Abstract
Microalgae-bacteria consortia application to wastewater treatment is considered as a potential and cheap strategy towards a self-sustaining oxygen-carbon dioxide gas exchange. However, microalgae can also carry out mixotrophy, thus reducing the net oxygen production, due to consumption of organic substrates. In this work, respirometric tests were used to quantify the oxygen reduction in the presence of biodegradable COD (chemical oxygen demand), which resulted up to 70%, depending on the biodegradability of the carbon substrate. The implication of mixotrophic metabolism on nutrient removal in urban wastewater was also measured by co-cultivating C. protothecoides with bacteria from activated sludge. To better understand the contribution of different populations, ad hoc experiments under controlled conditions were designed to quantify the nutrient consumption of bacteria and microalgae. Microalgae and bacteria were cultivated together and separately, with and without external bubbling, so to better ascertain the specific role of gas production and nutrient removal. Results showed that microalgae can remove up to 100 and 85% of P and N respectively, but the contribution on COD consumption may affect the net O2 supply to heterotrophic bacteria. However, a mutual COD consumption by microalgae and bacteria was proved by both experimental growth curves and mass balance application, based on stoichiometry experimentally adjusted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The continuous growth of the population and the increasing amount of wastewater generated by human activities from one side, the water scarcity and the increasing demand for high quality from the other one, make freshwater availability as a huge future global challenge. Conventional wastewater treatments (WWTs), although efficient and implemented for a long time, are usually rather expensive. One of the major issues of current WWT processes is related to the energy consumption due to aeration, which is an essential process in biological WWT systems, by usually accounting for more than 50% of the treatment energy demand (Longo et al. 2016). Therefore, the development of an efficient, cheap and green WWT process is one of the major challenges of this century.
The successful use of microalgae in WWT has been investigated by several authors and has been recognised as a simple and environmental-friendly technology (de-Bashan and Bashan 2010; Olguín 2012; He et al. 2013; Karya et al. 2013; Bertucco et al. 2014; Gupta et al. 2016). In particular, the application of microalgae-bacteria co-cultivation, where microalgae supply the oxygen required by bacteria for organic matter oxidation, could represent a promising technology for WWT reducing the energy demand for aeration (Su et al. 2012; Boelee et al. 2014; Alcántara et al. 2015).
In addition, since microalgae assimilate nitrogen and phosphorous for their growth, they can support and/or replace other conventional biological processes of nutrient removal (e.g. nitrification, denitrification or enhanced P uptake) (Cho et al. 2011; Ruiz-Martinez et al. 2012).
Another concept currently catching on is linked to the circular economy strategy, where a closed loop of product lifecycle through recycling and re-use of materials and energy may bring benefits for both the environment and the economy. From this perspective, the growth of microalgal biomass on wastewater ensures many advantages, as photosynthetic organisms are a carbon/energy-free source of many products of interest, including biofuels and biofertilizers (Olguín 2012; Gutzeit et al. 2015; Gupta et al. 2016).
The gas exchange between algae and bacteria has been considered so far, from both experimental and mass balance point of view, but microalgae are highly flexible organisms, able to acclimate their metabolism to environmental conditions: in particular, the capability to switch among autotrophic, mixotrophic and heterotrophic metabolism according to specific environmental conditions (Chojnacka and Marquez-Rocha 2004; Sforza et al. 2012; Abinandan and Shanthakumar 2015) should be assessed. Although the capacity of algae to mixotrophically exploit the organic carbon present in wastewater has been observed (Gupta et al. 2016; Liu et al. 2018; Nur and Buma 2018), it needs to be better investigated when a consortium with bacteria is applied. In particular, the reduction of oxygen production due to algal mixotrophic metabolism is usually not considered when applying such a consortium in wastewater treatment.
In this work, an integrated microalgae-bacteria system to efficiently treat wastewater is investigated, with the aim to better understand the possibility to exploit the oxygen produced by photosynthesis, in order to support the aerobic removal of organic compounds by the microbial community. To this aim, experiments were designed to possibly discriminate the growth and nutrient removal performances of axenic algae and bacteria, separately. These observations were then used for the interpretation of the results obtained when these organisms are cultivated together. In addition, the mixotrophic capability of microalgal species used was assessed to account for this metabolism in the gas exchange with bacteria.
To the best of the author’s knowledge, even though microalgal mixotrophy was extensively described, it is generally neglected when microalgae-bacteria consortia are applied in wastewater treatment, particularly related to the gas mass balance. Accordingly, the reduction of the net oxygen production due to microalgal mixotrophic metabolism was assessed by respirometric tests. In addition, a simplified model based on the stoichiometric growth equations of microalgae and bacteria, also accounting for the mixotrophic algal metabolism, was proposed. The final aim of this paper is to provide details about microalgae-bacteria interactions in wastewater, to possibly understand some of the complex phenomena that may occur in co-cultures.
Materials and methods
Microalgal, bacteria species, growth medium and wastewater
Chlorella protothecoides 33.80 (obtained from SAG Goettingen, Germany) was maintained axenically in BG11 medium for the inoculum. C. protothecoides was previously cultivated in urban wastewater, both sterilised and not, with promising performances about N and P removal (Ramos Tercero et al. 2013) and a good resistance to competition with endogenous microflora. Thus, it was chosen as a promising species for wastewater treatment, and it was tested as a possible candidate for studying the interactions in microalgae-bacteria consortia.
Bacteria inoculum was obtained from the activated sludge sampled at the full-scale municipal wastewater treatment plant of Montebello Vicentino (Vicenza, Italy; latitude 45°27′26″64 N, longitude 11°23′4″20 E). The plant can potentially serve up to 470,000 population equivalent (PE). Preinocula were maintained in liquid flasks with synthetic wastewater (recipe reported below), and inoculated in fresh medium at 30 °C, 3 days before each experiment. This allowed obtaining an exponentially growing inoculum, vital and with low content of inert solids.
A first series of experiments, aimed to ascertain the growth capability of microalga species in such a medium, and the possibility of co-cultivation with bacteria, was carried out with real wastewater, sampled in the same wastewater plant located in Montebello Vicentino, before the primary treatment. It was then filtered with laboratory paper to remove the gross-suspended solids and was subdivided into aliquots of 0.4 L that were stored at − 20 °C. The aliquots were defrosted just before starting each experiment and the nutrient composition was re-measured before inoculation, to ascertain variation due to preservation. The wastewater pollutants had the following main composition: NH4+-N 21.04 mg L−1, total nitrogen (TN) 33.09 mg L−1, total phosphorus (TP) 2.52 mg L−1, COD 496 mg L−1 (analysis provided by the Montebello WWTP, and confirmed in the lab).
As the main focus of this work is to investigate the specific relations between populations involved, due to the high variability of real wastewater characteristics (including possible variations due to preservation method in the laboratory), a synthetic one was formulated to obtain a medium with constant quality to improve the reproducibility of the experiments, in particular in the case of axenic experiments with microalgae alone. A standard synthetic wastewater with complex organic carbon (OECD 2001) was slightly modified in order to adjust the composition by making it similar to common wastewater used in the first part of the work. The synthetic wastewater was prepared using the following chemicals: peptone 80 mg L−1, meat extract 110 mg L−1, NH4Cl 40 mg L−1, CH3COONa 159 mg L−1, K2HPO4 23 mg L−1, NaCl 7 mg L−1, CaCl2*2H2O 4 mg L−1 and Mg2SO4*7H2O 2 mg L−1, and it was sterilised by autoclave.
The final characterisation of the synthetic sewage is the following: NH4+-N 13 mg L−1, TN 29.5 mg L−1, PO43−-P 3.75 mg L−1, COD 313 mg L−1.
During batch growth curves, pH was checked daily and manually adjusted in the range 7–9, if needed.
Analytical methods
The microalgal and bacterial growth was monitored by spectrophotometric analysis of the optical density (measured at 750 nm, by double-beam spectrophotometer UV-Visible UV 500 from Spectronic Unicam, UK), which however did not allow to discriminate between bacteria and microalgae, as it is just a measure of light scattering. To specifically quantify microalgal concentration, cell amount was also measured by cell counting with a Bürker Chamber (HBG, Germany). Microalgal-specific growth rates in batch experiments were measured by linear regression of 6–8 experimental points of cells concentration during the logarithmic phase of growth, from three independent biological replicates.
At the end of the growth curve, the final concentration of biomass of each experiment was also measured as dry weight (DW) in terms of g L−1. This was done gravimetrically on biomass filtered (10 mL sample filtered through nitro-cellulose membrane with 0.22 μm of pore size) and dried at 100 °C in a laboratory oven for 2 h. Nitrate (NO3−-N), nitrites (NO2−-N), ammonium (NH4+-N), Ntot, orthophosphate (PO43−-P) and COD were determined at the beginning (just after the inoculation) and at the end of each growth curve, in order to verify the consumption by microalgae, bacteria or consortium, after biomass removal by filtration (after an acidification procedure, if needed, up to pH = 7 so to re-suspend phosphorus possibly precipitated), to measure dissolved compounds only. Nitrate, nitrites, ammonium and orthophosphate (PO43−-P) were analysed using Hydrocheck Spectratest kits by Reasol®, while Aquanal® kit by Sigma-Aldrich was used for COD. Ntot was measured as nitrate, after persulfate digestion in autoclave for 1 h.
Experimental set-up and procedures
Batch experiments with C. protothecoides and activated sludge bacteria grown in real and synthetic wastewater were carried out in order to investigate their growth and their nutrient removal capacity under continuous light and under photoperiod. Each experiment started with an initial biomass inoculation of OD750 = 0.6.
Growth curves were carried out in Drechsel bottles of 250 mL (200 mL of working volume) with 5 cm diameter, continuously mixed by a magnetic stirrer. Before starting each experiment, the volume of preinoculum was centrifuged for 5 min at 1500 rpm in the case of microalgae, and 2 min at 8000 rpm in the case of bacteria, in order to remove the basal medium that could modify the nutrient concentration after inoculation. The biomass inoculated was then quantified to have reliable information of the starting point for each batch growth curve.
Five conditions were studied in order to better understand the effect of non limiting gas supply and the possible exploitation of gas produced by biomasses in the case of consortium: (a) microalgae alone (axenic conditions) with air-CO2 bubbling; (b) microalgae (axenic conditions) alone without bubbling; (c) bacteria and microalgae cultivated together without bubbling; (d) bacteria alone without bubbling; (e) bacteria alone with air bubbling.
Aeration (air alone, or enriched with 5% v/v of CO2) was provided at a total flow rate of 1 L h−1. The temperature was controlled at 24 °C in an incubator (Frigomeccanica Andreaus, Padova, Italy) and artificial light (white neon lamps OSRAM) was provided continuously at intensity of 30 μmol photons m−2 s−1 of PAR (photosynthetic active radiation), measured by a photoradiometer (Model LI-189, LI-COR, USA). This light intensity was chosen due to the high dilution of the cultures (so to avoid photoinhibition phenomena), and to be more consistent with real systems, where light penetration may be lowered by high thickness of cultivating systems and the presence of suspended solids.
Respirometric assays
In order to evaluate the oxygen production rate of C. protothecoides in the presence of both inorganic and organic carbon substrates, some tests based on respirometry were carried out. A similar approach was previously reported by Decostere et al. (2013), under autotrophic conditions only, and not considering the dark respiration phase. Respirometry was also recently applied as a tool to evaluate microalgal performances in wastewater (Rossi et al. 2018). In this work, a new protocol was proposed.
Dissolved oxygen (DO) concentration was continuously measured by means of a Handylab Ox 12 SCHOTT® oximeter connected to a PC by using Multi/ACHAT II software provided by WTW. The oxygen measurement was carried out in airtight flasks of 25 mL, in order to prevent oxygen transfer between the liquid and the external air. In addition, no gas headspace was left to avoid gas losses. The liquid was continuously mixed by a magnetic stirrer and the temperature was maintained constant at 25 °C by using a thermostatic water bath.
Each respirometric test was started with fresh culture medium (BG11), where a constant biomass inoculum (about 0.44 g L−1 of DW), previously centrifuged, was resuspended. To ensure a constant microalgal biomass composition and acclimation, all preinocula were sampled from a continuous reactor, working at steady state, at 2.6 days of residence time, carried out under autotrophic and axenic conditions (refer to Bertucco et al. 2014 for detailed description of the continuous system).
A first series of experiments was carried out by supplying bicarbonate to the culture, to measure the oxygen evolution and consumption in autotrophic conditions, as supported by Decostere et al. (2013). Further experiments were carried out by providing organic substrate to the culture to allow mixotrophic growth. In these cases, as the preinoculum had to be adapted to autotrophic conditions, the first light-dark repeat was not considered, as its trend resulted strongly different with respect to the three following ones.
Inorganic carbon source, to stimulate autotrophy, was added as sodium bicarbonate, while sodium acetate, meat extract and peptone were used as organic substrates. All of these substrates were supplied at a concentration of about 200 mg L−1 of C. The same test was also carried out in the presence of real and synthetic wastewater.
Each test lasted about 90 min and consisted in alternating cycles of light and dark (15:15 min), obtained thanks to a digital controller connected to a neon lamp at the irradiation of 45 μmol photons m−2 s−1. Each run started in the dark phase, and the first 15 min of data acquisition was discarded to allow the acclimation of the microorganisms to the applied environmental conditions. This protocol resulted in DO trends of which an example is reported in the supplementary materials (Fig. S1).
The pH of the medium, when it consisted in BG11 with the addition of bicarbonate or organic compounds, was buffered at 8 with HEPES 1 M. When wastewater was used, the chemical buffer was not added, since a sufficient amount of buffer capacity was assumed for the wastewater. Nevertheless, pH was always continuously checked in all experiments by means of a pH probe and no significant variations were recorded during the full duration of experimental runs (about 2 h). When the DO values increased, that is when light is on, the oxygen production rate (OPR) can be calculated from the slope. The same procedure for the oxygen consumption rate (OCR, mgO2 L−1 min−1) when light is off and DO values decrease. Every OPR and OCR was estimated as the average of at least three measurements. The specific oxygen production and consumption rates (mgO2 min−1 gM−1) were obtained dividing by initial biomass concentration measured as DW (gM L−1).
Statistical analysis
Student t tests were applied to ascertain meaningful differences in oxygen production and consumption rates under mixotrophic conditions. The level of statistical significance was assumed for P < 0.05, and significantly different results are highlighted with an asterisk in the figures.
Mass balance and stoichiometry
To account for the effect of microalgae application in consortia for wastewater treatment, in terms of gas exchange and pollutant removal, the stoichiometry of both heterotrophs and photosynthetic organisms was considered.
For bacteria, a typical biomass composition of CH1.4O0.4N0.2P0.017 was assumed (Metcalf and Eddy et al. 2014) and the growth equation for bacteria based on acetate (CH3COO−) as carbon source, \( \mathrm{N}{\mathrm{H}}_4^{+} \), \( {\mathrm{H}}_2\mathrm{P}{\mathrm{O}}_4^{-} \)and O2 consumption can be written as follows (Boelee et al. 2014):
Typical microalgal biomass composition of C1H1.78N0.12O0.36P0.01 has been reported, based on CO2, \( \mathrm{N}{\mathrm{H}}_4^{+} \) and \( {\mathrm{H}}_2\mathrm{P}{\mathrm{O}}_4^{-} \) consumption (Boelee et al. 2014), which can be written as
Even though mixotrophic metabolism of microalgae is well known and experimentally studied, to the authors’ knowledge, little information is available to account for it in mass balances. Chojnacka and Marquez-Rocha (2004) proposed a combination of autotrophic and heterotrophic stoichiometry, to analytically describe mixotrophy.
Thus, the part of metabolism corresponding to the respiration of organic material can be written as (Chojnacka and Marquez-Rocha 2004)
where a, b, c, d, e and f should be experimentally determined.
Stoichiometric equations above were used to assess the feasibility of gas exchange between microalgae and microbial community, also accounting for the mixotrophic metabolisms of microalgae. Equations of microalgae stoichiometry (2 and 3) were modified according to experimental results (see the “Microalgae-bacteria consortium in synthetic wastewater: growth and pollutant removal” section).
To assess the impact of algal mixotrophy on microalgae-bacteria growth and nutrient removal, a simple scenario case was preliminary studied, aimed at quantifying the possible gas exchange among different populations. The operating variables used to develop a simple mass balance of a hypothetical process were taken from Metcalf and Eddy et al. (2014), based on a normalised flow rate of incoming wastewater Q = 1 m3 day−1. Typical values of organic matter, nitrogen and phosphorous were assumed, corresponding to COD 300 mg L−1, BOD 140 mg L−1, N-NH4+ 25 mg L−1, TN 30 mg L−1 and TP 5 mg L−1 (Metcalf and Eddy et al. 2014). Details are reported in Supplementary materials, as well as the values of kinetic parameters (Metcalf and Eddy et al. 2014). The O2 demand needed by heterotrophic bacteria to decompose completely the organic matter resulted in an oxygen uptake rate (OUR) of 28.5 mg L−1 h−1 of O2, corresponding to 1.78 mmol L−1 h−1, which is also the base calculation applied by Boelee et al. (2014).
By applying the stoichiometry of microalgae growth, it was calculated the photoautotrophic microalgal biomass needed to produce that amount of oxygen. Based on microalgal composition, the availability of nutrients in wastewater was also checked. The nitrogen and phosphorus uptake by heterotrophic bacteria and microalgae was calculated based on the yield values, \( {Y}_{\raisebox{1ex}{$i$}\!\left/ \!\raisebox{-1ex}{$x$}\right.} \):
where Cx is the biomass concentration (mg L−1, the subscript x is indicated as M or B for microalgae and bacteria respectively) and Ci the concentration of nutrient i (mg L−1). The nutrient yield values for bacteria were taken from literature (Metcalf and Eddy et al. 2014), while those of microalgae where measured, based on actual consumption in batch growth experiments.
To calculate the microalgal carbon yield, in the case of mixotrophic growth, some specific batch experiments were carried out: a first growth curve was carried out in BG11 medium, without gas bubbling and additional carbon source, to measure the biomass production due to photoautotrophy based on CO2 dissolved in the medium, at equilibrium with the atmosphere and under limited gas exchange. Another growth curve was then carried out, by adding organic carbon to the medium, at the same concentration of synthetic wastewater. The additional biomass obtained, with respect to the case of autotrophic one, was assumed to be the one produced starting from dissolved organic carbon, whose consumption was measured.
Results and discussion
Growth of C. protothecoides in real wastewater: interaction with bacteria consortium
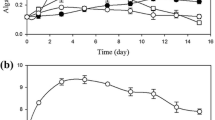
A batch growth curve was carried out by inoculating the microalgae-bacteria consortium in real wastewater, with an initial biomass ratio (based on dry weight) of 0.8 (microalgae-bacteria). As a control, the microalgal species was cultivated alone, in the same real wastewater, both untreated and after sterilisation. Results, displayed in Fig. 1, confirmed that this algal species is able to grow in real wastewater, even without any sterilisation pre-treatment, as already reported by Ramos Tercero et al. (2013). The growth in the presence of the consortium was found similar to the control (microalgae cultivated alone), with a specific growth rate of about 0.76 day−1 (data reported in Table S2 as supplementary materials) suggesting that no inhibition phenomena occurred. It was previously reported that microalgae and bacteria can interact through a range of mechanisms, both positive and negative (Fukami et al. 1997; Subashchandrabose et al. 2011; Unnithan et al. 2014). The positive interactions account mainly for nutrient exchange (Kouzuma and Watanabe 2015), facilitating, for instance, nitrogen assimilation by microalgae (Le Chevanton et al. 2013). However, the mechanisms of interactions may be also negative. He et al. (2013), for instance, reported a competition for resources with bacteria that inhibited algal growth. The presence of algae can inhibit bacteria as well, through the release of toxic metabolites (Kouzuma and Watanabe 2015). As no inhibition was observed, by adding bacterial community to microalgae inoculum in this first series of experiments, C. protothecoides resulted to be a very promising candidate for studying in depth the interactions and gas exchange in association with bacteria from activated sludge.
Concerning gas exchange, apparently, CO2 was still limiting despite the bacterial production, as the growth curve of microalgae with external CO2 supply resulted in higher specific growth rate (about 0.96 ± 0.09 day−1, reported in Table S2 of supplementary materials) and final biomass concentration (Fig. 1). This aspect will be better investigated in the “Microalgae-bacteria consortium in synthetic wastewater: growth and pollutant removal” section.
Nutrient consumption was also measured, with a reduction up to 80% of dissolved total nitrogen and a complete consumption of phosphorus (data reported in Table S2 as supplementary materials), when microalgae are present in the consortium. Similar results were obtained in other works (Ruiz-Martinez et al. 2012; Ramos Tercero et al. 2013), confirming that the N and P removal by microalgae is quite efficient.
Microalgae-bacteria consortium in synthetic wastewater: growth and pollutant removal
Once confirmed that C. protothecoides is able to grow in consortium with bacteria, further experiments with synthetic media were carried out, with the aim to investigate the interaction between microalgae and activated sludge bacteria in a stable and reproducible environment. In particular, the possibility to exploit gas exchange of oxygen produced by photosynthesis and CO2 by heterotrophic organisms was investigated.
To understand the effect of co-cultivation on the possible gas exchange, the growth curve in consortium was compared to the controls with algae and bacteria cultivated alone, with or without bubbling, as detailed in Fig. 2: it is clear that the growth curve of microalgae is not inhibited by the presence of bacteria, but the specific growth rate was slower than in the case of non-limiting CO2 supply. This is probably due to the simultaneous growth of bacteria which is fast in the first day, but is then strongly reduced, thus stopping the production of CO2.
Growth curve (OD data in a, cells count in b) of M + B consortium (dark triangles), microalgae alone without bubbling (reverse triangles) and with non-limiting CO2 supply (dark diamonds), bacteria alone without bubbling (open triangles) and with non-limiting air supply (open circles) in synthetic wastewater. In a, data of cultures with bacteria only (open circles and triangles) are referred to right Y axis, for graphical reasons only
Nutrient removal is reported in Fig. 3. Nitrogen, reported for all the chemical species analysed, showed interesting results in the case of microalgae cultivation. It appeared that, when CO2 is not limiting, microalgae prefer to exploit ammonium, while the organic fraction is not consumed. The total nitrogen reduction accounted for the 60% of the initial amount.
Initial (first column) and final concentration of nitrogen (a), phosphorus (b) and COD (c) of growth curve in synthetic wastewater of bacteria with non-limiting air supply (B + air), bacteria alone without bubbling (B), microalgal-bacterial consortium (M + B), microalgae alone without bubbling (M) and with non-limiting CO2 supply (M + CO2). In a, grey bars refer to N-NO2, white to N-NH3, dark to Norg and striped pattern to N-NO3. Dots refer to the percent consumption with the respect to initial value, measure for each single experiment
On the opposite, when CO2 is limiting (i.e. without forced aeration), the 85% of total nitrogen is removed, suggesting that also organic N can be used by microalgae. The possibility of exploiting organic nitrogen is well documented trough mixotrophic metabolism (Markou et al. 2014). Mixotrophy, on the other hand, is reduced when a non-limiting CO2 supply is provided (Sforza et al. 2012), which can explain the smaller organic nitrogen removal observed in this study. Concerning nitrogen consumption by bacteria alone, this was only about 30%, both in the presence and in the absence of aeration. A different speciation, however, can be observed when air is supplied to the culture. In fact, in an anoxic environment, the heterotrophic community prevailed and a fraction of organic nitrogen was transformed to ammonia. Under aerobic environment, instead, ammonia is converted to nitrate and nitrite by means of AOB (ammonia oxidising bacteria) and NOB (nitrite oxidising bacteria) populations.
When a microalgae-bacteria consortium is applied, a reduction of about 60% of total N was obtained, with a final concentration of about 10 mg L−1. In this condition, part of organic N is converted to ammonia, which is commonly efficiently removed by microalgae (Markou et al. 2014).
It is not clear if the presence of microalgae produced a significant amount of O2, allowing bacteria to perform a transient oxidation of ammonia to nitrites and nitrates, which are then consumed by algae. The main result, anyway, is that the coexistence of microalgae and bacteria allowed a promising nitrogen removal from wastewater, with a reduction of about 62% in a single step, thus simplifying the process, which conventionally requires two aerobic-anoxic steps for nitrification and denitrification. In addition, in view of nutrient recycling, nitrogen is stored in the algal biomass, and it is not released to the atmosphere, as it usually occurs in the denitrification process. Nitrogen stored in algal biomass can be subsequently recovered, for instance, by anaerobic digestion or by use as a biofertilizer (Sforza et al. 2017).
Also, phosphorus concentration is remarkably lowered when microalgae are present, as it can be observed in Fig. 3b. Microalgae alone completely removed phosphorus, while bacteria were able to remove only a 30–35% of initial P, both with and without aeration. With co-cultivation, instead, this value is about 73%, with a final concentration of 1.24 mg L−1 of P. Confirming that the presence of microalgae in the consortium helps to reduce both nitrogen and phosphorus concentration, in a single step of co-cultivation.
It also appeared that microalgae and bacteria may compete somehow for nutrients, as the removal of nitrogen and phosphorus in co-cultivation was always lower than in the case of microalgae alone. The occurrence of competition is more evident in the case of organic carbon consumption, as reported in Fig. 3c. In fact, the carbon removal by bacteria alone was efficient, with a reduction of about 90% of COD. Instead, microalgae alone removed about the 75% of COD, while, when co-cultivated with bacteria, overall, the reduction of COD was 55%. In other terms, when microalgae and bacteria are grown together, a lower organic carbon uptake is achieved. A possible explanation can be based on the comparison with nitrogen removal: the O2 produced by microalgae seems to be able to partially sustain the oxidation of ammonia by autotrophic bacteria (AOB and NOB), but both nitrates and nitrites are completely removed by microalgae, thus limiting the heterotrophic portion of bacterial community which exploits this N species as electron acceptors (Metcalf and Eddy et al. 2014). In fact, AOB and NOB growth rates are respectively 0.54 and 0.67 days−1, which are lower than the average value for C. protothecoides of 0.83 days−1 in real wastewater and 0.76 days−1 in synthetic wastewater.
Role of mixotrophy in oxygen production
As reported above, microalgae showed a consistent mixotrophic metabolism when cultivated in a wastewater containing organic carbon and nitrogen species. To understand the impact of mixotrophy on oxygen production and consumption, respirometric tests were performed, on the microalgal biomass alone, in the presence of inorganic and organic substrates, according to the protocol reported in the “Respirometric assays” section.
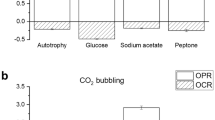
Figure 4a summarises the specific oxygen production and consumption rates under light and dark condition, respectively. The algal biomass used for all respirometric tests was sampled from steady-state continuous reactor so to have an actively replicating, constant quality biomass. The average data of specific oxygen production rate and consumption (Fig. 4a) showed that, with an inorganic carbon source, the autotrophic metabolism allowed a net oxygen production between light and dark conditions. On the other hand, when an organic carbon source was added, the oxygen production was much less, and the respiration rate strongly increased. In addition, it can be observed that respiration rates with simpler organic forms, like acetate, were higher than with complex substrates, like meat extract. The capability of exploitation of peptone under dark is noteworthy, confirming that microalgae are able to uptake organic nitrogen. Eventually, respirometric tests were also carried out in synthetic and real wastewaters, to prove the mixotrophic capability of microalgae in such a medium. It resulted that in wastewater, oxygen production rate is reduced by about 70%, while the respiration rate is increased if compared to autotrophic conditions. Based on the values in Fig. 4a, it can be concluded that the organic carbon present in real wastewater is less degradable by microalgae than the one contained in synthetic one, and this should be considered when accounting for mixotrophic metabolism. Anyway, the capability of carrying out mixotrophic metabolism by microalgae in wastewater is confirmed, and quantified.
a Specific oxygen production and consumption rates of C. protothecoides in autotrophic conditions (with bicarbonate) and mixotrophic conditions with different organic substrate and wastewaters under light (black) and dark (grey) conditions are reported. Asterisks refer to significantly different value with respect to the autotrophic control. Specific oxygen consumption rates of C. protothecoides and bacteria in synthetic wastewater under light (black) and dark (grey) conditions (b)
Another interesting result concerns the oxygen produced by microalgae compared to the one consumed by bacteria. As reported in Fig. 4b, when measuring the dissolved oxygen concentration in a co-culture with synthetic wastewater, a fast reduction was found, suggesting that the consumption rate by bacteria is much faster than microalgal production. When a biomass ratio of 1:1 was applied, no difference in the oxygen consumption rate could be observed, suggesting that autotrophic O2 production is too slow to compensate the consumption by bacteria. When increasing the algal/biomass ratio (with constant algal biomass, the bacterial inoculum was much less), a smaller slope of oxygen consumption was observed in light periods, suggesting that the oxygen produced by microalgae can in part reduce the overall decrease of dissolved oxygen concentration. On the other hand, no net production of oxygen was observed in all the cases considered, suggesting that bacterial heterotrophic metabolism strongly prevails.
Mass balance of gas exchange and pollutant removal in microalgae-bacteria consortium
In this section, based on the experimental results presented previously, a discussion about the feasibility of the microalgae-bacteria integrated process is carried out, by considering the mass balances between the O2 and CO2 produced and also the nutrients consumed by both microalgae and bacteria, according to stoichiometric growth equations. The process of treating urban wastewater through the combination of microalgae and heterotrophic bacteria may be feasible and self-sustainable only if these balances are satisfied. More specifically, microalgae must be able to produce the sufficient amount of oxygen needed to the bacteria to degrade the organic matter present in a typical urban wastewater. Starting from the stoichiometric equation reported in the “Mass balance and stoichiometry” section, and calculating the bacterial OUR (see supplementary materials), the microalgal biomass productivity necessary to sustain the process is calculated: to produce 1.78 mmol L−1 h−1 of oxygen, a microalgal productivity of 0.81 g L−1 day−1 is required, which is a value of production actually obtainable by microalgae (Sforza et al. 2015). At the same time, the amount of CO2 requested by microalgae (1.52 mmol L−1 h−1) is satisfied by bacterial biomass.
Moreover, the amount of nutrients (mainly N and P) present in the wastewater should be enough to sustain both the microalgal and bacterial growth and they should be consumed at least under the law limit concentrations values.
The elemental composition of microalgal biomass may remarkably differ from the canonical Redfield ratio, not only due to the specific organisms studied, but also as a response of environmental conditions. To account for these aspects, based on experimentally determined elemental composition of Chlorella protothecoides (reported in Table S4 in supplementary materials, for axenic cultures) and by adjusting the nitrogen and phosphorus yields on experimental results obtained in this work, the autotrophic algal stoichiometry (Eq. 1), proposed by Boelee et al. (2014), was modified as follows:
Thus, if nutrient yields and biomass productivity are considered, it appeared that the consumption by bacteria and microalgae resulted in 8.24 and 20.64 mgN L−1 and 1.58 and 2.70 mgP L−1 respectively, with final residual concentrations of 1.12 mgN L−1 and 0.72 mgP L−1 and 76.5 mg L−1 for COD. Accordingly, the 69% of the nitrogen and the 54% on phosphorus are actually removed by microalgae. Thus, the application of microalgae-bacteria consortia appeared to be feasible, as both the gas exchange and nutrient removal are satisfied. These calculations, however, did not account for the kinetic of the two populations involved. In fact, the OUR calculation is based on a conventional wastewater treatment plant, where the HRT corresponds to 4.4 h. The growth rate constant required to avoid washout from this system is remarkably higher than that of microalgal species, suggesting that the process design should be based on microalgal growth capability.
Moreover, microalgae, in spite of being mainly autotrophic organisms, can assimilate organic carbon and develop a mixotrophic metabolism. This is particularly true in the case of growth in wastewater, where both carbon and organic nitrogen are present, as shown in the previous part of this work. As reported in Fig. 4, by exploiting organic C and N, microalgae actually consume an amount of oxygen due to respiration phenomena leading to a lower net oxygen production rate. This results in a lower net oxygen production, on one side, but also a cooperative COD removal, on the other one. As a consequence, stoichiometric equations should be rewritten to account for the effect of mixotrophy. The mixotrophic metabolism was described, based on works of Chojnacka and Marquez-Rocha (2004), as a combination of two equations representing separately the autotrophic (see Eq. (5)) and heterotrophic growth, written as
In Eq. 6, the exploitation of organic nitrogen was introduced, by accounting for an averaged formula of amminoacids and peptides, derived from Torabizadeh (2011). Equations 5 and 6 can be combined to describe mixotrophy. To balance Eq. 6, the biomass/carbon yield experimentally measured was applied, corresponding to 0.78 mgC,M mgC−1 (comparable to values reported by Barros et al. (2017)). This value accounts for the ratio of organic carbon which is actually fixed in biomass.
To balance the ratio of biomass produced by photosynthesis with respect to that obtained from organic carbon, the data of oxygen production were used, as reported in the “Role of mixotrophy in oxygen production” section. Based on the oxygen produced under autotrophic condition and with wastewater as carbon source (corresponding to 0.56 and 0.17 mgO2 min−1 gM−1, respectively), it resulted that, when microalgae exploit organic carbon and nitrogen in a complex medium, a reduction of about 78% of oxygen can be observed. Thus, 78% of the O2 produced by autotrophy (Eq. 5) is then exploited for organic substrate respiration, as described by Eq. 6. This results in a ratio of biomass produced by autotrophy and heterotrophy of 1:9.2, which can be used to calculate the carbon actually consumed by microalgae in the process.
Consequently, it appeared that the net oxygen production is enough to sustain only a portion of the bacterial OUR. However, if a portion of COD is removed by microalgae, this net O2 production is potentially enough to sustain a production of bacteria needed to remove the remaining organic matter. Of course, the carbon and nitrogen specifically removed by bacteria or microalgae is dependent on the growth and uptake kinetic, and, consequently, on the hydraulic retention time of the system, which actually could also change the ratio of the populations involved.
Of course, this discussion is based only on mass balances, starting from the stoichiometry of the organisms involved, and a comprehensive study of the kinetic should be carried out, with particular attention to the effect of light. In fact, the growth rate of bacteria is remarkably higher than that of microalgae, thus possibly affecting the coexistence in a continuous system. Accordingly, the SRT of the system should be carefully set, or other technical solutions, like the co-immobilisation of the community (De-Bashan et al. 2002; de-Bashan and Bashan 2010; Boelee et al. 2011), should be considered, with the drawback of reducing the light available for microalgae. In this context, deeper attention should be paid to the effect of light on microalgae removal capability, in particular in the case of mixotrophic metabolism.
In summary, even if mixotrophic metabolism strongly reduces the oxygen supply to heterotrophic bacteria, the co-cultivation is promising, because microalgae contribute to the removal of organic matter, also exploiting nitrogen and phosphorus in a single step.
Conclusions
C. protothecoides was used in consortium with bacteria form activated sludge, in order to describe complex interactions and gas exchange among populations involved. This algal species, in the presence of bacteria from activated sludge, showed no inhibition of growth, but the oxygen produced by microalgae did not allow a complete reduction of COD by heterotrophic bacteria. Biodegradable COD content in wastewater stimulates the mixotrophic metabolism of microalgae, thus reducing the net oxygen availability. Stoichiometric equations were implemented to describe mixotrophy, based on quantitative measurements of oxygen production rate in respirometric tests performed with both inorganic and organic carbon sources. Mass balance demonstrated that, even though the net photosynthetic oxygen production is reduced when mixotrophy occurs, the contribution of microalgae to pollutant removal is significant.
References
Abinandan S, Shanthakumar S (2015) Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: a review. Renew Sust Energ Rev 52:123–132. https://doi.org/10.1016/j.rser.2015.07.086
Alcántara C, Fernández C, García-Encina PA, Muñoz R (2015) Mixotrophic metabolism of Chlorella sorokiniana and algal-bacterial consortia under extended dark-light periods and nutrient starvation. Appl Microbiol Biotechnol 99:2393–2404. https://doi.org/10.1007/s00253-014-6125-5
Barros A, Guerra LT, Simoes M et al (2017) Mass balance analysis of carbon and nitrogen in industrial scale mixotrophic microalgae cultures. Algal Res 21:35–41. https://doi.org/10.1016/j.algal.2016.10.014
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101:1611–1627. https://doi.org/10.1016/j.biortech.2009.09.043
Bertucco A, Beraldi M, Sforza E (2014) Continuous microalgal cultivation in a laboratory-scale photobioreactor under seasonal day-night irradiation: experiments and simulation. Bioprocess Biosyst Eng 37:1535–1542. https://doi.org/10.1007/s00449-014-1125-5
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH (2011) Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res 45:5925–5933. https://doi.org/10.1016/j.watres.2011.08.044
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH (2014) Balancing the organic load and light supply in symbiotic microalgal-bacterial biofilm reactors treating synthetic municipal wastewater. Ecol Eng 64:213–221. https://doi.org/10.1016/j.ecoleng.2013.12.035
Cho S, Luong TT, Lee D, Oh YK, Lee T (2011) Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour Technol 102:8639–8645. https://doi.org/10.1016/j.biortech.2011.03.037
Chojnacka K, Marquez-Rocha F-J (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–34
De-Bashan LE, Moreno M, Hernandez JP, Bashan Y (2002) Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res 36:2941–2948. https://doi.org/10.1016/S0043-1354(01)00522-X
Decostere B, Janssens N, Alvarado A, Maere T, Goethals P, van Hulle SWH, Nopens I (2013) A combined respirometer-titrimeter for the determination of microalgae kinetics: experimental data collection and modelling. Chem Eng J 222:85–93. https://doi.org/10.1016/j.cej.2013.01.103
Fukami K, Nishijima T, Ishida Y (1997) Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia 358:185–191. https://doi.org/10.1023/A:1003139402315
Gupta PL, Choi HJ, Pawar RR, Jung SP, Lee SM (2016) Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J Environ Manag 184:585–595. https://doi.org/10.1016/j.jenvman.2016.10.018
Gutzeit G, Lorch D, Weber A et al (2015) Bioflocculent algal-bacterial biomass improves low-cost wastewater treatment. Water Sci Technol 52:9–18
He PJ, Mao B, Lu F et al (2013) The combined effect of bacteria and Chlorella vulgaris on the treatment of municipal wastewaters. Bioresour Technol 146:562–568. https://doi.org/10.1016/j.biortech.2013.07.111
Karya NGAI, van der Steen NP, Lens PNL (2013) Photo-oxygenation to support nitrification in an algal-bacterial consortium treating artificial wastewater. Bioresour Technol 134:244–250. https://doi.org/10.1016/j.biortech.2013.02.005
Kouzuma A, Watanabe K (2015) Exploring the potential of algae/bacteria interactions. Curr Opin Biotechnol 33:125–129. https://doi.org/10.1016/j.copbio.2015.02.007
Le Chevanton M, Garnier M, Bougaran G et al (2013) Screening and selection of growth-promoting bacteria for Dunaliella cultures. Algal Res 2:212–222. https://doi.org/10.1016/j.algal.2013.05.003
Liu L, Zhao Y, Jiang X, Wang X, Liang W (2018) Lipid accumulation of Chlorella pyrenoidosa under mixotrophic cultivation using acetate and ammonium. Bioresour Technol 262:342–346. https://doi.org/10.1016/j.biortech.2018.04.092
Longo S, d’Antoni BM, Bongards M, Chaparro A, Cronrath A, Fatone F, Lema JM, Mauricio-Iglesias M, Soares A, Hospido A (2016) Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl Energy 179:1251–1268. https://doi.org/10.1016/j.apenergy.2016.07.043
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Metcalf & Eddy, Tchobanoglous G, Stendel HD, et al (2014) Wastewater engineering: treatment and resource recovery, 5th edn
Nur MMA, Buma AGJ (2018) Opportunities and challenges of microalgal cultivation on wastewater, with special focus on palm oil mill effluent and the production of high value compounds. Waste Biomass Valoriz 1–19. doi:https://doi.org/10.1007/s12649-018-0256-3
OECD (2001) http://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm
Olguín EJ (2012) Dual purpose microalgae-bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a biorefinery. Biotechnol Adv 30:1031–1046. https://doi.org/10.1016/j.biotechadv.2012.05.001
Ramos Tercero EA, Sforza E, Morandini M, Bertucco A (2013) Cultivation of Chlorella protothecoides with urban wastewater in continuous photobioreactor: biomass productivity and nutrient removal. Appl Biochem Biotechnol 172:1470–1485. https://doi.org/10.1007/s12010-013-0629-9
Rossi S, Bellucci M, Marazzi F, et al (2018) Activity assessment of microalgal-bacterial consortia based on respirometric tests. Water Sci Technol wst2018078. doi:https://doi.org/10.2166/wst.2018.078
Ruiz-Martinez A, Martin Garcia N, Romero I et al (2012) Microalgae cultivation in wastewater: nutrient removal from anaerobic membrane bioreactor effluent. Bioresour Technol 126C:247–253. https://doi.org/10.1016/j.biortech.2012.09.022
Sforza E, Cipriani R, Morosinotto T, Bertucco A, Giacometti GM (2012) Excess CO2 supply inhibits mixotrophic growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour Technol 104:523–529. https://doi.org/10.1016/j.biortech.2011.10.025
Sforza E, Urbani S, Bertucco A (2015) Evaluation of maintenance energy requirements in the cultivation of Scenedesmus obliquus: effect of light intensity and regime. J Appl Phycol 27:1453–1462. https://doi.org/10.1007/s10811-014-0460-x
Sforza E, Barbera E, Girotto F, Cossu R, Bertucco A (2017) Anaerobic digestion of lipid-extracted microalgae: enhancing nutrient recovery towards a closed loop recycling. Biochem Eng J 121:139–146. https://doi.org/10.1016/j.bej.2017.02.004
Su Y, Mennerich A, Urban B (2012) Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: influence of algae and sludge inoculation ratios. Bioresour Technol 105:67–73. https://doi.org/10.1016/j.biortech.2011.11.113
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29:896–907. https://doi.org/10.1016/j.biotechadv.2011.07.009
Torabizadeh H (2011) All proteins have a basic molecular formula. Int J Chem Mol Nucl Mater Metall Eng 5:501–505
Unnithan VV, Unc A, Smith GB (2014) Mini-review: a priori considerations for bacteria-algae interactions in algal biofuel systems receiving municipal wastewaters. Algal Res 4:35–40. https://doi.org/10.1016/j.algal.2013.11.009
Acknowledgements
The authors would like to thank Montebello Vicentino (VI, Italy) treatment plants for providing the wastewaters and activated sludge inoculum for the experiments. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerald Thouand
Electronic supplementary material
ESM 1
(DOCX 145 kb)
Rights and permissions
About this article
Cite this article
Sforza, E., Pastore, M., Spagni, A. et al. Microalgae-bacteria gas exchange in wastewater: how mixotrophy may reduce the oxygen supply for bacteria. Environ Sci Pollut Res 25, 28004–28014 (2018). https://doi.org/10.1007/s11356-018-2834-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2834-0