Abstract
Plant–plant interactions via volatile organic compounds (VOCs) have received much attention, but how abiotic stresses affect these interactions is poorly understood. We tested the effect of VOCs exposure from damaged conspecifics on the production of extra-floral nectar (EFN) in wild cotton plants (Gossypium hirsutum), a coastal species in northern Yucatan (Mexico), and whether soil salinization affected these responses. We placed plants in mesh cages, and within each cage assigned plants as emitters or receivers. We exposed emitters to either ambient or augmented soil salinity to simulate a salinity shock, and within each group subjected half of the emitters to no damage or artificial leaf damage with caterpillar regurgitant. Damage increased the emission of sesquiterpenes and aromatic compounds under ambient but not under augmented salinity. Correspondingly, exposure to VOCs from damaged emitters had effect on receiver EFN induction, but this effect was contingent on salinization. Receivers produced more EFN in response to damage after being exposed to VOCs from damaged emitters when the latter were grown under ambient salinity, but not when they were subjected to salinization. These results suggest complex effects of abiotic factors on VOC-mediated plant interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) emitted by plants play key roles in ecological communities (Kessler and Baldwin 2001; Turlings and Erb 2018; Bouwmeester et al. 2019). Studies conducted over the last decade have shown that plants respond to VOCs emitted by herbivore-damaged plants, a phenomenon often referred to as “communication” (Karban 2015; Ninkovic et al. 2019), hereafter “plant–plant signaling”. For example, studies with species such as Artemisia tridentata (sagebrush), Arabidopsis thaliana, Zea mays (maize), and Solanum lycopersicum (tomato) have shown that VOCs released by damaged plants trigger defensive responses against attackers in nearby undamaged plants (reviewed by Karban et al. 2014). Plant-plant signaling via VOCs has also been shown to influence plant traits implicated in indirect defense, including VOC emissions and extra-floral nectar (EFN) production which attract natural enemies (Heil and Kost 2006; Turlings and Wäckers 2004; Bouwmeester et al. 2019). This form of plant–plant signaling can thus mediate complex interactions involving both plant antagonists and mutualists, affecting plant performance and shaping associated food webs.
Extra-floral nectar contains mainly sugars, as well as amino acids, lipids, and different types of secondary metabolites (Bentley 1977; Heil 2015). It is a highly inducible trait in response to herbivory and has been shown to increase recruitment of natural enemies (e.g., predators and parasitoids) which feed on this secretion, resulting in enhanced herbivore suppression (Rico-Gray and Oliveira 2007; Heil 2015). Extra-floral nectar has been well studied within the context of ant-plant interactions, whereby ants actively consume EFN and in turn defend plants against both herbivores and pathogens (Heil and McKey 2003; Rudgers 2004; Rico-Gray and Oliveira 2007). At least two studies have shown that VOCs emitted by attacked plants can trigger increased production of EFN in nearby intact plants, thereby enhancing indirect defense (Kost and Heil 2006; Heil and Silva-Bueno 2007). They found that lima bean (Phaseolus lunatus) plants exposed to VOCs from damaged emitter conspecifics produced more EFN (i.e., volatiles triggered nectar induction), but VOC-exposed plants were also primed such that they produced a greater amount of EFN when they themselves were damaged (Kost and Heil 2006; Heil and Silva-Bueno 2007). Despite these key findings, the effects of plant–plant signaling on indirect defenses, particularly on EFN, remain largely understudied, thus limiting our understanding of VOCs-mediated signaling on plant indirect defense and multi-trophic interactions.
Abiotic factors modify VOCs emissions in plants (Gouinguené and Turlings 2002; Holopainen and Gershenzon 2010) which can have important implications for plant–plant signaling (Moreira and Abdala-Roberts 2019). Such effects are increasingly relevant for both crops and wild plant populations due to more extreme and frequent abiotic disturbances including heat waves, severe storms, drought events, as well as coastal flooding (Bellard et al. 2012; Rosenzweig et al. 2014). However, as of yet the effects of abiotic stresses on VOCs-mediated plant–plant signaling are poorly understood. Among the few available studies, in a study with tomato (S. lycopersicum) Catola et al. (2018) found that drought stress in emitter plants increased VOCs emissions and parasitoid attraction in receiver plants, whereas Pezzola et al. (2017) found that water stress in receiver plants influenced induced responses to emitter VOCs in sagebrush (A. tridentata). In addition, several other studies have found that pollutants such as ozone can disrupt VOCs-mediated plant signaling, in some cases presumably by degrading volatiles (e.g., Girón-Calva et al. 2016; reviewed by Blande 2021). However, studies on the effects of abiotic factors on plant–plant signaling remain limited and various types of pervasive drivers remain unstudied.
Soil salinity is a key factor modulating plant induced responses (Parihar et al. 2014; Forieri et al. 2016; Landi et al. 2020) and is likely to become an increasingly important abiotic force affecting plant populations and communities, particularly in coastal habitats due to sea level rise and flooding (Sweet and Park 2014). Negative impacts of salinity on resource uptake and use (e.g., via effects on cell water relations, hormonal balance, and carbon supply; reviewed by Munns 2002) have been shown to impact the synthesis of secondary metabolites involved in plant defense (Baldwin and Preston 1999; Quijano-Medina et al. 2021). Indeed, several studies have addressed the effects of salinization on herbivore-induced plant defenses, including VOCs in species such as maize (Z. mays), cotton (Gossypium hirsutum), and poplar (Populus x canescens) (e.g., Teuber et al. 2008; Forieri et al. 2016; Quijano-Medina et al. 2021), and at least one study has shown that salinization affects plant–plant signaling but this did not involve herbivory (i.e., signaling effects via constitutive volatile emissions). Specifically, in a study with Vicia faba, Caparrotta et al. (2018) found that soil salinization in emitter plants can cause VOCs-mediated physiological changes in undamaged receivers which in turn primed them to better cope with salinity stress. Still, current understanding of the biochemistry and physiology behind such effects on VOCs and plant signaling remains very limited, and to our knowledge, no study to date has addressed the effects of soil salinization effects on plant–plant signaling mediated by herbivore-induced VOCs.
We studied the effect of soil salinization on airborne VOCs-mediated signaling between wild cotton (Gossypium hirsutum) plants, with a focus on EFN responses. This species is distributed on the coastal shrubland of the Yucatan Peninsula (Mexico), where, as in other coastal areas, climate change is expected to lead to more severe and frequent sea flooding events, thereby increasing salinity exposure of cotton populations. Wild cotton possesses effective direct (terpenoids, phenolics) and indirect (VOCs, EFN) defensive traits (Hagenbucher et al. 2013). Previous work shows that a salinization shock treatment causes changes in the total emission of VOCs and reduces the induction of some volatile compounds in response to leaf damage (Quijano-Medina et al. 2021). The present study builds on these findings and addresses the following questions: (1) Does exposure to VOCs from damaged emitter plants increase the amount and concentration of EFN produced by intact receiver plants (i.e., induction effect)? (2) Does VOCs exposure increase the strength of induction of EFN in response to leaf damage in receivers (i.e., priming effect on induction)? And (3) Do changes in VOC emissions due to soil salinization alter this plant–plant signaling effect on EFN production? To this end, we performed a greenhouse experiment in which we compared the VOC emissions from control emitter cotton plants with plants that had been subjected to soil salinization. Within each group plants were either left intact or subjected to artificial leaf damage plus application of caterpillar (Spodoptera frugiperda) regurgitant. We then measured the amount and concentration of EFN produced by receiver plants that had been exposed to VOCs released by the emitters, before and after they themselves were damaged. We predicted physiological effects of salinization (e.g., via reduced water uptake, carbon supply) would hamper resource allocation and synthesis of induced VOCs and that this would weaken plant-plant signaling effects on EFN. Overall, this study reveals that increased soil salinity can have considerable disruptive effects on VOCs-mediated plant–plant interactions with possible significant consequences for associated food webs.
Materials and methods
Study species
Wild cotton, Gossypium hirsutum, is myrmecophitic shrub distributed in Central America, Mexico, and the Caribbean Basin (D’Eeckenbrugge and Lacape 2014; Wendel and Grover 2015), where it is found mainly in coastal habitats. In particular, populations found in the northern coast of the Yucatan Peninsula (Mexico) are exposed from moderate to high levels of soil salinity within and across sites (0.05 to 3.53‰ salinity across locations, mean = 0.8 ± 0.17‰ [SE]; N = 6 sites; Quijano-Medina et al. 2021). At these sites, wild cotton is attacked by a diverse community of herbivorous insects, including leaf chewers (e.g., caterpillars and grasshoppers) which cause c. 25% of leaf area loss on average (Abdala-Roberts et al. 2019a), and to a lesser extent phloem-feeding (e.g., bugs and aphids) species. To resist these attacks, G. hirsutum produces several inducible traits that are associated with direct defense (e.g., phenolic compounds and non-volatile terpenoids; Mansour et al. 1997; Agrawal and Karban 2000; Opitz et al. 2008; Nix et al. 2017), as well as indirect defense such as VOCs (Loughrin et al. 1994; McCall et al. 1994; Chappuis and Egger 2016) and EFN (Wäckers and Bonifay 2004; Abdala‐Roberts et al. 2019b). In addition, work with wild cotton reported increases in both EFN amount and concentration in response to artificial leaf damage (Abdala-Roberts et al. 2019b), and ant abundance positively correlates with both EFN variables (Reyes-Hernández et al. 2022). Relatedly, a recent study showed that soil salinization did not affect wild cotton EFN induction in response to leaf damage (Quijano-Medina et al. 2021). Finally, work with cultivated cotton found that exposure to VOCs from damaged plants increase resistance in undamaged receiver plants (Bruin et al. 1992; Zakir et al. 2013a, b). These signaling effects have not been investigated for EFN induction in this species.
Plant material
We used seeds collected in 2019 and 2020 from four wild cotton populations located along the northern coast of Yucatan, two near the town of Chicxulub (21° 17′ 46.0752ʺN, − 89° \(34^{\prime}\) 45.4832ʺW and 21° 18′ 14.2697ʺN, − 89° 32′ 29.9137ʺ) and two near the town of Sisal (21° 18′ 14.2697ʺN, − 89° 32′ 29.9137ʺW and 21° 11′ 38.6700ʺN, − 89° 57′ 28.1088ʺW). Across sites, seeds from a total of seven mother plants were used (hereafter genotypes). In April 2021, we exposed seeds to coat scarification and germinated them with wet cotton wool in Petri dishes at 35 ℃. Seedlings were individually transplanted to 25 × 30 cm low-density polyethylene nursery bags containing a mix of sandy soil (from the seed source sites), native forest soil, and perlite (1:2:1). After transplantation, we kept all cotton seedlings in a greenhouse at the Campus de Ciencias Biológicas y Agropecuarias of the Universidad Autónoma de Yucatán (México, 20°52′00.6ʺN, 89°37′29.5ʺW) for two months prior to starting the experiment. During this time, plants were watered with 300 ml three times per week and had 10–12 leaves.
Experimental design
In late May 2021, we conducted a factorial experiment in which we manipulated soil salinity and leaf damage of emitter plants. In total, we included 44 emitter plants and 88 receiver plants allocated in triplets to mesh cages (cylindrical shape: 60 cm diameter × 80 cm high) each containing one emitter and two receiver plants separated by 20 cm. All plants in a cage were of the same genotype and genotypes were approximately equally represented across treatments. Within each cage, we randomly assigned the emitter plant to either tap water (i.e., control or ambient salinity) or salinized tap water. For salinized plants, we placed the plastic bag in a plastic container with 2 L of tap water at 1% salinity (by adding NaCl) for 24 h until saturation was achieved. We chose this concentration based on previous work showing that this NaCL concentration results in a level of soil salinization comparable to in situ levels (Quijano-Medina et al. 2021; see ahead). The salinity treatment represented a saline shock treatment that simulated an event of flooding due to sea water or coastal lagoon surges, which occurs periodically, especially during winter months (Quijano-Medina et al. 2021). These events and the accompanying stresses due to salinization are expected to increase in duration and frequency due to sea level rise (Sweet and Park 2014). Control emitter plants under ambient salinity were treated the same way but with non-salinized water. We opted for this emitter-centered test because prior work showed that salinization influences wild cotton induced VOCs emissions (Quijano-Medina et al. 2021), making it the next logical step to test the extended consequences of such effects on plant–plant signaling.
Three days after salinizing emitters, we randomly subjected emitter plants of each salinity level to one of two leaf damage treatments: undamaged control or artificial leaf damage. At the time of damage application, soil salinity was significantly greater (t = − 6.91, P = 0.0002; N = 10, subset of five control and five salinized plants from which samples were taken) for salinized emitters (0.468 ± 0.039‰ or 0.008 ± 0.0006 mol/l) relative to those with ambient salinity (0.154 ± 0.026‰, i.e., 0.0026 ± 0.0004 mol/l). Soil salinity was estimated by direct measurements of water potential (see Quijano-Medina et al. 2021). Importantly, the measured soil salinization levels were within the natural range of soil salinity observed in situ, and in the case of salinized soil it was close to the mean value observed across natural cotton populations (Quijano-Medina et al. 2021). The damage treatment consisted in removing 50% of leaf area of half of the leaves per plant by cutting off the lobes of each leaf with a scissor, as well as puncturing the remaining leaf tissue with a micro-needle bearing 32 points (Dermapen®, FD Holdings) and exposing this area (ca. 1 cm2) to oral secretions of third instar larvae of Spodoptera frugiperda (Turlings et al. 1993; Abdala-Roberts et al. 2019c; Quijano-Medina et al. 2021). Larvae were sourced from a colony reared at the Chemical Ecology Lab in ECOSUR (Chiapas, Mexico), fed a wheat germ-based artificial diet. Damage was applied over two consecutive days, each day removing half of the total amount of damage to mimic a gradual increase in leaf consumption under natural conditions. We obtained caterpillar secretions by gently poking the abdomen of each larva until it regurgitated (Turlings et al. 1993). This insect (as other Spodoptera species; see Arce et al. 2021) is known to attack cultivated cotton and has been shown to induce both direct and indirect defenses (VOCs, EFN) in both wild and cultivated G. hirsutum (Quijano-Medina et al. 2021).
Starting from the first day of damage (see above), receiver plants were exposed to emitters for 48 h. At the end of this period, we removed emitters and collected their VOCs (N = 44) as well as EFN from all receivers to test for “initial” effects of exposure to emitter VOCs on receiver EFN. This timing of VOCs collection and exposure time for receivers was based on previous work showing that VOCs are highly induced after two days of damage (including compounds of known or suspected role in signaling; Loughrin et al. 1994; Arce et al. 2021). Hence, our sampling design ensured that receiver plants were exposed to these blends of induced cotton volatiles. After the first EFN collection, on that same day, we removed one receiver from each cage, which was used for another experiment, and we damaged the remaining receiver to test whether exposure to VOCs from damaged emitters primed EFN induction in receivers. Specifically, we damaged two fully expanded leaves located in the upper portion of the plant (same procedure as above, the same day after initial collection of EFN) and 24 h later collected EFN. We collected EFN from two damaged and two undamaged leaves per plant to test for treatment effects on local vs. systemic induction (Abdala-Roberts et al. 2019b). We consistently damaged leaves 2 and 4 (counting from the apical meristem downward), and undamaged leaves sampled were in positions 1 and 3, thus alternating leaf position/age between leaf types to avoid confounding leaf ontogeny with damage.
VOCs and EFN collection
We used 5-μl capillary tubes (Micropipettes Blaubrand ® intraMARK, Germany) to collect the EFN found on two fully or almost fully expanded leaves per receiver plant. Chosen leaves were close to the apical meristem. Nectar was collected between 06.00 and 08.00 h, and samples were taken to the laboratory to measure the amount (in µl) of EFN and its sugar content (expressed in °Brix) with a refractometer (Atago Master T 0 to 33°Brix, Germany). For statistical analyses, we used the total volume across both leaves per plant in the case of EFN production, whereas for concentration we used the mean value per plant.
We collected above-ground VOC emissions following Turlings et al. (1998). Briefly, plants were bagged within a nalophan bag (Reynolds, Inc), and VOCs were adsorbed on filters containing 25 mg of 80/100 mesh Hayesep-Q adsorbent (Sigma, Switzerland). One of the filter ends was inserted into the bag and the other end was connected to a micro air sampler (Supelco PAS-500) at a flow rate of 500 ml/min. For each sampling period, we also collected an air sample from empty bags which served as an ambient control. After collecting volatiles for 2 h, traps were eluted with 150 μl dichloromethane and then spiked with 10-μl internal standard solution [nonyl-acetate, (20 μg/μl) each]. Samples were sealed with polytetrafluoroethylene (PTFE) caps and Teflon, stored at − 30 ℃ and sent to University of Neuchâtel (Neuchâtel Switzerland) for GC–MS analysis. Samples were analyzed with a gas chromatograph (Agilent7890B) coupled with a mass spectrometer detector (Agilent 5977B). A 1.5-μl aliquot of each sample was injected in pulsed splitless mode onto an Agilent HP-5MS column (30-m length × 250-μm diameter and 0.25-μm film thickness). After injection, temperature was maintained at 40 ℃ for 3.5 min, increased to 100 ℃ at a rate of 8 ℃ per min, and subsequently to 230 ℃ at a rate of 5 ℃ per min followed by a post run of 3 min at 250 ℃. Helium was used as carrier gas and kept at constant flow of 0.9 ml/min. Compounds were subsequently identified by comparing their mass spectra with those from the NIST mass spectral library and comparisons with authentic standards. Quantification was based on peak areas relative to internal standard and measured in nanogram.
Statistical analyses
We ran general linear mixed models testing for effects of emitter leaf damage, salinity, and their interaction (all fixed factors) on total VOCs from emitter plants, the main volatile groups, and the concentration and volume of EFN produced by receiver plants. In addition, we analyzed variation in eight emitter VOCs that are highly inducible (Paré and Tumlinson 1997) namely: β-ocimene, linalool, DMNT, TMTT, E-β-farnesene, humulene, bicyclogermacrene, benzyl isonitrile, methyl salicylate, and indole. Analyses of EFN included the test of initial effects after 48 h of VOCs exposure as well as EFN induction in response to damage to test for priming effects. In this latter case, we ran separate models for damaged and undamaged leaves to tease apart effects on EFN local vs. systemic induction, respectively. We also included plant genotype (random) in all models to account for effects of genetic variation or maternal effects, as well as replicate cage (random) for models testing initial effects on receiver EFN (to account for non-independence of receiver pairs per cage). In most cases data exhibited a normal distribution, and we report model least-square means and standard errors as descriptive statistics. However, some models for volatile groups did not show a normal distribution of errors in which case a generalized linear mixed model with a Gamma distribution and log link was used. Analyses based on a normal distribution were run in PROC MIXED in SAS ver. 9.4 (SAS 2015), whereas Gamma models were run with ‘glmer’ function from lme4 package (Bates et al. 2015) in R version 4.1 (R Core Team 2021). A redundancy analysis (RDA) was used to explore the differences in VOCs composition between emitter treatments (salinity and damage) and their interaction, using the vegan package in R version 4.1 (R Core Team 2021). For this analysis, the normalized proportions of each volatile compound relative to the total VOC amount were used as the response matrix.
Results
Emitter VOCs
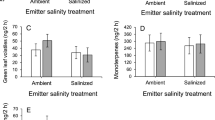
We identified 20 volatiles organic compounds (VOCs) emitted from cotton plants (Table S1). These compounds were classified in five groups: green leaf volatiles (GLVs), monoterpenes, homoterpenes, sesquiterpenes, and aromatic compounds. The RDA analysis testing the composition of the VOCs blend between salinization, leaf damage treatment, and their interaction was not statistically significant (permutation test: F3,36 = 0.80, P = 0.62). In addition, the amount of total VOCs did not differ between treatments (Table 1). When each compound group was analyzed separately, we found that for GLVs or monoterpenes there were no significant effects of any factor (Table S2). However, damaged plants emitted significantly more homoterpenes (DMNT and TMTT) than undamaged plants (Table S2). In addition, the interaction between salinization and herbivory treatments affected the release of sesquiterpenes and aromatic compounds (Table S2). Under ambient soil conditions, damaged plants showed higher emissions than control plants for these groups, whereas for plants that had been exposed to soil salinization no such differences were found (Fig. 1). Analysis of individual VOCs showed a significant interaction for the sesquiterpene bicyclogermacrene and the aromatic compound benzyl isonitrile (Table S3).
Effects of leaf damage under ambient vs. salinized soil on releases of A aromatic compounds and B sesquiterpenes produced (ng/2 h) by emitter wild cotton plants (Gossypium hirsutum). Values are back-transformed model least-square means and standard errors accounting for plant genotype and main effects. There were significant differences between control and damaged under ambient (sesquiterpenes: Z = 1.12, P = 0.0003; aromatics: Z = 1.32, P = 0.0001), but not under augmented salinity (sesquiterpenes: Z = − 0.045, P = 0.89; aromatics: Z = − 0.56, P = 0.12). ***P < 0.001
Receiver EFN
Initial effects of VOCs exposure
We found no significant effect of emitter leaf damage, salinity or their interaction on the volume or concentration of EFN produced by intact receiver plants after exposure to emitters (Table 1; Fig. 2A, B).
Effects of emitter leaf damage under ambient vs. salinized soil on A the volume (µl) and B concentration (°Brix) of extra-floral nectar (EFN) produced by unharmed receiver wild cotton (Gossypium hirsutum) plants (initial effects of VOCs exposure on EFN, see “Methods”). Values are model least-square means and standard errors accounting for plant genotype and main effects in the model
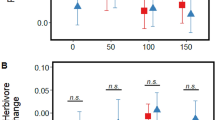
Effects of VOCs on EFN induction by damage in receiver plants. We found significant effects of emitter leaf damage and salinization on damage-induced EFN production in receiver undamaged leaves, i.e., systemic induction (Table 2A). Specifically, upon damage, undamaged leaves of receivers exposed to damaged emitters exhibited a 240% increase in EFN volume compared to damaged receivers exposed to intact emitters (control: 0.37 ± 0.21 µl; damage: 0.89 ± 0.21 µl) (Table 2A). In contrast, emitter salinization caused a significant (62%) decrease in receiver EFN volume produced after damage compared to receivers exposed to emitters with ambient salinity (control: 0.91 ± 0.21 µl; salinized: 0.35 ± 0.20 µl) (Table 2A). Furthermore, we observed a significant damage by salinity interaction (Table 2A), whereby under ambient salinity receivers exposed to damaged emitters produced significantly more EFN after damage (470%) compared to receivers exposed to undamaged emitters (control: 0.32 ± 0.27 µl; damaged: 1.50 ± 0.26 µl), but when emitters were salinized this effect was no longer present (control: 0.41 ± 0.25 µl; damaged: 0.29 ± 0.26 µl) (Fig. 3A). On the other hand, for receiver damaged leaves (local induction) there were no significant main effects or interaction (Table 2B), though in the latter case a similar trend relative to undamaged leaves was observed (Fig. 3C). The effect of emitter damage on EFN volume was marginally significant (Table 2B), with receivers exposed to damaged emitters exhibiting a 172% greater mean value compared to receivers exposed to control emitters (control: 0.73 ± 0.21 µl; damage: 1.26 ± 0.22 µl).
Effects of emitter leaf damage under ambient vs. salinized soil on the volume (µl) and concentration (°Brix) of extra-floral nectar (EFN) produced by receiver plants after leaf damage (effect of priming on EFN induction) in wild cotton (Gossypium hirsutum. Panels A, B show results for undamaged leaves, C, D for damaged leaves. Values are model least-square means and standard errors accounting for plant genotype and main effects in the model. There were significant differences between control and damaged under ambient (volume: t = − 3.21, P = 0.003; concentration: t = − 2.09, P = 0.04) but not augmented salinity (volume: t = 0.34, P = 0.74; concentration: t = 0.84, P = 0.41). *P < 0.05, **P < 0.01
There were no significant main effects of emitter leaf damage (control: 4.95 ± 3.05°Brix; damaged: 8.98 ± 3.03°Brix) or salinization (control: 10.84 ± 3.12°Brix; salinized: 3.08 ± 2.97°Brix) on the concentration of EFN after damage for undamaged leaves (marginally significant in the latter case; Table 2B). However, mirroring patterns for EFN volume, there was a significant emitter damage by salinity interaction (Table 2B), whereby under ambient salinization receivers exposed to damaged emitters exhibited a significant (398%) increase in EFN concentration after damage relative to receivers exposed to intact emitters (control: 4.35 ± 4.51°Brix; damaged: 17.33 ± 4.28°Brix), but again the effect of emitter damage was lost under augmented salinization (control: 5.56 ± 4.08°Brix; damaged: 0.61 ± 4.28°Brix) (Fig. 3B). In the case of damaged leaves, analyses indicated a significant effect of emitter salinization on receiver EFN concentration in response to damage (Table 2B), with receivers exposed to salinized emitters showing a 68% lower mean value compared to receivers exposed to emitters with ambient salinity (control: 16.58 ± 3.37°Brix; salinized: 5.20 ± 3.46°Brix). The effects of emitter damage (marginally significant) and the interaction with salinity on EFN concentration of receiver plants exhibited similar trends to those observed for undamaged leaves but were not significant (Table 2B; Fig. 3D).
Discussion
Our findings indicated that the salinization treatment impaired the induction of two groups of VOCs in wild cotton (sesquiterpenes and aromatic compounds, 48 h after damage initiation), but that volatile composition did not differ between treatments. Exposure to VOCs from damaged emitters did not initially affect either the volume or concentration of EFN produced by unharmed receiver plants. However, after receiver plants were damaged, we found a significant interaction between the effects of emitter leaf damage and salinization. Receivers exposed to damaged emitters induced a greater amount and concentration of EFN after damage when emitters had ambient salinization but there was no such effect when the emitters had been submitted to salinity shock treatment, i.e., salinization impaired the plant–plant signaling effects on EFN. This result was strongest (and only significant) when analyzing undamaged leaves, suggesting that salinity effects on plant–plant signaling mainly impacted systemic EFN induction.
Although salinization did not affect volatile composition or total VOCs emissions in response to leaf damage, it did suppress the induction of specific groups of VOCs, namely sesquiterpenes and aromatic compounds (Table S2, S3). This result is in line with Quijano-Medina et al. (2021) who found that high salinity disrupted the induction of phenolics and shaped potential trade-offs between defensive traits. These results provide robust evidence that salinity directly affects the induction of defenses in wild cotton, and in the case of VOCs this can have important consequences for cotton-associated interactions. The compound groups affected by salinization have been previously reported to play an active role as indirect defenses, either in predator attraction or plant–plant signaling. For example, in maize the sesquiterpene caryophyllene is essential to attract root entomopathogenic nematodes (Rasmann et al. 2005) and the aromatic indole released by damaged plants primes neighboring plants to above-ground herbivore attack (Erb et al. 2015). It is therefore likely that these sesquiterpenes and aromatic compounds are involved in VOCs-mediated signaling between cotton plants. It is important to stress the need for further work assessing the biochemical and physiological mechanisms by which salinization affects VOCs emissions to better understand outcomes of plant-associated interactions mediated by these compounds.
We observed no significant increase in either the amount or concentration of EFN produced by intact receiver plants upon 48 h of exposure to damaged vs. undamaged emitters. In contrast, heightened resistance in intact cotton plants due to exposure to VOCs from damaged emitters has been reported previously and has been attributed to direct chemical defenses. For example, Bruin et al. (1992) reported for cultivated cotton that exposure to HIPVs increased resistance to mites (Tetranychus urticae) in undamaged plants. Likewise, a study by Zakir et al. (2013b) found that oviposition by S. littoralis females was lower on receiver cotton plants previously exposed to VOCs from damaged emitters (i.e., induced resistance). In addition, exposure to VOCs from damaged emitters increased the concentration of gossypol and heliocides in receiver plants of cultivated cotton, as well as the expression of defense-related genes (Grandi 2020). These findings, together with results for other plant species (e.g., Karban et al. 2000; Engelberth et al. 2004; Moreira et al. 2016), indicate that exposure to VOCs from damaged plants commonly drives increases in the expression of secondary metabolites involved in direct defense in receiver plants. However, there is still limited information about plant–plant signaling effects on indirect defenses, particularly EFN. A notable exception is work with lima bean (P. lunatus) showing that VOCs from damaged emitters increased EFN secretion as well as primed EFN for increased induction in response to damage in receiver plants (Heil and Silva-Bueno 2007). While our results suggest that there was no change in EFN upon exposure to emitter induced VOCs for the studied wild cotton genotypes, further work assessing changes in the expression of defensive genes linked to EFN is needed, as well as the use of more prolonged VOCs exposure times (see Moreira et al. 2021) and greater amounts of herbivory (including natural damage). Indeed, cotton plants may respond slower than other species, as has been shown for HIPV emissions (Loughrin et al. 1994), for instance compared to maize plants (Turlings et al. 1998).
Although there were no effects on intact plants of exposure to damaged emitters on receiver EFN, there was a marked effect on receiver EFN production in plants that were subsequently damaged. This implies that VOCs from damaged emitters primed receiver EFN responses to damage, resulting in a stronger induction after being damaged. This finding is consistent with findings for EFN production in lima bean (Kost and Heil 2006; Heil and Silva-Bueno 2007), as well for priming of secondary metabolites involved in direct and indirect defense in other species (e.g., Engelberth et al. 2004; Ton et al. 2006; Catola et al. 2018). Nonetheless, our study is the first to test for plant–plant signaling effects on the induction of EFN in cotton, G. hirsutum. Preliminary work with this species found no evidence of priming of induced responses involving secondary metabolites mediating direct defense (gossypol, heliocides) in receivers exposed to VOCs from damaged emitters (Grandi 2020). Further investigations that simultaneously measure these direct defenses and EFN are needed to assess similarities and differences in the modus operandi (e.g., direct increase due to VOCs vs. priming of induced responses) and mechanisms by which VOCs-mediated signaling influences direct and indirect defenses in wild cotton.
There are some studies which have evaluated the effects of soil salinity on the expression direct defenses in plants (e.g., Forieri et al. 2016; Han et al. 2016), but fewer have looked at its effects on indirect defenses such as induced VOCs and EFN (e.g., Teuber et al. 2008; Quijano-Medina et al. 2021). Previous work with wild cotton indicates that soil salinization has no significant effect on EFN volume or concentration (Quijano-Medina et al. 2021). In comparison, here we found a significant reduction in EFN volume produced by undamaged leaves of receivers exposed to salinized emitters, suggesting that salinization effects via changes in (constitutive) VOCs emissions are more important than its direct effects on EFN induction, a possibility that merits further investigation. More importantly, to our knowledge, the present study is the first to test for and find soil salinity effects on plant–plant signaling via VOCs. Indeed, salinization weakened or disrupted plant–plant signaling, whereby effects of exposure to VOCs from damaged emitters on both EFN volume and concentration were observed under ambient (control) soil salinity but not under augmented salinity. This could be explained by the reduced induction of sesquiterpenes and aromatic compounds in emitter plants under salinization, the only volatile compounds that were affected by salinization, which suggests they play an important role in cotton plant–plant signaling. This finding is consistent with previous study on wild cotton, which reported a trend for a reduction of induced VOCs in response to leaf damage when plants had been exposed to augmented salinization (Quijano-Medina et al. 2021; see Forieri et al. 2016 for maize example). Interestingly, in the present study the salinization effect on EFN induction was strongest (and only significant) for undamaged leaves of damaged plants (though damaged leaves showed the same trend). This could be explained by the fact that EFN is more strongly induced locally (damaged leaf) than systemically (Abdala-Roberts et al. 2019b).
Overall, our results call for more research aimed at understanding the physiological mechanisms by which abiotic factors affect plant–plant VOCs signaling, as well as the ecological consequences for multi-trophic interactions. In the case of wild cotton, we envision work addressing the effects of soil salinity on plant–plant signaling via VOCs and its effects on ant-plant–herbivore interactions. The results also have implications for pest control strategies in cultivated cotton. It has been proposed that volatile-mediated induced resistance to pest insects can be a sustainable alternative to pesticide use (Renou et al. 2011; Llandres et al. 2018). The presented work reveals that such an approach may be hampered by unfavorable soil conditions, but also implies that proper soil management can help to optimize this mechanism for cotton induced resistance. Specifically, practices that keep soil salinity in check or mitigate increases could potentially strengthen plant–plant signaling effects on direct chemical defenses (Llandres et al. 2023) as well as EFN via predator recruitment such as in the case of ants (Llandres et al. 2019). This idea requires testing under field conditions.
Future work
Results from the present study call for further experimental work measuring effects on both direct and indirect defenses in situ to obtain a complete understanding of abiotic context-dependency of cotton signaling and its consequences for multi-trophic interactions. In addition, follow-up studies are needed to identify key VOCs (e.g., specific sesquiterpenes or aromatic compounds) that mediate signaling between cotton plants in combination with defense gene expression measurements to achieve a mechanistic understanding of these effects. Finally, testing for effects of salinity on receivers (jointly with emitters) would be a logical next step to achieve a more complete understanding of how this abiotic factor modulates plant–plant signaling, particularly given microhabitat-level variation in levels of salinity stress between neighboring plants or plant patches in wild cotton populations.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon request.
Code availability
We have used standardized modeling so no code us provided.
References
Abdala-Roberts L, Quijano-Medina T, Moreira X, Vázquez-González C, Parra-Tabla V, Teran MY, B, Turlings TCJ, Benrey B, (2019a) Bottom-up control of geographic variation in insect herbivory on wild cotton (Gossypium hirsutum) by plant defenses and climate. Am J Bot 106:1059–1067. https://doi.org/10.1002/ajb2.1330
Abdala-Roberts L, Pérez-Niño B, Moreira X, Parra-Tabla V, Grandi L, Glauser G, Benrey B, Turlings TCJ (2019b) Effects of early-season insect herbivory on subsequent pathogen infection and ant abundance on wild cotton (Gossypium hirsutum). J Ecol 107:1518–1529. https://doi.org/10.1111/1365-2745.13131
Abdala-Roberts L, Reyes-Hernández M, Quijano-Medina T, Moreira X, Francisco D, Angulo M, Parra-Tabla V, Virgen A, Rojas J (2019c) Effects of amount and recurrence of leaf herbivory on the induction of direct and indirect defences in wild cotton. Plant Biol 21:1063–1071. https://doi.org/10.1111/plb.13022
Agrawal AA, Karban R (2000) Specificity of constitutive and induced resistance: pigment glands influence mites and caterpillars on cotton plants. Entomol Exp Appl 96:39–49. https://doi.org/10.1046/j.1570-7458.2000.00677.x
Arce CM, Besomi G, Glauser G, Turlings TCJ (2021) Caterpillar-induced volatile emissions in cotton: the importance of damage and insect-derived factors. Front Plant Sci 12:709858. https://doi.org/10.3389/fpls.2021.709858
Baldwin IT, Preston CA (1999) The eco-physiological complexity of plant responses to insect herbivores. Planta 208:137–145. https://doi.org/10.1007/s004250050543
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–8
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377. https://doi.org/10.1111/j.1461-0248.2011.01736.x
Bentley BL (1977) Extrafloral nectaries and protection by pugnacious bodyguards. Annu Rev Ecol Syst 8:407–427. https://doi.org/10.1146/annurev.es.08.110177.002203
Blande JD (2021) Effects of air pollution on plant-insect interactions mediated by olfactory and visual cues. Curr Opin Environ Sci Health 19:100228. https://doi.org/10.1016/j.coesh.2020.100228
Bouwmeester H, Schuurink RC, Bleeker PM, Schiestl F (2019) The role of volatiles in plant communication. Plant J 100:892–907. https://doi.org/10.1111/tpj.14496
Bruin J, Dicke M, Sabelis MW (1992) Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics. Experientia 48:525–529. https://doi.org/10.1007/BF01928181
Caparrotta S, Boni S, Taiti C, Palm E, Mancuso S, Pandol C (2018) Induction of priming by salt stress in neighboring plants. Environ Exp Bot 147:261–270. https://doi.org/10.1016/j.envexpbot.2017.12.017
Catola S, Centritto M, Cascone P, Ranieri A, Loreto F, Calamai L, Balestrini R, Guerrieri E (2018) Effects of single or combined water deficit and aphid attack on tomato volatile organic compound (VOC) emission and plant-plant communication. Environ Exp Bot 153:54–62. https://doi.org/10.1016/j.envexpbot.2018.05.001
Chappuis L, Egger A (2016) Direct and indirect chemical defenses in cotton plants. M. Sc. Thesis. University of Neuchatel.
D’Eeckenbrugge G, Lacape J (2014) Distribution and differentiation of wild, feral, and cultivated populations of perennial upland cotton (Gossypium hirsutum L.) in Mesoamerica and the Caribbean. PLoS ONE 9:e107458. https://doi.org/10.1371/journal.pone.0107458
Engelberth J, Alborn H, Schmelz E, Tumlinson J (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A 101:1781–1785. https://doi.org/10.1073/pnas.0308037100
Erb M, Veyrat N, Robert CAM, Xu H, Frey M, Ton J, Turlings TCJ (2015) Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun 6:6273. https://doi.org/10.1038/ncomms7273
Forieri I, Hildebrandt U, Rostás M (2016) Salinity stress effects on direct and indirect defence metabolites in maize. Environ Exp Bot 122:68–77. https://doi.org/10.1016/j.envexpbot.2015.09.007
Girón-Calva PS, Li T, Blande JD (2016) Plant-plant interactions affect the susceptibility of plants to oviposition by pests but are disrupted by ozone pollution. Agr Ecosyst Environ 233:352–360. https://doi.org/10.1016/j.agee.2016.09.028
Gouinguené S, Turlings TCJ (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol 129:1296–1307. https://doi.org/10.1104/pp.001941
Grandi L (2020) Interaction between cotton plants mediated by volatile organic compounds: prospects for pest control? PhD thesis, University of Neuchâtel
Hagenbucher S, Olson D, Ruberson J, Wäckers F, Romeis J (2013) Resistance mechanisms against arthropod herbivores in cotton and their interactions with natural enemies. Crit Rev Plant Sci 32:458–482. https://doi.org/10.1080/07352689.2013.809293
Han P, Wang Z, Lavoir A, Michel T, Seassau A, Zheng W, Niu C-Y, Desneux N (2016) Increased water salinity applied to tomato plants accelerates the development of the leaf miner Tuta absoluta through bottom-up effects. Sci Rep 6:32403. https://doi.org/10.1038/srep32403
Heil M (2015) Extrafloral nectar at the plant-insect interface: a spotlight on chemical ecology, phenotypic plasticity, and food webs. Annu Rev Entomol 60:213–232. https://doi.org/10.1146/annurev-ento-010814-020753
Heil M, Kost C (2006) Priming of indirect defences. Ecol Lett 9:813–817. https://doi.org/10.1111/j.1461-0248.2006.00932.x
Heil M, McKey D (2003) Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Evol Syst 34:425–553. https://doi.org/10.1146/annurev.ecolsys.34.011802.132410
Heil M, Silva-Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci U S A 104:5467–5472. https://doi.org/10.1073/pnas.0610266104
Holopainen JK, Gershenzon J (2010) Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 15:176–184. https://doi.org/10.1016/j.tplants.2010.01.006
Karban R (2015) Plant Sensing and Communication. University of Chicago Press, Chicago, IL. https://doi.org/10.7208/9780226264844
Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125:66–71. https://doi.org/10.1007/PL00008892
Karban R, Yang LH, Edwards KF (2014) Volatile communication between plants that affects herbivory: a meta-analysis. Ecol Lett 17:44–52. https://doi.org/10.1111/ele.12205
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144. https://doi.org/10.1126/science.291.5511.2141
Kost C, Heil M (2006) Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94:619–628. https://doi.org/10.1111/j.1365-2745.2006.01120.x
Landi M, Araniti F, Flamini G, Piccolo EL, Trivellini A, Abenavoli MR, Guidi L (2020) “Help is in the air”: volatiles from salt-stressed plants increase the reproductive success of receivers under salinity. Planta 251:48. https://doi.org/10.1007/s00425-020-03344-y
Llandres AL, Almohamad R, Brévault T, Renou A, Téréta I, Jean J, Goebel F-R (2018) Plant training for induced defense against insect pests: a promising tool for integrated pest management in cotton: cotton training for induced defense against pests. Pest Manag Sci 74:2004–2012. https://doi.org/10.1002/ps.5039
Llandres AL, Verdeny-Vilalta O, Jean J, Goebel F-R, Seydi O, Brévault T (2019) Cotton extrafloral nectaries as indirect defence against insect pests. Basic Appl Ecol 37:24–34. https://doi.org/10.1016/j.baae.2019.05.001
Llandres AL, Verdeny-Vilalta O, Brévault T, Goebel F-R, Jean J (2023) Cotton topping reduces the performance of aphids on topped and neighbor plants under greenhouse conditions. Arthropod-Plant Interact 17:173–184
Loughrin J, Manukian A, Heath R, Turlings TCJ, Tumlinson J (1994) Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci U S A 91:11836–11840. https://doi.org/10.1073/pnas.91.25.11836
Mansour MH, Zohdy NM, ElGengaihi SE, Amr AE (1997) The relationship between tannins concentration in some cotton varieties and susceptibility to piercing sucking insects. J Appl Entomol 121:321–325. https://doi.org/10.1111/j.1439-0418.1997.tb01413.x
McCall PJ, Turlings TCJ, Loughrin J, Proveaux AT, Tumlinson JH (1994) Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L.) seedlings. J Chem Ecol 20:3039–3050. https://doi.org/10.1007/BF02033709
Moreira X, Abdala-Roberts L (2019) Specificity and context-dependency of plant-plant communication in response to insect herbivory. Curr Opin Insect Sci 32:15–21. https://doi.org/10.1016/j.cois.2018.09.003
Moreira X, Nell CS, Katsanis A, Rasmann S, Mooney KA (2016) Herbivore specificity and the chemical basis of plant-plant communication in Baccharis salicifolia (Asteraceae). New Phytol 220:703–713. https://doi.org/10.1111/nph.14164
Moreira X, Abdala-Roberts L, Granjel RR, Pasch V, Soengas P, Vázquez-González C, Turlings TCJ, Rasmann S (2021) Test of communication between potato plants in response to infection by a pathogenic fungus and its chemical and molecular correlates. Plant Cell Environ 44:1192–1201. https://doi.org/10.1111/afe.12484
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
Ninkovic V, Rensing M, Dahlin I, Markovic D (2019) Who is my neighbor? volatile cues in plant interactions. Plant Signal Behav 14:1634993. https://doi.org/10.1080/15592324.2019.1634993
Nix A, Paull C, Colgrave M (2017) Flavonoid profile of the cotton plant, Gossypium hirsutum: a review. Plants 6:1–14. https://doi.org/10.3390/plants6040043
Opitz S, Kunert G, Gershenzon J (2008) Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J Chem Ecol 34:508–522. https://doi.org/10.1007/s10886-008-9453-z
Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:1161–1167. https://doi.org/10.1104/pp.114.4.1161
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2014) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Pezzola E, Mancuso S, Karban R (2017) Precipitation affects plant communication and defense. Ecology 98:1693–1699. https://doi.org/10.1002/ecy.1846
Quijano-Medina T, Turlings TCJ, Sosenski P, Grandi L, Cervera JC, Moreira X, Abdala-Roberts L (2021) Effects of soil salinity on the expression of direct and indirect defences in wild cotton Gossypium hirsutum. J Ecol 109:354–368. https://doi.org/10.1111/1365-2745.13483
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737. https://doi.org/10.1038/nature03451
Renou A, Téréta I, Togola M (2011) Manual topping decreases bollworm infestations in cotton cultivation in Mali. Crop Prot 30:1370–1375. https://doi.org/10.1016/j.cropro.2011.05.020
Reyes-Hernández M, Quijano-Medina T, Angulo-Pérez D, Moreira X, Parra-Tabla V, Abdala-Roberts L (2022) Experimental test of ant effects on insect herbivory and pathogen infection on wild cotton (Gossypium hirsutum). Arthropod-Plant Interact 16:77–86. https://doi.org/10.1007/s11829-021-09876-8
Rico-Gray V, Oliveira PS (2007) The ecology and evolution of ant-plant interactions. University of Chicago Press, Chicago
Rosenzweig C, Elliott J, Deryng D, Ruane AC, Müller C, Arneth A, Boote KJ, Folberth C, Gloter M, Khabarov N, Neumann K, Piontek F, Pugh TAM, Schmid E, Stehfest E, Yang H, Jones JW (2014) Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc Natl Acad Sci U S A 111:3268–3273. https://doi.org/10.1073/pnas.1222463110
Rudgers JA (2004) Enemies of herbivores can shape plant traits: selection in a facultative ant–plant mutualism. Ecology 85:192–205. https://doi.org/10.1890/02-0625
SAS (2015) SAS Institute Inc., ver. 9.4. Cary, NC
Sweet WV, Park J (2014) From the extreme to the mean: acceleration and tipping points of coastal inundation from sea level rise. Earth’s Future 2:579–600. https://doi.org/10.1002/2014EF000272
Teuber M, Zimmer I, Kreuzwieser J, Ache P, Polle A, Rennenberg H, Schnitzler JP (2008) VOC emissions of grey poplar leaves as affected by salt stress and different N sources. Plant Biol 10:86–96. https://doi.org/10.1111/j.1438-8677.2007.00015.x
Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2006) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49:16–26. https://doi.org/10.1111/j.1365-313X.2006.02935.x
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452. https://doi.org/10.1146/annurev-ento-020117-043507
Turlings TCJ, Wäckers F (2004) Recruitment of predators and parasitoids by herbivore-injured plants. In: Cardés RT, Millar JG (eds) Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge, UK
Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH (1993) An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J Chem Ecol 19:411–425. https://doi.org/10.1007/s004250050466
Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D (1998) Timing of induced volatile emissions in maize seedlings. Planta 207:146–152
Wäckers FL, Bonifay C (2004) How to be sweet? extrafloral nectar allocation by Gossypium hirsutum fits optimal defense theory predictions. Ecology 85:1512–1518. https://doi.org/10.1890/03-0422
Wendel J, Grover C (2015) Taxonomy and Evolution of the Cotton Genus. Agronomy Monograph, Gossypium. https://doi.org/10.2134/agronmonogr57.2013.002
Zakir A, Bengtsson M, Sadek MM, Hansson BS, Witzgall P, Anderson P (2013a) Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J Exp Biol 216:3257–3263. https://doi.org/10.1242/jeb.083188
Zakir A, Sadek MM, Bengtsson M, Hansson BS, Witzgall P, Anderson P (2013b) Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore. J Ecol 101:410–417. https://doi.org/10.1111/1365-2745.12041
Acknowledgements
We thank A. Virgen for providing S. frugiperda larvae, and P. Sosenski for the use of pumps for VOCs collections. This study was supported by a project awarded to TCJT by the Swiss National Science Foundation (315230_185319). The authors also thank the Universidad Autónoma de Yucatán for providing greenhouse infrastructure and logistic support. The authors declare no conflict of interest.
Funding
This research was supported by the Swiss National Science Foundation (315230_185319).
Author information
Authors and Affiliations
Contributions
TT. obtained the funding. YBM. and LAR. conceived the study and designed the experiment. YBM, TQM., and BPN. performed the experiment. CBS. performed the chemical analyses. LAR, CBS, and YBM. performed the statistical analyses and LAR. and YBM. wrote the first version of the manuscript. CBS, TT, BPN, and BB. contributed to the revision of the manuscript and the interpretation of the results.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Evan H DeLucia.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1:
(DOCX 25 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Briones-May, Y., Quijano-Medina, T., Pérez-Niño, B. et al. Soil salinization disrupts plant–plant signaling effects on extra-floral nectar induction in wild cotton. Oecologia 202, 313–323 (2023). https://doi.org/10.1007/s00442-023-05395-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-023-05395-w