Abstract
Plants respond to complex blends of above- and below-ground volatile organic compounds (VOCs) emitted by neighboring plants. These responses often involve priming (i.e., preparation) or induction (i.e., increase) of defenses by “receiver” plants upon exposure to VOCs released by herbivore-damaged neighboring “emitters.” However, recent work has shown that induction of VOC emissions by herbivory is modulated by abiotic factors, potentially affecting plant–plant signaling. We tested the effect of soil salinization on the induction of VOC emissions in wild cotton (Gossypium hirsutum) due to leaf damage and its consequences for the induction of defenses in neighboring plants. To this end, we performed a greenhouse factorial experiment where emitter plants were subjected to augmented soil salinity (vs. ambient salinity) and within each group emitter plants were subsequently exposed to simulated caterpillar damage (mechanical leaf damage treated with Spodoptera frugiperda oral secretion) or no damage (control). After 48 h of exposure, we collected VOCs released by emitter plants and then damaged the receivers and collected their leaves to measure levels of chemical defenses (terpenoid aldehydes of known insecticidal effects). We found an interaction between leaf damage and salinization for two groups of VOCs released by emitters (sesquiterpenes and other aromatic compounds), whereby damaged receivers had higher emissions than control plants under ambient but not salinized soil conditions. We also found that, upon being damaged, receiver plants exposed to damaged emitters exhibited a significantly higher concentration of heliocides (but not gossypol) than control plants. However, salinization did not alter this VOC exposure effect on receiver induced responses to damage. Overall, we show that exposure to induced VOC emissions from damaged plants magnifies the induction of chemical defenses due to leaf damage in neighboring individuals and that this is not contingent on the level of soil salinity despite the latter's effect on VOC induction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) play a key role in plant defense against herbivory. Numerous studies have shown that intact plants (“receivers”) increase defense-related traits and in turn herbivore resistance when exposed to VOCs from damaged neighbors (“emitters”), a form of plant-plant signaling frequently termed “plant communication” (Baldwin and Schultz 1983; Heil and Karban 2010; Moreira and Abdala-Roberts 2019; Ninkovic et al. 2020). These responses often involve priming (i.e., preparation) or induction (i.e., increase) of defenses by “receiver” plants upon exposure to VOCs released by herbivore-damaged neighboring plants. This plant–plant VOC-mediated signaling is now well recognized and has been reported in more than 40 plant species distributed in over 15 families, including both wild and cultivated species (Karban et al. 2014; Pickett and Khan 2016; Stenberg et al. 2015; Turlings and Erb 2018).
Studies have shown specificity of plant VOC-mediated signaling contingent on biotic factors, such as the identity of the attacker (Moreira et al. 2018) and the plant species or genotype (Karban et al. 2006). Likewise, abiotic factors also modulate VOC emissions (Becker et al. 2015; Holopainen and Gershenzon 2010) and, given ubiquitous variation in abiotic conditions, presumably play a pervasive role in shaping the outcome of VOCs plant–plant signaling. For example, a study with tomato (Solanum lycopersicum) by Catola et al. (2018) found that drought stress in emitter plants increased VOC emissions and parasitoid attraction to receiver plants, whereas Pezzola et al. (2017) found that water stress in receiver plants influenced induced responses to emitter VOCs in sagebrush (Artemisia tridentata). In contrast, Vázquez-González et al. (2022) found that low water availability reduced volatile emissions, but this did not influence induced resistance to herbivory in receiver potato plants (Solanum tuberosum), suggesting buffering mechanisms whereby signaling effects remain consistent despite VOC changes. Additionally, other studies have found that pollutants such as ozone can disrupt VOC-mediated plant signaling, in some cases presumably by degrading volatiles (e.g., Girón-Calva et al. 2016; reviewed by Blande 2021). Collectively, these investigations highlight the importance of studying abiotic influences on VOC-mediated plant signaling, but research on this topic is still limited and several sources of abiotic variation, besides water availability and pollutants, remain to be studied.
Soil salinity is a good example of an overlooked and yet key abiotic factor shaping plant induced responses and VOC-mediated signaling (Parihar et al. 2014; Forieri et al. 2016; Landi et al. 2020). Several studies have found qualitative and quantitative effects of soil salinity on VOC emissions, including herbivore-induced plant VOC emissions in wild and cultivated plant species (e.g., Teuber et al. 2008; Forieri et al. 2016; Quijano-Medina et al. 2021). At least one investigation has shown that salinization affects plant–plant signaling but this did not involve herbivory (i.e., effects via constitutive emissions; Caparrotta et al. 2018). These findings are highly relevant given that the impacts of soil salinity are likely to become increasingly important, particularly in coastal habitats where climate change is expected to lead to more severe and frequent sea flooding events (Sweet and Park 2014). Currently, however, the effects of soil salinization on plant–plant signaling mediated by herbivore-induced VOC emissions are poorly understood.
Here we studied the effect of soil salinization on airborne VOC-mediated signaling between wild cotton (Gossypium hirsutum) plants. This species naturally grows in the coastal shrubland of the Yucatan Peninsula (Mexico) where it is exposed from moderate to high levels of soil salinity which hamper its ability to defend itself against insect herbivores. Specifically, this plant possesses effective direct (terpenoid aldehydes and phenolic compounds) and indirect (VOCs, extra-floral nectar [EFN hereafter]) defensive traits against insect herbivores (reviewed by Hagenbucher et al. 2013), with recent work showing that increases in soil salinity weaken the induction of cotton secondary metabolites – including VOCs – in response to leaf damage (Quijano-Medina et al. 2021) and also hamper plant–plant signaling effects on EFN induction (Briones-May et al. 2023). We expand on this work by testing for salinity-driven changes in VOC signaling affecting the induction of terpenoid aldehydes in wild cotton (gossypol and heliocides), which are known to have insecticidal effects (Mansour et al. 1997; Agrawal and Karban 2000; Opitz et al. 2008; Nix et al. 2017). The data used in this study come from the same (dual-purpose) experiment used to test for salinization effects on VOC-mediated effect on EFN induction in response to leaf damage (Briones-May et al. 2023). Specifically, we asked: 1) Do receiver plants exposed to VOCs of damaged emitters exhibit greater induction of chemical defenses compared to receivers exposed to undamaged emitters (i.e., a signaling effect)? 2) Does soil salinization affect any such VOC-mediated effects on the induction of receiver defenses (i.e., signaling by salinization interaction)? To this end, we performed a greenhouse factorial experiment where emitter cotton plants were subjected to augmented soil salinity (vs. ambient salinity) and then within each group plants were subjected to either mechanical damage with application of oral secretion from the generalist caterpillar Spodoptera frugiperda or no damage (control). We expected that receiver plants exposed to the damaged emitters would exhibit a greater induction of defenses in response to damage (i.e., a priming effect of exposure to emitter induced VOC emissions) but that this effect would be weaker under soil salinization because salinization hampers the induction of VOC emissions (Quijano-Medina et al. 2021; Briones-May et al. 2023). Overall, this study contributes to a better understanding of how abiotic factors affect plant–plant signaling and its consequences for plant defense induction.
Materials and methods
Study species
Wild cotton, G. hirsutum (Malvaceae), is a perennial shrub that is distributed in Central America, Mexico, and the Caribbean Basin (Wendel et al. 1992; D’Eeckenbrugge and Lacape 2014). Its likely center of origin and domestication is the coast of the Yucatan Peninsula in Mexico, where wild populations are abundant (Yuan et al. 2021). Within this region, wild cotton populations are found in the coastal scrubland of northern Yucatan (Abdala-Roberts et al. 2019a), where plants are exposed from moderate to high levels of soil salinity, namely 0.05 to 3.53‰ salinity across locations (N = 6 sites; mean = 0.8 ± 0.17‰; Quijano-Medina et al. 2021). Salinization effects will likely strengthen with predicted sea level rises in coastal areas such as the northern Yucatan Peninsula where this species is found.
Studies on wild cotton in coastal Yucatan have shown that it is attacked by several species of native insect herbivores, among which leaf chewers are the most common (mainly Orthoptera and Lepidoptera), with leaf damage averaging 23 ± 2.08% (mean ± SE) leaf area consumed per plant at the end of the growing season (range: 9%-52%; Abdala-Roberts et al. 2019a, b). This species produces leaf traits that play a defensive role against herbivores (Loughrin et al. 1994; McCall et al. 1994; McAuslane et al. 1997; Agrawal et al. 2000; Opitz et al. 2008), including phenolic compounds and terpenoid aldehydes which provide resistance against insect herbivores (e.g., piercing sucking insects and caterpillars), both of which are highly inducible (Mansour et al. 1997; Agrawal and Karban 2000; Opitz et al. 2008; Nix et al. 2017). In addition, herbivory or artificial leaf damage induce VOC emissions (Loughrin et al. 1994; McCall et al. 1994; Arce et al. 2021; Quijano-Medina et al. 2021) and extra-floral nectar (Wäckers and Bonifay 2004; Abdala‐Roberts et al. 2019c), traits shown to attract natural enemies such as parasitoids and ants (e.g., Reyes-Hernández et al. 2022). Finally, work mainly with cultivated cotton has shown that exposure to VOCs from damaged plants increases resistance against herbivory in undamaged receiver plants (Bruin et al. 1992; Zakir et al. 2013a, b) and a recent study with wild cotton found that soil salinization hampers plant–plant signaling effects on EFN induction (Briones-May et al. 2023).
Plant material
In January and July of 2019 and 2020, we collected seeds from four wild cotton populations located along the northern coast of Yucatan, two near the town of Chicxulub (21° 17′ 46.0752"N, -89° 34′ 45.4832"W and 21° 18′ 14.2697"N, -89° 32′ 29.9137") and two near the town of Sisal (21° 18′ 14.2697"N, -89° 32′ 29.9137"W and 21° 11′ 38.6700"N, -89° 57′ 28.1088"W). Across sites, seeds from a total of seven mother plants were used (hereafter genotypes). Prior to germination, we stored seeds in paper bags in the laboratory. In early April 2021, we exposed seeds to coat scarification and germinated them with wet cotton wool in Petri dishes at 35 °C. We then individually sowed two-week seedlings in 25 × 30 cm low-density polyethylene nursery bags containing calcareous sandy soil collected from a coastal site in Yucatan where wild cotton was found naturally growing, mixed with perlite and soil from local secondary forests (2:1:1). Plants were kept for two months in a greenhouse at the Campus de Ciencias Biológicas y Agropecuarias of the University of Yucatan (Yucatan, Mexico; 20° 52′ 03.4″ N 89° 37′ 16.1″ W) prior to starting the experiment, and watered with 300 ml of tap water three times per week. Plants had 10–12 fully expanded leaves at the start of the experiment (BBCH stage 3, i.e., stem elongation and shoot development; Zadoks et al. 1974). Greenhouse conditions were 70% mean relative humidity and 22 °C/35 °C minimum/maximum mean temperature. Plants were exposed to 60% natural sunlight inside the greenhouse (13-h light, 11-h dark).
Experimental design and treatments
The experiment included 44 emitter plants and 88 receiver plants allocated in triplets to mesh cages (cylindrical shape: 60 cm diameter × 80 cm high; see Briones-May et al. 2023 for more details), each containing one emitter and two receiver plants separated by 20 cm. All plants in a cage were of the same genotype and genotypes were similarly represented across treatments. In late May 2021, we started the experiment by manipulating emitter soil salinity by randomly assigning each emitter plant to either irrigation with tap water (control or ambient salinity) or with salinized tap water (augmented salinity). In the latter case, we placed the potted plant in a plastic container with 2 L of water at 1% salinity (by adding NaCl) for 24 h to achieve saturation. The day emitter induction was initiated (see ahead), soil salinity was significantly (3x) greater (t = − 6.91, P = 0.0002; N = 10) for salinized emitters (0.468 ± 0.039‰ or 0.008 ± 0.0006 mol L−1) compared to emitters with ambient salinity (0.154 ± 0.026‰ or 0.0026 ± 0.0004 mol L−1). This treatment was aimed to mimic a saline shock due to a flooding event caused by sea water or coastal lagoon surges, which are common events during winter months (Quijano-Medina et al. 2021). Soil salinity was estimated by direct measurements of water potential (see Quijano-Medina et al. 2021). Both levels of salinity were within the natural range of soil salinity observed in situ (see above) and in the case of salinized plants was close to the mean value observed across wild cotton populations (Quijano-Medina et al. 2021). Emitter plants assigned to ambient salinity were subjected to the same procedure but with non-salinized water. We opted for a test centered on salinization effects on emitters as prior work showed that salinization influences wild cotton-induced VOC emissions (Quijano-Medina et al. 2021), based on which we hypothesized that such an effect would have downstream consequences for plant–plant signaling.
Three days after applying the salinization treatment, emitter plants of each salinity level were randomly subjected to one of two leaf damage (i.e., induction) treatments: undamaged control or artificial leaf damage. Damage consisted in removing 50% of leaf area of half of the leaves per plant by cutting off the leaf lobes with scissors, as well as puncturing the remaining leaf tissue with a micro-needle bearing 32 points (Dermapen®, Sydney, Australia) and exposing this punctured area (ca. 1 cm2) to oral secretions of third instar Spodoptera frugiperda larvae (Turlings et al. 1993; Abdala-Roberts et al. 2019c; Quijano-Medina et al. 2021). Larvae were sourced from a colony reared at the Chemical Ecology Lab in ECOSUR (Chiapas, Mexico) and fed with a wheat germ-based artificial diet. Damage was applied over two consecutive days, each day removing half of the intended total amount of damage (i.e., 25% of total leaf removed per day, totaling in 50%) to mimic a gradual progression of leaf consumption due to real herbivory. We obtained the caterpillar oral secretions by gently applying pressure to the abdomen of each larva until it regurgitated (Turlings et al. 1993). As for other Spodoptera species (see Arce et al. 2021), S. frugiperda is known to attack cultivated cotton and has been used previously to induce both direct and indirect defenses (VOCs, EFN) in wild G. hirsutum (Quijano-Medina et al. 2021). Although applying mechanical damage and insect regurgitant is less realistic compared to natural herbivory, previous studies have demonstrated that this treatment provides an effective proxy of natural damage in cotton (Chappuis et al. 2023).
Receiver plants were exposed to emitters for 48 h starting from the first day of leaf damage. At the end of this period, we removed emitters and collected their VOCs (N = 44). This timing of VOCs collection and exposure time for receivers were chosen based on previous work showing that VOC emissions are highly induced after two days of damage, including compounds that have known or suspected roles in signaling (Loughrin et al. 1994; Arce et al. 2021). Hence, our sampling design ensured that we collected these blends of induced VOC emissions and that receiver plants were exposed to them. Results from analyses of VOC data can be found in Briones-May et al. (2023) and a subset of the results published therein are included here as supplementary material. After VOCs had been collected from emitter plants, they were removed from the cages. Likewise, one receiver plant was also removed, from which one apical fully expanded leaf was harvested to measure initial effects of exposure to emitter VOCs (i.e., prior to receiver induction). Immediately after, also on the same day, we damaged the remaining receiver plants (kept inside cages) to test whether exposure to VOCs from damaged emitters primed the induction of chemical defenses. For this, we damaged two fully expanded leaves located in the upper portion of the plant (same procedure as for emitters), removing ca. 50% of the leaf area of half of the leaves of each plant, and the following day collected two damaged and two undamaged leaves per plant to differentiate treatment effects on local vs. systemic defense induction (see Briones-May et al. 2023). Whereas previous work has shown that cotton-induced defenses take several days to build up (see Bezemer et al. 2004), by sampling leaves 24 h after damage we aimed to test for early effects on induced responses as VOC priming was expected to lead to faster induction after damage. We always damaged leaves 2 and 4 (counting from the apical meristem downward), whereas sampled undamaged leaves were in positions 1 and 3, thus alternating leaf position/age between leaf types to average out effects of leaf ontogeny (i.e, to avoid confounding this factor with damage).
Chemical analyses
We collected aboveground VOCs released by emitter plants following Turlings et al. (1998). Briefly, we bagged plants within a nalophan bag (Reynolds, Inc.) and adsorbed VOCs onto HayeSep-Q adsorbent filters (Sigma, Switzerland). One of the filter ends was inserted into the bag and the other end was connected to a micro air sampler (Supelco PAS-500) at a flow rate of 500 ml min−1. Filters were eluted with 150-μl dichloromethane and spiked with 10-μl internal standard (nonyl acetate 20 μg μl−1). Vials were sealed with polytetrafluoroethylene (PTFE) caps and Teflon, stored at − 30 °C and sent to University of Neuchâtel (Neuchâtel Switzerland) for GC–MS analysis with a gas chromatograph (Agilent7890B) coupled with a mass spectrometer detector (Agilent 5977B). Further details on the VOC collection methodology and quantification can be found in Briones-May et al. (2023).
To quantify receiver leaf chemical defenses (terpenoid aldehydes), harvested leaves were immediately frozen on dry ice and subsequently stored at -80 °C. Quantification of terpenoid aldehydes was conducted at the Fundamental and Applied Research in Chemical Ecology (FARCE Lab) and the Neuchâtel Platform of Analytical Chemistry (NPAC) at the University of Neuchâtel (Switzerland), in November 2022. Briefly, frozen leaves were ground under liquid nitrogen, and 50 mg of frozen leaf powder was extracted with 200 μL of a solution of acetonitrile, MilliQ water, and formic acid (80: 18.5:1.5). Samples were homogenized with three to five glass beads (1.25–1.65 mm diameter) in a mixer mill for 3 min at 30 Hz (TissueLyser II, Qiagen, Germany) and ultrasonicated for 5 min. They were then centrifuged for 3 min at 8000×g. The recovered supernatant was centrifuged a second time before being transferred to amber glass vials. Samples were directly analyzed using ultra-performance liquid chromatography with diode array detection (UPLC-DAD, Ultimate 3000 Dionex, Thermo Fisher Scientific, MA, United States). DAD detector was set at 288 ± 2 nm. 10-μL sample was injected onto an ACQUITY UPLC® BEH C18 column (2.1 × 100 mm, 1.7 μm; Waters, MA, United States). Flow rate was held constant at 0.45 mL min−1 and the temperature was kept at 40 °C. The mobile phase solvent A consisted of 0.05% formic acid in MilliQ water (18 Ω) and the mobile phase solvent B of 0.05% formic acid in acetonitrile (HiPerSolv, VWR Chemicals®, France). Solvent B was increased from 45 to 90% in 8 min, then to 100% in 0.5 min, and held at 100% for 2.5 min, which was followed by re-equilibration at 45% solvent B for 3.5 min. Gossypol and heliocides (grouped together) were identified by their retention time. Quantification was based on linear regression from six calibration points (5–250 μg mL−1) in gossypol equivalents. Concentrations were expressed in μg g−1 tissue on a fresh weight basis.
Statistical analyses
We ran general or generalized (depending on the response, see below) linear mixed models testing for effects of emitter leaf damage, emitter soil salinization, and their interaction (all fixed factors) on the concentrations of gossypol and heliocides of receiver plants. We ran models separately for initial effects of signaling on defenses, as well as post-damage effects to test for effects of exposure to induced VOC emissions on defense induction for damaged and undamaged leaves. All models included plant genotype (treated as random) to account for genetic variation and/or maternal effects. Residuals were normally distributed in most cases except for some groups of VOCs which were run with generalized linear mixed models using a gamma distribution. Analyses based on a normal distribution were run in PROC MIXED in SAS ver. 9.4 (SAS 2015), whereas gamma models were run with ‘glmer’ function from lme4 package (Bates et al. 2015) in R version 4.1 (R Core Team 2021).
Results
Emitter VOCs
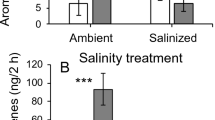
We detected a total of 20 VOCs released by emitter plants belonging to five groups: green leaf volatiles, monoterpenes, homoterpenes, sesquiterpenes, and aromatic compounds. Separate analyses for each type of compounds showed significant effects of emitter damage and salinity in several instances (Table S1 in the Supplementary Material), with damaged plants showing 72 to 89% increases (depending on the compound group) relative to undamaged plants and salinized plants having 29 to 42% lower emissions relative to plants with ambient salinity (Fig. 1). More importantly, we also found a significant interaction between emitter leaf damage and salinization for two groups, namely sesquiterpenes and aromatic compounds (Fig. 1). These interactions depicted a pattern whereby damaged emitters had, on average, significantly higher emissions (up to 4 times) than control plants under ambient soil conditions, whereas for emitters exposed to soil salinization no significant difference was found (Fig. 1A, B). The other VOC groups did not show a significant interaction (Table S1), although homoterpenes exhibited a similar pattern suggestive that induction was present under ambient but not augmented salinity (Fig. 1E).

Modified from: Briones-May et al. (2023)
Effects of leaf damage under ambient vs. augmented soil salinity on the main groups of volatile compounds released (ng/2 h) by emitter wild cotton plants (Gossypium hirsutum). Values are model least-square means (back transformed for sesquiterpenes, homoterpenes, and aromatic compounds) and standard errors accounting for plant genotype and main effects. For two groups, sesquiterpenes and aromatic compounds, there were significant differences between control and damaged under ambient (sesquiterpenes: Z = 1.12, P = 0.0003; aromatics: Z = 1.32, P = 0.0001) but not augmented salinity (sesquiterpenes: Z = − 0.045, P = 0.89; aromatics: Z = − 0.56, P = 0.12). ***P < 0.001.
Emitter treatment effects on receiver chemical defenses
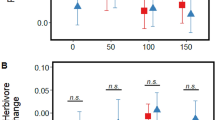
We found no effect of salinity on how VOCs from emitter plants affected terpenoid aldehyde levels in leaves of undamaged receivers (i.e., no initial effects of VOC exposure; see Table 1). However, upon damage, receiver plants showed a significant increase in leaf heliocides concentration after exposure to damaged emitters (Table 1), whereby damaged leaves of plants exposed to damaged emitters exhibited a significantly (22%) higher concentration of these compounds (304.30 ± 31.13 μg g−1) relative to those exposed to control plants (251.86 ± 31.22 μg g−1) emitters (Fig. 2). Undamaged leaves showed the same trend, although the difference was not significant (Table 1; Fig. 2). In contrast, there was no effect of emitter damage on gossypol for either damaged (control emitter: 129.31 ± 16.28 μg g−1, damaged emitter: 129.48 ± 16.23 μg g−1) or undamaged (control: 207.58 ± 30.85 μg g−1, damaged: 198.10 ± 30.72 μg g−1) leaves of receiver plants (Table 1). Finally, and contrary to expectations, we found no significant effects of salinity or emitter leaf damage by salinity interaction for either of these defense compounds, regardless of leaf damage status (Table 1; Fig. 3).
Effects of emitter leaf damage treatment (undamaged vs. mechanical damage and Spodoptera frugiperda oral secretion) on the concentration (μg g−1 of f.w.) of leaf heliocides in receiver wild cotton (Gossypium hirsutum) plants. Shown are results for damaged and undamaged leaves after damaging receiver plants. Values are model least-square means and standard errors. *P < 0.05
Effects of emitter leaf damage (undamaged vs. mechanical damage and Spodoptera frugiperda oral secretion) shown separately under each level of soil salinity (ambient vs. augmented) on the concentration (μg g−1 of f.w.) of gossypol and heliocides in receiver wild cotton (Gossypium hirsutum) plants. Shown are results for A–B initial effects (48-h exposure to VOC emissions, pre-damage), as well as C–D undamaged and E–F damaged leaves after damaging receiver plants. Values are model least-square means and standard errors
Discussion
We found that exposure to VOCs from damaged emitters primed receiver cotton plants such that, after they were damaged themselves, they produced significantly more heliocides (but not gossypol). However, in contrast to VOC induction patterns, salinization did not reduce the effects of VOC exposure on the induction of receiver chemical defenses, suggesting that the effect of salinization on induced VOCs emissions is not relevant for signaling effects on terpenoid aldehyde levels.
The lack of effect of VOCs from damaged emitter plants on leaf chemical defenses in undamaged receivers (i.e., initial effects of exposure to induced VOC emissions) suggests that volatiles do not directly induce these compounds, which is consistent with results found for EFN volume or concentration from the same experiment (Briones-May et al. 2023). In contrast, work with other plant species found that exposure to VOCs from damaged plants can directly elevate the expression of secondary metabolites associated with direct defense in undamaged neighboring plants (sagebrush: Karban et al. 2000; maize: Engelberth et al. 2004; Baccharis: Moreira et al. 2016), as well as indirect defenses (EFN in lima bean: Heil and Silva-Bueno 2007). While further tests are needed to reach stronger conclusions for wild cotton (e.g., higher levels of herbivory on emitters, longer VOC exposure times, and gene expression measurements), follow-up work with wild cotton genotypes sourced from Yucatan similarly shows a lack of effect of VOC exposure on terpenoid aldehyde levels in undamaged plants (C. Bustos-Segura, unpublished), supporting our results. Collectively, these findings suggest a limited potential for associational resistance in wild cotton, either through direct (chemical) or indirect (EFN) defensive traits, from exposure to induced VOC emissions from undamaged neighboring plants.
There was, on the other hand, evidence for VOC-mediated signaling effects on the induction of receiver heliocides in response to damage, whereby plants exposed to damaged emitters produced higher concentrations of these compounds after being damaged themselves compared to damaged receivers that had been exposed to unharmed emitters. This result is indicative of a priming effect of exposure to emitter induced VOC emissions on these compounds, agreeing with responses reported for other plant species (reviewed by Frost et al. 2008; Erb 2015), for which exposure to inducible VOCs results in a stronger and/or faster induction of defense metabolites in response to subsequent leaf damage. We also found such priming effects on EFN induction (Briones-May et al. 2023), suggesting that VOC-mediated signaling consistently influences the induction of direct as well as indirect defenses in wild cotton. Interestingly, the observed signaling effect on heliocides was found only in damaged leaves whereas for EFN it attended to be stronger in undamaged leaves (Briones-May et al. 2023), possibly explained by differences in the relative strength of local vs. systemic induction for each trait. Strong local induction of EFN (Abdala-Roberts et al. 2019c) could offset the signaling effect when comparing damaged leaves (Briones-May et al. 2023). In addition, higher heliocides levels for receiver undamaged than damaged leaves (Fig. 2) suggest stronger systemic induction. That said, undamaged leaves showed a similar (yet non-significant) trend for increased heliocides induction when exposed to damaged emitters, suggesting similar effects of signaling on damaged and undamaged leaves. Therefore results are not conclusive in this regard, warranting further detailed work to disentangle the effects of signaling on local vs. systemic induction on these secondary metabolites.
In contrast, the lack of signaling effects on gossypol in damaged receivers suggests compound type-specific responses which require further testing. One possibility is that our timing of leaf sampling after leaf damage was too short (24 h) to measure the induction of these defense compounds (Bezemer et al. 2004; Eisenring et al. 2018), even precluding the detection of early or faster induction due to VOCs priming. Also, the induced accumulation of gossypol and heliocides also takes place in young developing leaves, requiring several days of leaf growth to detect effects on these tissues (Bezemer et al. 2004). Hence, our short-term sampling of older, fully expanded leaves may not have captured the full gossypol defensive response due to sampling time and leaf stage mismatches. Priming is expected to result in quicker induced responses after damage (Mauch-Mani et al. 2017), increasing the chance to detect enhanced induction shortly after the onset of herbivory. Thus, further work incorporating multiple time points of leaf collection post-damage (see Quijano-Medina et al. 2021) is needed to obtain a better understanding of the nature and extent of VOC signaling effects (including priming mechanisms) on the induction of different types of terpenoid aldehydes in wild cotton.
Finally, we found no effect of emitter salinization on receiver chemical defenses, counter to previous studies with other species (e.g., Forieri et al. 2016; Han et al. 2016), as well as to our own work with wild cotton showing that emitter salinization reduces EFN volume secretion in receivers (Briones-May et al. 2023). More importantly, we also found no evidence that salinization influenced leaf damage-induced plant-plant signaling for any of the chemical compounds analyzed. This indicates that the dampening of VOC induction due to salinization did not have extended consequences for signaling effects on early induced chemical defenses in wild cotton, in contrast to impaired signaling effects on the induction of EFN under emitter salinization (Briones-May et al. 2023). This might imply that differently induced VOCs or VOC mixtures (as modulated by salinization) are involved in priming EFN vs. terpenoid aldehydes. Together, these results also suggest soil salinity leads to contrasting outcomes in signaling effects on direct vs. indirect defense, whereby plants found in highly saline soils would be expected to rely more on signaling effects of direct chemical defenses (than on EFN) to boost resistance to herbivory. Further, signaling effects on direct defenses might be spatially more consistent across plant patches varying in soil salinity, whereas those involving EFN would be patchier in response to within-site heterogeneity in salinity levels. Field studies manipulating salinity at within- and among-patch scales, combined with measurements of interaction outcomes (herbivory, predation) and plant resistance, are needed to test this.
Conclusions and future work
Our results call for further research aimed at understanding the physiological mechanisms by which abiotic factors affect plant–plant VOCs signaling, and the ecological consequences for plant–arthropod interactions. In the case of wild cotton, further work testing for salinity effects on induced VOCs emissions and plant signaling in situ is required, as well as efforts to disentangle the relative contributions of direct and indirect defense to plant resistance against herbivory under contrasting abiotic conditions. An important feature in such endeavors will be to manipulate soil salinity in both emitter and receiver plants given plausible local-scale variation in levels of salinity stress between neighboring plants. In addition, while our results indicate that salinization does affect signaling effects on early induction of defensive metabolites, further work involving measurements at multiple time points after induction is needed to fully assess salinization effects on VOCs priming of induced defenses and its consequences for herbivory and plant fitness. The identification of key VOCs and measuring underlying gene expression patterns should provide mechanistic insight into plant–plant signaling. Knowledge gained on VOC-mediated signaling effects in wild cotton could also potentially benefit sustainable pest and soil management strategies in cultivated cotton that seek to reduce pesticide use.
References
Abdala-Roberts L, Pérez Niño B, Moreira X, Parra-Tabla V, Grandi L, Glauser G, Benrey B, Turlings TCJ (2019a) Effects of early-season insect herbivory on subsequent pathogen infection and ant abundance on wild cotton (Gossypium hirsutum). J Ecol 107:1518–1529. https://doi.org/10.1111/1365-2745.13131
Abdala-Roberts L, Quijano-Medina T, Reyes-Hernández M, Moreira X, Francisco M, Angulo-Pérez D, Parra-Tabla V, Virgen A, Rojas JC (2019b) Effects of amount and recurrence of herbivory on the induction of direct and indirect defences in wild cotton. Plant Biol 21:1063–1071. https://doi.org/10.1111/plb.13022
Abdala-Roberts L, Quijano-Medina T, Moreira X, Vázquez-González C, Parra-Tabla V, Mier B, y Teran J, Grandi L, Glauser G, Turlings TCJ, Benrey B, (2019c) Bottom-up control of geographic variation in insect herbivory on wild cotton (Gossypium hirsutum) by plant defenses and climate. Am J Bot 106:1059–1067. https://doi.org/10.1002/ajb2.1330
Agrawal AA, Karban R (2000) Specificity of constitutive and induced resistance: pigment glands influence mites and caterpillars on cotton plants. Entomol Exp Appl 96:39–49. https://doi.org/10.1046/j.1570-7458.2000.00677.x
Agrawal AA, Karban R, Colfer RG (2000) How leaf domatia and induced plant resistance affect herbivores, natural enemies, and plant performance. Oikos 89:70–80. https://doi.org/10.1034/j.1600-0706.2000.890108.x
Arce CM, Besomi G, Glauser G, Turlings TCJ (2021) Caterpillar-induced volatile emissions in cotton: the importance of damage and insect-derived factors. Front Plant Sci 12:709858. https://doi.org/10.3389/fpls.2021.709858
Baldwin IT, Schultz JC (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221:277–279. https://doi.org/10.1126/science.221.4607.277
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed effects models using lme4. J Stat Softw 67:1–8. https://doi.org/10.18637/jss.v067.i01
Becker C, Desneux N, Monticelli L, Fernandez X, Michel T, Lavoir AV (2015) Effects of abiotic factors on HIPV-mediated interactions between plants and parasitoids. Biomed Res Int 2015:342982. https://doi.org/10.1155/2015/342982
Bezemer TM, Wagenaar IR, van Dam NM, van der Putten WH, Wäckers FL (2004) Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J Chem Ecol 30:53–67
Blande JD (2021) Effects of air pollution on plant-insect interactions mediated by olfactory and visual cues. Curr Opin Environ Sci Health 19:100228. https://doi.org/10.1016/j.coesh.2020.100228
Briones-May Y, Quijano-Medina T, Pérez-Niño B, Turlings TCJ, Bustos-Segura C, Abdala-Roberts L (2023) Soil salinization disrupts plant-plant signaling effects on extra-floral nectar induction in wild cotton. Oecologia 202:313–323. https://doi.org/10.1007/s00442-023-05395-w
Brui J, Dicke M, Sabelis MW (1992) Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics. Experientia 48:525–529. https://doi.org/10.1007/BF01928181
Caparrotta S, Boni S, Taiti C, Palm E, Mancuso S, Pandol C (2018) Induction of priming by salt stress in neighboring plants. Environ Exp Bot 147:261–270. https://doi.org/10.1016/j.envexpbot.2017.12.017
Catola AS, Centritto M, Cascone P, Ranieri A, Loreto F, Calamai L, Balestrini R, Guerrieri E (2018) Effects of single or combined water deficit and aphid attack on tomato volatile organic compound (VOC) emission and plant-plant communication. Environ Exp Bot 153:54–62. https://doi.org/10.1016/j.envexpbot.2018.05.001
Chappuis L, Egger A, Röeder G, Glauser G, Jaffuel G, Benrey B, Abdala-Roberts L, Clancy M, Turlings TCJ, Bustos-Segura C (2023) Effects of experimental growth conditions on the chemical defences of two cotton species, Gossypium hirsutum and G. herbaceum. J Chem Ecol 49:340–352. https://doi.org/10.1007/s10886-023-01422-5
D’Eeckenbrugge G, Lacape JM (2014) Distribution and differentiation of wild, feral, and cultivated populations of perennial upland cotton (Gossypium hirsutum L.) in Mesoamerica and the Caribbean. PLoS ONE 9:e107458. https://doi.org/10.1371/journal.pone.0107458
Engelberth J, Alborn H, Schmelz E, Tumlinson J (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 101:1781–1785. https://doi.org/10.1073/pnas.0308037100
Eisenring M, Glauser G, Meissle M, Romeis J (2018) Differential impact of herbivores from three feeding guilds on systemic secondary metabolite induction, phytohormone levels and plant-mediated herbivore interactions. J Chem Ecol 44:1178–1189. https://doi.org/10.1007/s10886-018-1015-4
Forieri I, Hildebrandt U, Rostás M (2016) Salinity stress effects on direct and indirect defence metabolites in maize. Environ Exp Bot 122:68–77. https://doi.org/10.1016/j.envexpbot.2015.09.007
Frost CJ, Mescher MC, Carlson JE (2008) Plant defence priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824. https://doi.org/10.1104/pp.107.113027
Girón-Calva PS, Li T, Blande JD (2016) Plant-plant interactions affect the susceptibility of plants to oviposition by pests but are disrupted by ozone pollution. Agr Ecosyst Environ 233:352–360. https://doi.org/10.1016/j.agee.2016.09.028
Hagenbucher S, Olson D, Ruberson J, Wäckers F, Romeis J (2013) Resistance mechanisms against arthropod herbivores in cotton and their interactions with natural enemies. Crit Rev Plant Sci 32:458–482
Han P, Wang Z, Lavoir A, Michel T, Seassau A, Zheng W, Niu C-Y, Desneux N (2016) Increased water salinity applied to tomato plants accelerates the development of the leaf miner Tuta absoluta through bottom-up effects. Sci Rep 6:32403. https://doi.org/10.1038/srep32403
Heil M, Karban R (2010) Explaining evolution of plant communication by airborne signals. Trends Ecol Evol 25:137–144. https://doi.org/10.1016/j.tree.2009.09.010
Heil M, Silva-Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 104:5467–5472. https://doi.org/10.1073/pnas.0610266104
Holopainen JK, Gershenzon J (2010) Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 15:176–184. https://doi.org/10.1016/j.tplants.2010.01.006
Karban R, Yang LH, Edwards KF (2014) Volatile communication between plants that affects herbivory: a meta-analysis. Ecol Lett 17:44–52. https://doi.org/10.1111/ele.12205
Karban R, Shiojiri K, Huntzinger M, McCall AC (2006) Damage-induced resistance in sagebrush: volatiles are key to intra-and interplant communication. Ecology 87:922–930. https://doi.org/10.1890/0012-9658(2006)87[922:DRISVA]2.0.CO;2
Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125:66–71. https://doi.org/10.1007/PL00008892
Landi M, Araniti F, Flamini G, Piccolo EL, Trivellini A, Abenavoli MR, Guidi L (2020) “Help is in the air”: volatiles from salt-stressed plants increase the reproductive success of receivers under salinity. Planta 251:48. https://doi.org/10.1007/s00425-020-03344-y
Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH (1994) Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci USA 91:11836–11840. https://doi.org/10.1073/pnas.91.25.11836
Mansour MH, Zohdy NM, El-Gengaihi SE, Amr AE (1997) The relationship between tannins concentration in some cotton varieties and susceptibility to piercing sucking insects. J Appl Entomol 121:321–325. https://doi.org/10.1111/j.1439-0418.1997.tb01413.x
Mauch-Mani B, Baccelli I, Luna E, Flors V (2017) Defense priming: an adaptive part of induced resistance. Annu Rev Plant Biol 68:485–512. https://doi.org/10.1146/annurev-arplant-042916-041132
McAuslane HJ, Alborn HT, Toth JP (1997) Systemic induction of terpenoid aldehydes in cotton pigment glands by feeding of larval Spodoptera exigua. J Chem Ecol 23:2861–2879. https://doi.org/10.1023/A:1022575313325
McCall PJ, Turlings TCJ, Loughrin J, Proveaux AT, Tumlinson JH (1994) Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L.) seedlings. J Chem Ecol 20:3039–3050. https://doi.org/10.1007/bf02033709
Moreira X, Nell CS, Katsanis A, Rasmann S, Mooney KA (2018) Herbivore specificity and the chemical basis of plant-plant communication in Baccharis salicifolia (Asteraceae). New Phytol 220:703–713. https://doi.org/10.1111/nph.14164
Moreira X, Abdala-Roberts L (2019) Specificity and context-dependency of plant–plant communication in response to insect herbivory. Curr Opin Insect Sci 32:15–21. https://doi.org/10.1016/j.cois.2018.09.003
Ninkovic V, Markovic D, Rensing M (2020) Plant volatiles as cues and signals in plant communication. Plant Cell Environ 44:1030–1043. https://doi.org/10.1111/pce.13910
Nix A, Paull C, Colgrave M (2017) Flavonoid profile of the cotton plant, Gossypium hirsutum: a review. Plants 6:1–14. https://doi.org/10.3390/plants6040043
Opitz S, Kunert G, Gershenzon J (2008) Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J Chem Ecol 34:508–522. https://doi.org/10.1007/s10886-008-9453-z
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2014) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Pezzola E, Mancuso S, Karban R (2017) Precipitation affects plant communication and defense. Ecology 98:1693–1699. https://doi.org/10.1002/ecy.1846
Pickett JA, Khan ZR (2016) Plant volatile-mediated signaling and its application in agriculture: successes and challenges. New Phytol 212:856–870. https://doi.org/10.1111/nph.14274
Quijano-Medina T, Turlings TCJ, Sosenski P, Grandi L, Cervera JC, Moreira X, Abdala-Roberts L (2021) Effects of soil salinity on the expression of direct and indirect defences in wild cotton, Gossypium hirsutum. J Ecol 109:354–368. https://doi.org/10.1111/1365-2745.13483
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reyes-Hernández M, Quijano-Medina T, Angulo-Pérez D, Moreira X, Parra-Tabla V, Abdala-Roberts L (2022) Experimental test of ant effects on insect herbivory and pathogen infection on wild cotton (Gossypium hirsutum). Arthropod-Plant Interact 16:77–86. https://doi.org/10.1007/s11829-021-09876-8
SAS (2015) SAS Institute Inc., ver. 9.4. Cary, NC
Stenberg JA, Heil M, Åhman I, Björkman C (2015) Optimizing crops for biocontrol of pests and disease. Trends Plant Sci 20:698–712. https://doi.org/10.1016/j.tplants.2015.08.0073
Sweet WV, Park J (2014) From the extreme to the mean: Acceleration and tipping points of coastal inundation from sea level rise. Earth’s Future 2:579–600. https://doi.org/10.1002/2014EF000272
Teuber M, Zimmer I, Kreuzwieser J, Ache P, Polle A, Rennenberg H, Schnitzler JP (2008) VOC emissions of grey poplar leaves as affected by salt stress and different N sources. Plant Biol 10:86–96. https://doi.org/10.1111/j.1438-8677.2007.00015.x
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452. https://doi.org/10.1146/annurev-ento-020117-043507
Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH (1993) An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J Chem Ecol 19:411–425. https://doi.org/10.1007/s004250050466
Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D (1998) Timing of induced volatile emissions in maize seedlings. Planta 207:146–152. https://doi.org/10.1007/s004250050466
Vázquez-González C, Pombo L, Martín-Cacheda L, Rassman S, Röder G, Abdala-Roberts L, Mooney K, Moreira X (2022) Effect of water availability on communication between potato plants in response to herbivory. Funct Ecol 36:2763–2773. https://doi.org/10.1111/1365-2435.14159
Wäckers FL, Bonifay C (2004) How to be sweet? Extrafloral nectar allocation by Gossypium hirsutum fits optimal defense theory predictions. Ecology 85:1512–1518. https://doi.org/10.1890/03-0422
Wendel JF, Brubaker CL, Percival AE (1992) Genetic diversity in Gossypium hirsutum and the origin of upland cotton. Am J Bot 79:1291–1310. https://doi.org/10.1002/j.1537-2197.1992.tb13734.x
Yuan D, Grover CE, Hu G, Pan M, Miller ER, Conover JL, Hunt SP, Udall JA, Wendel JF (2021) Parallel and intertwining threads of domestication in allopolyploid cotton. Adv Sci 8:2003634. https://doi.org/10.1002/advs.202003634
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zakir A, Bengtsson M, Sadek MM, Hansson BS, Witzgall P, Anderson P (2013a) Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J Exp Biol 216:3257–3263. https://doi.org/10.1242/jeb.083188
Zakir A, Sadek MM, Bengtsson M, Hansson BS, Witzgall P, Anderson P (2013b) Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore. J Ecol 101:410–417. https://doi.org/10.1111/1365-2745.12041
Acknowledgements
This study was in part supported by funds from the Swiss Science Foundation awarded to TCJT (315230_185319). TQM was supported by a CONAHCYT PhD scholarship. XM and LAR were financially supported by two grants from the Spanish National Research Council (COOPA20477, INCGL20004). Permit information for sampling of plant material in Yucatan: SEMARNAT- SCPA/DGVS/00585/20 and SEMARNAT-SCPA/DGVS/2974/20. We thank A. Vallat for help with running samples with the HPLC, as well as B. Perez, Jonathan Interian, B. Suárez, and D. Franco for their kind help in the field. The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Contributions
TQM, LAR, and XM conceived the ideas and designed the methodology; TQM collected the data; TQM and LAR analyzed the data; USR, MM, MC, and CBS performed the chemical analyses; TQM, LAR, and XM wrote the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the content of this article.
Additional information
Handling Editor: Islam Sobhy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quijano-Medina, T., Briones-May, Y., Solís-Rodríguez, U. et al. Soil salinization effects on volatile signals that mediate the induction of chemical defenses in wild cotton. Arthropod-Plant Interactions (2024). https://doi.org/10.1007/s11829-024-10062-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11829-024-10062-9