least 48 h to ensure complete pigments extraction. After further centrifugation, the absorption spectrum on the extract was measured, using DMF as reference. The final concentration of chlorophyll a and carotenoid was determined according to Ref. [13]. By linear regression of experimental points of chlorophyll a concentration measured during the logarithmic phase of growth it is possible to calculate the cyanobacterial specific growth rate. Finally, in the first and in the last day of all batch experiments dry cell weight (DW) in terms of g L−1 was determined. DW was measured gravimetrically in cells previously harvested with a 0.22 μm pore size filter (GVS LIFE SCIENCES), and then dried for 2 h at 105 °C in a laboratory oven. Every day the pH was verified using a Hanna portable pH-meter (Code HI9124) and it was kept in optimal range using the solutions of sodium hydroxide (NaOH) or hydrochloric acid (HCl). For the calculation of the growth rate constant, only the first days where there is no substrate limitation were fully representative of the exponential phase, and were taken into account. Nutrient analysis was carried out on the culture medium and on the filtered sample obtained in the final day of the batch experiment. Standard procedures for wastewater based on colorimetric tests have been used (APHA-AWWA-WEF 1992). The nutrients analysed were ammonium (N-NH4+), ortophosphates (P-PO43−) and COD. To measure ammonium, the Hydrocheck Spectratest diagnostic kit (code 6201) was used. Innamorati et al. [14] described the method used for the detection of orthophosphates in Nova Thalassia vol. 11. The COD was quantified using potassium dichromate, according to DIN ISO 15705. Student’s t tests were applied to ascertain significant differences in nutrients contents in all the experimental conditions employed. The level of statistical significance was p < 0.05.
Acidogenically fermented sludge
The medium used as real wastewater was provided by the University of Verona, obtained by the acidogenic fermentation of mixed primary and secondary sewage sludge [15]. This process, performed at 37 °C under alkaline conditions, yielded a medium rich in volatile short-chain fatty acids (VFA), with a high content of nutrients released [16]. This was stored in 500 mL aliquots at a temperature of − 20 °C. Before use, it was necessary to centrifuge it (12,745 rcf, 15 min) to remove most of the suspended solids, and the nutrient compositions were measured every time to verify variations due to preservations. The liquid medium has the following main compositions: NH3-N 606.2 ± 36.9 mg/L, NO3-N 15.4 ± 0.9 mg/L, PO43−-P 98.4 ± 0.6 mg/L, COD 6742.6 ± 423.3 mg/L. Acetic acid and propionic acid are the most abundant acids in the liquid from sludge fermentation, consisting of 60–70% of total VFA. In batch growth curves, the microorganisms were inoculated into 100 mL of non-sterilized medium with an initial OD750 of about 0.5.
Respirometric tests
To evaluate the effect of different metabolism (autotrophy, mixotrophy) and environmental conditions (different light intensities) of Synechocystis sp., some respirometric test was carried out in axenic conditions. Similar approaches were already applied by other authors [17,18,19]. The details of the respirometric apparatus are described in Sforza et al. [19]. Each test started with fresh culture medium (BG11), in which a constant biomass inoculum (about 0.2 g L−1 of DW) was resuspended. To ensure a constant cyanobacterial biomass composition and acclimation, biomass was always sampled from preinocula cultivated for 3 days, corresponding to the exponential phase of the growth curve. Axenic conditions were verified by Luria Broth plating. To evaluate autotrophic metabolism, the inorganic carbon source was supplied as sodium bicarbonate at a concentration of 200 mg L−1 of C. The same concentration (based on carbon balance) of organic substrates, specifically glucose, sodium acetate and peptone, was used to investigate mixotrophic growth. To verify the effect of CO2 under autotrophic and mixotrophic metabolism, two sets of experiments were carried out. In the first one, CO2 was supplied prior the experimental run, by means of bubbling, which was carried out at the beginning of the test, to have almost 270 mg L−1 of dissolved carbon (220 mg L−1 as bicarbonate ions and 50 mg L−1 as dissolved CO2). In this way, it is also possible to reduce the oxygen dissolved in the culture to about 4 mg L−1. Sforza et al. [19] reported that the half-saturation constant for CO2 is equal to 1.3 mg L−1, so the amount of CO2 supplied by bubbling is well above the limiting one. On the other hand, in the second set of experiment, CO2 was replaced by N2. This way, the bicarbonate ion was the only carbon species available for growth. To measure the amount of carbonate and bicarbonate present in the medium and so verify the initial condition of carbon species, a titration with hydrochloric acid using two indicators was performed. Phenolphthalein (0.1% in ethanol) is used as an indicator for carbonate ion, whereas bromocresol green (0.04% in ethanol) is the indicator for both carbonate and bicarbonate ions. The difference between the last one and the measure of the carbonate ions gives the amount of the bicarbonate ions present in the medium. The whole procedure depends strictly on knowing the exact molarity of the hydrochloric acid used. For this reason, it was verified by titration with NaOH 1 M before each analysis, which was then carried out in triplicate. The amount of dissolved carbon dioxide was measured based on mass transfer calculations of gaseous compounds. Knowing the mass transfer coefficient equal to 172 day−1 [19] and the bubbling time (1.12 min), it is possible to quantify the amount of CO2 as dissolved carbon (see Figure S1 of supplementary materials). The bubbling was then stopped to minimize the mass transfer coefficient and measure the oxygen production and consumption due to cyanobacterial activity only. Each respirometric test lasted about 180 min and consisted in alternating cycles of light and dark (5:5 min), with a digital controller connected to a LED lamp at the irradiation of 150 μmol photons m−2 s−1. The first 20 min of data acquisition was discarded to allow the acclimation of the microorganisms to the applied environmental conditions, including the possible effect of light intensity supplied. Then, the tests are repeated under autotrophic growth and with sodium acetate as organic substrates, since the fermentate is mainly composed of this, with N2 bubbling, using different light intensities (50, 100 and 250 μmol photons m−2 s−1). Results of respirometric tests are reported as specific oxygen production and consumption rate and are summarized as an average of at least three measurements.
Results and discussion
Effect of CO2 on mixotrophic metabolism of Synechocystis sp.
This section reports the results of both growth curves and respirometric tests aiming at investigating the effect of CO2 addition on the mixotrophic exploitation of organic carbon by Synechocystis.
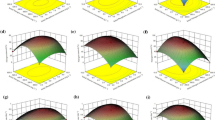
Respirometric tests, performed under autotrophic and mixotrophic metabolism, with and without CO2 bubbling were carried out and the results are summarized in Fig. 1. Here, the specific oxygen production and consumption rate under light and dark conditions showed a remarkable capability of Synechocystis to perform mixotrophy. In fact, in all the tests, in the presence of an organic carbon source oxygen production has always been greater with respect to autotrophic conditions. This also suggests that the presence of organic carbon generally increases the metabolism of biomass, differently from what was observed in the case of Chlorella protothecoides, where an increased respiration was measured when providing organic carbon, with a general decrease of the net oxygen produced [3].
Among the different sources of organic carbon tested, glucose has the major effect with an average oxygen production 10% higher than the control, and a respiration rate that is almost doubled.
A remarkable difference was observed when using carbon dioxide: analysing the tests performed with only nitrogen bubbling (Fig. 1a), and those performed with CO2 bubbling (Fig. 1b), the consumption, and especially the production of oxygen were significantly lower, especially during autotrophic metabolism and in the presence of glucose. So in the presence of an organic carbon source in the medium, it seems reasonable not to apply CO2 bubbling, because it inhibits the capability to exploit organic carbon of this species.
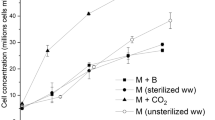
To confirm the outcome of respirometric tests, growth curves in real wastewater were carried out, then fed with a mixture air-CO2 (5% v/v) or without bubbling. Synechocystis sp. did not required initial time to acclimate to this highly concentrated and non-sterilized medium, showing a strong resistance to complex media. Other species were tested (C. protothecoides, Synechococcus sp.) which required a long lag phase of about 11 days and also resulted in a very low nutrient removal (data not shown). For Synechocystis sp. both the OD750 and the chlorophyll a content revealed an increasing trend from the first day, thus confirming that no lag phase occurred. The measured growth rate constant was equal to 0.58 day−1.
Concerning the effect of the presence of CO2, a larger final OD750 value was measured when CO2 was provided, but Synechocystis needed more time to reach the stationary phase (Fig. 2a). This seems reasonable, because in that case microorganisms may exploit both types of carbon, the organic and the inorganic one. However, looking at the trend of chlorophyll a and carotenoid (Fig. 2b), which represents the growth of the cyanobacterial species only, it appears that Synechocystis grew much more when CO2 was not supplied, reaching higher final pigment contents. The growth rate constant measured when the culture was supplied with CO2 was similar to the previous one and was equal to 0.6 day−1. On the opposite, when inorganic carbon was not supplied, the growth rate measured was significantly greater (1.02 day−1), which is remarkably high considering the dense colour of the culture and the possible competition with endogenous microflora present in the wastewater. The final biomass concentration was higher when CO2 was not supplied (3.63 ± 1.117 g L−1 instead of 2.76 ± 0.113 g L−1), as well as the final contents of pigments. This is particularly relevant in a context of circular economy, as a high concentration of valuable biomass can be obtained in such a complex waste stream. Apparently, when CO2 was not supplied, the growth of cyanobacteria was favoured with respect to other microorganisms, and, consequently, the consumption of the COD, the only source of carbon in this case, can be mostly attributed to them. Obviously, the pH values in the two experimental conditions were different. In the presence of CO2 it varied between 7 and 7.5 while in the absence of CO2 it was always measured within range 8–8.5. This might have also played a role in the results obtained, as reported in Ref. [20], but looking also at nutrient removal, and considering the huge increase in growth rate were no CO2 was supplied, it is reasonable that this result is mostly due to a possible inhibition of mixotrophy when CO2 is provided in excess [21]. In summary, it could be concluded that providing limiting CO2 concentration was more favourable to the growth of Synechocystis in that specific medium.
Nutrients analysis revealed no significant differences in the removal between the two experimental conditions. In both cases, on average 97.7% of ortophosphates, 67.6% of ammonia, and 76.8% of COD were removed from wastewater (see Fig. 3). However, in the case of no CO2 bubbling, this was obtained in less time than with CO2 supply, according to the difference measured for the growth rate. Yu et al. [11] already reported the capability of Synechocystis to grow either photoautotrophically or photoheterotrophically. Moreover, they noticed that it may co-metabolize different organic acids, including pyruvate, acetate and succinate, when inorganic carbon was insufficient in the medium. However, Synechocystis sp. is unable to growth heterotrophically, because it lacks the two glyoxylate shunt enzymes, isocitrate lyase (EC 4.1.3.1) and malate synthase (EC 2.3.3.9) required for incorporate acetate into the central carbon metabolism through the glyoxylate pathway (glyoxylate shunt) and gluconeogenesis [22]. Under mixotrophic conditions, it can simultaneously utilize bot inorganic and organic carbon sources, but thinking to an economic alternative to common wastewater treatment plant, CO2 aeration would imply additional costs [23]. For this reason, the results of our work are encouraging, suggesting the cyanobacterial cultivation could be performed without any additional supply of inorganic carbon.
In particular, the great potential of Synechocystis sp. in consortium with bacteria in wastewater treatment was confirmed and a remarkable removal of nutrients was achieved although the medium was highly concentrated, with COD reaching up to 6700 mg L−1 and ammonium 600 mg L−1 (see Fig. 3).
This kind of wastewater may contain many substances apart from VFA which are potential inhibitors of microalgae growth [24]. Furthermore, Liu et al. [25] have detected the same growth inhibition problem on other species, which occurs depending on the composition of the VFA mixture in the fermentation effluent. Specifically, this was found at certain concentrations of butyrate, lactate and HCO3−.
Finally, though many microalgae species could use different carbon sources such as glucose, glycerol, acetate [26], Chalima et al. [24] reported that, based on literature, only a limited number of microalgae are known to be able to use VFA for their growth. In this context, our results are very promising, as Synechocystis showed no inhibition and a remarkable removal of nutrients. Even though the overall removal of nutrients was obtained in consortium with bacteria, based on the results from respirometric tests it appeared that Synechocystis may have a relevant role in such a removal.
An experimental section was also dedicated to the effect of temperature on the growth rate constant of Synechocystis in such wastewater. At 28 °C and 37 °C the measured growth rate constant was almost the same (0.99 days−1 and 1.02 days−1), but at 32 °C, it increased significantly (1.77 days−1, see Table 2). In literature, a similar value was achieved cultivating Synechocystis sp. in an ideal PBR [11]. This result confirms also the one by Sheng et al. [10] who demonstrated that compared with an optimal temperature of 30–33 °C, a higher and a lower temperature severely inhibited the specific growth rate, the biomass production rate, the nutrient utilization rates and the lipid production rate of Synechocystis. For this reason, to evaluate only the effect of light, experiments at different light intensities were performed at 32 °C.
Based on results of a partial inhibition of growth when CO2 is supplied in excess, all the following experiments were performed without the addition of an external inorganic carbon source.
Effect of different light intensities on mixotrophic culture of Synechocystis sp. PCC6803
Three light intensities (50, 100 and 250 μmol photons m−2 s−1) were tested. To evaluate separately the effect of light on the growth of the axenic cyanobacterial species, a number of respirometric tests were carried out, under autotrophic and mixotrophic growth, with sodium acetate as organic substrates, since the fermentate is mainly composed by that. Results are shown in Fig. 4. At lower light intensities (50 and 100 μmol photons m−2 s−1), under mixotrophic metabolism, both the oxygen production and consumption rate are greater than under autotrophic metabolism, confirming the results reported in the previous section. On the other hand, under higher light intensity (250 μmol photons m−2 s−1), the presence of acetate in the medium seems to lower the capability to perform both respiration and photosynthesis of Synechocystis sp. These results are consistent with the one of Thiel et al. [22], who reported that an enhanced acetate intake can efficiently promote the growth of cyanobacteria. Such a behavior is specifically clear under low-light conditions, when photosynthetic activity is low and expected to result from the increased availability of acetyl-CoA precursors. Moreover, changes in cellular glycogen metabolism are induced, which may include the allocation of resources towards enhanced growth instead of glycogen accumulation. So, it seems that acetate suppresses the activity of the photosynthetic apparatus, further emphasizing the contribution of glycolytic metabolism in the acetate-induced effect [22].
The results obtained were also confirmed through two sets of batch experiments in the fermentate. The measured growth curves are reported in Fig. 5. The largest concentration of biomass was obtained at 100 μmol photons m−2 s−1 (3.06 ± 0.028 g L−1), while the lower one was at 50 μmol photons m−2 s−1 (2.45 ± 0.099 g L−1). It appears that the maximum chlorophyll a and carotenoid contents were identified under 100 μmol photons m−2 s−1. Growth rate constant calculation confirmed that the highest value (1.77 day−1) can be obtained under 100 μmol photons m−2 s−1. At 50 μmol photons m−2 s−1 it decreased to 0.85 day−1. Anyway, this value was higher than the one found at 100 μmol photons m−2 s−1 when the culture was supplied with CO2 (0.6 day−1), confirming that the presence or absence of inorganic carbon sources is crucial for the growth of Synechocystis.
As reported in Fig. 6a, no significant difference were found in the removal of phosphorus under 100 and 250 μmol photons m−2 s−1, while at 50 μmol photons m−2 s−1 it resulted in a lower value. This is consistent with the fact the growth of microorganisms, and so the final biomass measured was lower. A similar trend was found for ammonium (Fig. 6b). At 100 and 250 μmol photons m−2 s−1 no significant differences were found in the removal of ammonium, instead at 50 μmol photons m−2 s−1 it was lower. Figure 6c shows also the initial and final concentration of COD measured in all the experimental conditions tested. There was no significant difference between the measured values and, on average, 67.5% of COD was removed by the microorganisms, despite the relevant concentrations measured at the inlet.
In summary, the analysis carried out showed that the best conditions for the growth of the cyanobacteria Synechocystis sp. PCC6803 in rich wastewater were the absence of CO2 supply, a temperature of 32 °C and a constant light intensity of 100 μmol photons m−2 s−1. In that way the growth rate, the biomass concentration and the pigments contents could be maximized. At the same time, the growth of the other microorganisms was reduced. Therefore, Synechocystis proved to have a great potential in wastewater treatment, as it was able to survive and grow even if cultivated in a highly concentrated and non-sterilized medium. It was shown that Synechocystis grows efficiently without bubbling of air enriched with CO2, confirming its capability to perform both photoautotrophic and mixotrophic metabolism. A relevant growth rate was measured (1.77 day−1) and from experiments carried out in batch mode, the productivity reached was equal to 0.34 g L−1 day−1. This suggests that such a species may potentially be operated in a continuous system with lower residence time than microalgal species such as Chlorella sp. and Scenedesmus sp., as the washout can be approximately calculated around 0.56 day of HRT. Lower residence times means also lower reactor volumes. The possibility to carry out wastewater treatment without using external sources of inorganic carbon is very attractive, as it allows to reduce both operation and plant costs. Another interesting feature is related to the low light demand in the case of mixotrophic metabolism, thus also possibly reducing the areal requirement. Light, in fact, is the main limiting factor for the growth of photosynthetic microorganisms [27], but in this case it seems to inhibit the capability to exploit organic carbon by Synechocystis. It should be noted that the light intensities used are not fully comparable between respirometric tests and growth curve, due to different geometry and higher turbidity of the fermentate, which has to be considered in the discussion of results.
Conclusions
In this work, the possibility of exploiting Synechocystis sp. in wastewater treatment processes was assessed. In particular, the work was aimed at exploiting the remarkable capability of mixotrophy of this species so to optimize the removal of N, P and also COD. To better highlight this feature, an effluent obtained from acidogenic fermentation of primary sludge with a relevant pollutants concentration was used. The species showed great potential for phycoremediation achieving an almost complete removal of phosphorus (96%), and a good reduction of both nitrogen (66%) and COD (68%) in such highly concentrated wastewater. Respirometric tests under axenic conditions and batch experiments in real medium confirmed that CO2 insufflation and light intensity should be carefully considered so to optimize the organic carbon removal. CO2 limitation strongly increased the growth of the species in organic carbon-rich media, suggesting that mixotrophy is reduced in the presence of high content of CO2. Experiments carried out at different light intensities showed a better capability of Synechocystis to exploit organic carbon source at a lower light intensity.
References
Rawat I, Gupta SK, Shriwastav A, Singh P, Kumari S, Bux F (2016) Microalgae applications in wastewater treatment. In: Bux F, Chisti Y (eds) Algae biotechnology. Green energy and technology. Springer, Cham, pp 249–268
Lema JM, Bongards M, Chaparro A et al (2016) Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl Energy 179:1251–1268. https://doi.org/10.1016/j.apenergy.2016.07.043
Sforza E, Pastore M, Spagni A, Bertucco A (2018) Microalgae-bacteria gas exchange in wastewater: how mixotrophy may reduce the oxygen supply for bacteria. Environ Sci Pollut Res 25:28004–28014. https://doi.org/10.1007/s11356-018-2834-0
Percival SL, Williams DW (2014) Chapter Five - Cyanobacteria. In: Percival SL, Yates MV, Williams DW et al (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 79–88
El-Bestawy E (2008) Treatment of mixed domestic-industrial wastewater using cyanobacteria. J Ind Microbiol Biotechnol 35:1503–1516. https://doi.org/10.1007/s10295-008-0452-4
Vijayakumar S (2012) Potential applications of cyanobacteria in industrial effluents—a review. J Bioremediation Biodegrad. https://doi.org/10.4172/2155-6199.1000154
Panda B, Mallick N (2007) Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett Appl Microbiol 44:194–198. https://doi.org/10.1111/j.1472-765X.2006.02048.x
Ansari S, Fatma T (2016) Cyanobacterial polyhydroxybutyrate (PHB): screening, optimization and characterization. PLoS One 11:1–20. https://doi.org/10.1371/journal.pone.0158168
Balaji S, Gopi K, Muthuvelan B (2013) A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res 2:278–285. https://doi.org/10.1016/j.algal.2013.03.002
Sheng J, Kim HW, Badalamenti JP et al (2011) Effects of temperature shifts on growth rate and lipid characteristics of Synechocystis sp. PCC6803 in a bench-top photobioreactor. Bioresour Technol 102:11218–11225. https://doi.org/10.1016/j.biortech.2011.09.083
Yu Y, You L, Liu D et al (2013) Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar Drugs 11:2894–2916. https://doi.org/10.3390/md11082894
Rippka R, Deruelles JJBW, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiol SGM 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Bryant DA (ed) (1994) The molecular biology of cyanobacteria. Advances in photosynthesis and respiration, vol 1. Springer, Dordrecht, pp 559–579
Innamorati M, Ferrari I, Marino D, Ribera D’Alcala M (1990) Metodi nell’ ecologia del plancton marino. Nova Thalassia, vol 11. Ed Lindt, Trieste, pp 123–132
Frison N, Fatone F, Longo S et al (2014) Recovery of volatile fatty acids from fermentation of sewage sludge in municipal wastewater treatment plants. Bioresour Technol 175:436–444. https://doi.org/10.1016/j.biortech.2014.09.107
Basset N, Katsou E, Frison N et al (2016) Integrating the selection of PHA storing biomass and nitrogen removal via nitrite in the main wastewater treatment line. Bioresour Technol 200:820–829. https://doi.org/10.1016/j.biortech.2015.10.063
Decostere B, Janssens N, Alvarado A et al (2013) A combined respirometer-titrimeter for the determination of microalgae kinetics: experimental data collection and modelling. Chem Eng J 222:85–93. https://doi.org/10.1016/j.cej.2013.01.103
Rossi S, Bellucci M, Marazzi F et al (2018) Activity assessment of microalgal-bacterial consortia based on respirometric tests. Water Sci Technol 87(1–2):207–215
Sforza E, Pastore M, Barbera E, Bertucco A (2019) Respirometry as a tool to quantify kinetic parameters of microalgal mixotrophic growth. Bioprocess Biosys Eng. https://doi.org/10.1007/s00449-019-02087-9
Nguyen BT, Rittmann BE (2016) Effects of inorganic carbon and pH on growth kinetics of Synechocystis sp. PCC 6803. Algal Res 19:363–369. https://doi.org/10.1016/j.algal.2016.03.011
Sforza E, Cipriani R, Morosinotto T et al (2012) Excess CO2 supply inhibits mixotrophic growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour Technol 104:523–529. https://doi.org/10.1016/j.biortech.2011.10.025
Thiel K, Vuorio E, Aro EM, Kallio PT (2017) The effect of enhanced acetate influx on Synechocystis sp. PCC 6803 metabolism. Microb Cell Fact 16:1–12. https://doi.org/10.1186/s12934-017-0640-x
Wan N, Abernathy M, Tang JKH et al (2015) Cyanobacterial photo-driven mixotrophic metabolism and its advantages for biosynthesis. Front Chem Sci Eng 9:308–316. https://doi.org/10.1007/s11705-015-1521-7
Chalima A, Oliver L, de Castro L et al (2017) Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 3:54. https://doi.org/10.3390/fermentation3040054
Liu CH, Chang CY, Liao Q et al (2013) Photoheterotrophic growth of Chlorella vulgaris ESP6 on organic acids from dark hydrogen fermentation effluents. Bioresour Technol 145:331–336. https://doi.org/10.1016/j.biortech.2012.12.111
Chiranjeevi P, Venkata Mohan S (2017) Diverse acidogenic effluents as feedstock for microalgae cultivation: dual phase metabolic transition on biomass growth and lipid synthesis. Bioresour Technol 242:191–196. https://doi.org/10.1016/j.biortech.2017.04.059
Yun YS, Park JM (2003) Kinetic modeling of the light-dependent photosynthetic activity of the green microalga Chlorella vulgaris. Biotechnol Bioeng 83:303–311. https://doi.org/10.1002/bit.10669
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors would like to acknowledge Dr. Nicola Frison, University of Verona for kindly providing the wastewater.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trentin, G., Bertucco, A. & Sforza, E. Mixotrophy in Synechocystis sp. for the treatment of wastewater with high nutrient content: effect of CO2 and light. Bioprocess Biosyst Eng 42, 1661–1669 (2019). https://doi.org/10.1007/s00449-019-02162-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02162-1