Abstract
Community assembly relies on deterministic niche-based processes (e.g., biotic interactions), and stochastic sources of unpredictable variation (e.g., colonization history), that combined will influence late-stage community structure. When community founders present distinct functional traits and a colonization–competition trade-off is not operating, initial colonization can result in late-stage assemblages of variable diversity and composed by different species sets, depending if early colonizers facilitate or inhibit subsequent colonization and survival. By experimentally manipulating the functional identity of founders and predators access during the development of fouling communities, we tested how founder traits constrain colonization history, species interactions and thereby regulate community diversity. We used as founders functionally different fouling organisms (colonial and solitary ascidians, and arborescent and flat-encrusting bryozoans) to build experimental communities that were exposed or protected against predation using a caging approach. Ascidians and bryozoans are pioneer colonizers in benthic communities and also good competitors, but the soft-body of ascidians makes them more susceptible to predators than mineralized bryozoans. When ascidians were founders, their dominance (but not richness) was reduced by predation, resulting in no effects of predators on overall diversity. Conversely, when bryozoans were founders, both space limitation and predator effects resulted in species-poor communities, with reduced number and cover of ascidian species and high overall dominance at the end of the experiment. We, thus, highlight that current species interactions and colonization contingencies related to founder identity should not be viewed as isolated drivers of community organization, but rather as strongly interacting processes underlying species distribution patterns and diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of species assembly during early stages of community development may greatly alter late-stage species composition and their abundance, often underlying large differences among mature communities at equilibrium, sometimes within sites nearby (Sousa 1984; Berlow 1997; Fukami 2015; Chang and Marshall 2016; Vieira et al. 2017). However, the dynamics of developing communities may be more or less predictable depending on the mechanisms driving temporal change. Some communities follow roughly the same pathway of organization through time, resulting in a predictable order of species replacements. This configurates a sequential succession, usually with species with traits that enhance colonization ability (e.g., dispersal) at early stage paving the way to species with traits that enhance competitive success (e.g., resource monopolization) at more advanced stages (Connell and Slatyer 1977; Jackson 1977; Berlow 1997), revealing a dominance-controlled process (Yodzis 1986). However, other communities are greatly affected by stochastic processes. Their temporal dynamics are much less predictable and may follow many different possible pathways (Berlow 1997; Fukami 2015). It may relate to when disturbing events take place (Sutherland and Karlson 1977; Dean and Hurd 1980; Berlow 1997), or how the identity of first colonizers results in distinct functional roles (Dean and Hurd 1980; Sousa 1984; Vieira et al. 2018a), leading to a founder-controlled dynamics (Yodzis 1986). In this case, stochastic drivers often explain the exceptionally large variability of assemblage structure that is observed at different spatial scales, even within just a few meters (Sousa 1984; Chang and Marshall 2016; Vieira et al. 2016). Currently, deterministic and stochastic forces are thought to exert joint effects on the process of community assembly (Adler et al. 2007; Chase 2007; Vellend 2010; Chase and Myers 2011) as observed for assemblages of several groups including microorganisms (Dini-Andreote et al. 2015; Hu et al. 2015), arthropods (Elwood et al. 2009), fouling species (Chang and Marshall 2016; Vieira et al. 2017), plants (Maren et al. 2018; Romme et al. 2016), and vertebrates (Willig and Moulton 1989).
Niche-based processes are related to functional traits that determine which species colonize and persist through community development (McGill et al. 2006; Cadotte et al. 2015).While environmental filtering operates at a broader scale by selecting species with traits that confer tolerance to regional conditions (Mittelbach and Schemske 2015), in a local scale, the way species interact with each other also becomes an important deterministic force guiding the development of communities (Berlow 1997; Mittelbach and Schemske 2015). Under a colonization/competition trade-off model (Connell and Slatyer 1977; Dean and Hurd 1980; Berlow 1997), facilitation provided by founder organisms would allow first colonization and early persistence of a given set of species [e.g., some species may modify microscale conditions, promoting environmental amelioration (Perea and Gil 2014; Vogt et al. 2014; Jurgens and Gaylord 2016) or even provide settlement surface and protection against predators with their tridimensional bodies (Russ 1980; Vieira et al. 2018a, b)]. In the other hand, negative effects of interspecific competition and predation pressure would determine the species remaining in later successional stages (Connell and Slatyer 1977; Sutherland 1978; Berlow 1997; Fukami 2015; Vieira et al. 2018a).
Altering the classic colonization/competition trade-off model, stochastic processes add uncertainty and broaden the range of alternative development pathways and assemblage structure at advanced stages (Berlow 1997; Fukami 2015). Surplus nutrient inputs, for example, are well-known stochastic episodes which facilitate species with specific functional traits, and thereby regulate later community dynamics structure (Coles and Brown 2007; Chase 2010; Wernberg et al. 2012; Smith et al. 2020). Disrupting events such as those may greatly depart patterns of species assembly from expectations (Berlow 1997). A specific case can be made when the regional species pool is large, and ‘realized’ diversity likely high, because the identity of first colonizers is both uncertain and paramount for later temporal dynamics. Different from the more predictable order of species replacements in an ecological succession through dominance control (niche-based), habitat patches prone to be colonized by several species with equal chances of arrival, but that diverge in their functional roles, may develop in variable and unpredictable ways that can be modeled based on lottery effects (Greene and Shoener 1982; Berlow 1997).
More than just two complementary components of community assembly, deterministic and stochastic processes may interact with each other (Berlow 1997; Dini-Andreote et al. 2015; Vieira et al. 2018a) because the variability imposed by different species colonizing an empty space will extend to niche-based regulation during community assembly, with limits set by the specific functional roles of different founder species (Dean and Hurd 1980; Cifuentes et al. 2010; Cleland et al. 2015; Sutherland 1978; Vieira et al. 2018a). Temporal dynamics would generally be ruled through dominance control when founder traits mostly facilitate the establishment of other species (Connell and Slatyer 1977; Jones et al. 1994; Fukami 2015; Vieira et al. 2018a, b). On the other hand, founder control is expected when founder traits actually tend to inhibit subsequent species arrivals (Connell and Slatyer 1977; Sutherland 1978; Fukami 2015; Vieira et al. 2018a). Examples of the latter include space preemption (Sutherland 1978; Fukami 2015; Vieira et al. 2018a), allelopathy (Jackson and Buss 1975; Sammarco et al. 1983), and interference competition (Buss and Jackson 1979; Bonnici et al. 2012).
Besides cascading founder effects through competition or facilitation, predation pressure on the founder species themselves may also modify patterns of species assembly. Higher-order predators may functionally shape community structure by controlling palatable founder species, or even pioneering fast-growing species trading energy from defensive strategies to increased clonal or sexual reproduction (Díaz et al. 2007; López and Freestone 2021). However, how founders with distinct functional attributes, considering both competitive and predation resistance traits, may alter the relevance of trophic interactions in the determination of late-stage community structure is still poorly known. As founders are often resource monopolizers (Buss and Jackson 1979; Fukami 2015) and unequally vulnerable to predation (Sousa 1984; Berlow 1997; Vieira et al. 2018a), direct predation effects may greatly depend on whether pioneers themselves are prone to predation or not. Also, founder traits other than those related to resistance against predators may underlie indirect predation effects on later stages of community assembly by either facilitating or inhibiting predation-vulnerable later-stage guilds.
Here, we explore how different colonization scenarios, imposed by founders with distinct functional identities, modulate predation effects on later stages of community organization through direct or indirect processes. To do so, we conducted an experiment simulating different colonization scenarios, and controlling the access of predators, to test how consumptive effects are modulated by the identity of founders and their functional traits. Marine fouling species were used as models, as they are functionally diverse, fast-growing, and easily manipulated (Dean and Hurd 1980; Osman and Whitlatch 2004; Freestone et al. 2011; Vieira et al. 2018a, b; Dias et al. 2020). Founder functional identity was determined by starting communities with species of four different groups, colonial and solitary ascidians, and arborescent and flat-encrusting bryozoans. Using these functionally different founders we could test for (I) phylogeny and the importance of resistance to predation (ascidians vs. bryozoans); (II) the effect of the rate of space occupation (colonial vs. solitary ascidians, and arborescent vs. flat-encrusting bryozoans); and (III) for the importance of potential facilitation (arborescent vs. flat-encrusting bryozoans). Trials for all founder identities were run with and without predation pressure (through caging manipulation) to evaluate how colonization history conditioned to founder identity modulates consumptive effects. We expected that communities founded by fragile soft-bodied ascidians, especially solitary species which occupy space at a lower rate and are more susceptible to predators, would be more affected by both direct and indirect effects of predation. Such effects would be smallest for communities founded by tougher mineralized bryozoans, such as flat-encrusting bryozoans, which cover available space fast and are more resistant to fish predators.
Methods
Study site and species
The experiment was conducted at the Yacht Club of Ilhabela (23º46′S, 45º21′W) during the austral summer of 2016 (Fig. S1a in Online Resource 1). The site is a recreational marina composed by floating platforms in which a diverse fouling community grows (Oricchio et al. 2016a, b; Vieira et al. 2016). These communities are usually dominated by colonial organisms that can monopolize space through asexual reproduction in a variable way, some of them being able to prevent the colonization of other species (Jackson 1977; Hiebert et al. 2019). Additionally, they are fast growing, easy to manipulate and provide clear results regarding the effect of interactions in a short time span (Osman and Whitlatch 2004; Freestone et al. 2011; Vieira et al. 2018a, b; Dias et al. 2020), which make them ideal for experiments testing the effect of founder identity and predation on community structure and diversity. Predation is one of the strongest forces shaping such communities in the area, affecting both richness and structure throughout community development (Vieira et al. 2012, 2016; Oricchio et al. 2016a, b; Dias et al. 2020). Fish are the main consumers (Oricchio et al. 2016b) in the studied system and region, while predation by other small organisms, such as gastropods, do not play a major role (Oricchio et al. 2016a).
As we wanted to test for the effect of colonization contingencies related to founder identity in a functional context, we selected the dominant fouling taxonomic groups in the area to build the experimental communities, ascidians, and bryozoans (Oricchio et al. 2016a, b; Vieira et al. 2018a, b), which also show contrasting life-history traits. While ascidians are best competitors (Kay and Keough 1981; Nandakumar et al. 1993; Vieira et al. 2012, 2016; Oricchio and Dias 2020), but as soft-bodied vulnerable to predation (Osman and Whitlatch 2004; Freestone et al. 2011; Vieira et al. 2012, 2016; Oricchio et al. 2016a,b; Dias et al. 2020; Oricchio and Dias 2020), bryozoans are second in line in terms of competition (Kay and Keough 1981; Nandakumar et al. 1993; Oricchio and Dias 2020), but very resistant to predation by having a tough mineralized body (Lidgard 2008; Oricchio and Dias 2020). Additionally, these groups are divided in different functional groups, which colonize and monopolize resources by quite different means. Colonial ascidians can monopolize space faster than solitary ones (Jackson 1977; Nandakumar et al. 1993; Vieira et al. 2012) and are susceptible to predation during their entire life (Hiebert et al. 2019). Still, they may easily recover if only a small part of the colony is consumed (Hiebert et al. 2019), while solitary species are less resistant to predation (Jackson and Hughes 1985), mostly when young (Osman and Whitlatch 2004). Arborescent bryozoans grow vertically, not monopolizing space (Nandakumar et al. 1993; Walters and Wethey 1996), and may be more susceptible to predation (Oricchio et al. 2016b; Dias et al. 2020) when compared to flat-encrusting forms that quickly cover a great amount of space by growing in two dimensions (Jackson 1977; Sutherland 1978; Nandakumar et al. 1993; Oricchio et al. 2016b; Vieira et al. 2018a, b). By building heavily calcified skeletons, this is the functional group most resistant to fish predators (Lidgard 2008; Oricchio and Dias 2020). In addition, solitary ascidians and arborescent bryozoans (but not colonial ascidians and flat-encrusting bryozoans) may facilitate other species recruitment by altering nearby water circulation (Koehl 1982, 1984), and by increasing survival through shelter provisioning (Russ 1980; Breitburg 1985; Vieira et al. 2018a,b). Therefore, we used the colonial ascidians Botrylloides niger and Didemnum perlucidum; the solitary ascidians Herdmania pallida and Phallusia nigra; the flat-encrusting bryozoans Schizoporella errata and Watersipora subtorquata; and the arborescent bryozoans Bugula neritina and Crisia pseudosolena to understand how founder identity in terms of functional traits will determine the effects of predation on community diversity (Fig. S1b in Online Resource 1).
Collection of organisms
The organisms were obtained from inventory panels covered with sanded acetate sheets and kept in the field for 1 month. We prevented the access of predators to the inventory panels with plastic screen cages (as the ones described below) to ensure that groups vulnerable to predation, such as ascidians, would be available to build the experimental communities. From those panels, we selected arborescent bryozoans with 2–3 bifurcations, and encrusting bryozoans, colonial and solitary ascidians with a diameter around 1.5 cm (see Vieira et al. 2018a, b for details). Those are the sizes of approximately 15-days old individuals and sufficed to allow a size advantage to experimental founders (Urban and De Meester 2009; Vieira et al. 2018a, b). As the solitary ascidian H. pallida is commonly found protected from predators in crevices but was not common on inventory panels, individuals were produced in the laboratory using in vitro fertilization (following Crean and Marshall 2008). Competent larvae were collected and individually put to settle in water drops over PVC panels covered by sanded acetate sheets (as those used in inventory panels). Panels were left in the dark for 12 h to ensure settlement and initial post-larval development, and then deployed in the field for 15 days to obtain H. pallida individuals of the target size and age.
Experimental design
To test the effects of founder functional identity on community assembly, we built experimental communities with four small organisms of the same functional group (two of each species) attached to the central area (10 × 10 cm) of sanded PVC settlement panels (15 × 15 × 0.5 cm; Fig. S2a in Online Resource 1). For each functional group (colonial or solitary forms for ascidians and flat-encrusting or arborescent forms for bryozoans), we built 12 experimental panels.
As we also wanted to test how the functional identity of the founders could modulate predation effects on the community development, we also manipulated the access of predators to experimental panels (Fig. S2b in Online Resource 1). For that, half of the panels of a given functional group (n = 6) were covered by a plastic screen cage (15 × 15 × 8 cm, 1 cm mesh), excluding larger predators such as fish, crustaceans and mollusks, and the other half (n = 6) were covered by partial cages of the same dimension but lacking the roof, allowing predator access while controlling for eventual cage effects (Osman and Whitlatch 2004; Freestone et al. 2011; Vieira et al. 2012, 2016; Dias et al. 2020). We are aware that exclusion experiments commonly have a third treatment with uncaged panels. However, as we had a limited number of founder organisms to build the experimental communities, and since previous studies in the same area showed no differences between communities developing in uncaged and partially caged panels (Vieira et al. 2012; Dias et al. 2020), we decided to use only the partial cage treatment as it is open to predators while still imposing any eventual alterations caused by plastic screens.
Deployment and sampling procedures
Replicate inventory panels were haphazardly suspended along marina floating platforms to ensure proper spatial interspersion of treatments. Panels were deployed in a horizontal position, with the experimental side facing the bottom, at a depth of 1.5 m and at least 2 m apart from each other and to the sandy bottom below (Fig. S2c in Online Resource 1). On a monthly basis cages were cleaned and replaced when needed.
We ended the experiment after 3 months. By then, most space was already covered and eventual effects of experimental manipulations on community structure were readily noticeable (as in Freestone et al. 2011; Vieira et al. 2016; Dias et al. 2020). Panels were retrieved and all organisms were identified to the lowest possible taxonomic level. Photographs were taken for later quantification of the area covered by each taxon, using a 100 points grid on the Coral Point Count with Excel extensions (CPCE) software (Kohler and Gill 2006). Grid points were restricted to the central 13 × 13 cm area of panels to avoid border effects of manipulative procedures.
Data analyses
The number of all species, as well as the number of ascidian, bryozoan and other species, Shannon diversity and Simpson dominance indices per community were obtained and separately analyzed with two-way orthogonal type III sum-of-square ANOVAs, considering the effects of ‘founder identity’ (fixed, four levels: colonial ascidians, solitary ascidians, flat-encrusting bryozoans, or arborescent bryozoans), ‘predation’ (fixed, two levels: predators allowed or excluded), and their interactions. Considering our hypotheses, we established the following planned contrasts for the founder identity factor: (I) ascidians vs. bryozoans—testing for the importance of phylogeny and resistance to predation; (II) colonial vs. solitary ascidians—testing for the effects of rate of space occupation; and III) flat-encrusting vs. arborescent bryozoans—testing for the combined effect of space occupation and potential facilitation. When the main model showed an effect for founder identity or for its interaction with predation, we then estimated the effects of each contrast and, if it was the case, the interaction of each contrast with predation. In the case of a significant effect of the interaction of any contrasts with predation, we explored it with Tukey tests. Overall, raw data followed normality and homoscedasticity assumptions, except for ascidian richness that needed to be log transformed. These analyses were run in R software (R Core Team 2019) with the package ‘car’ (Fox and Weisberg 2019).
For community structure, we followed a multivariate approach using square-root transformed cover data, a proxy for relative abundance (Vieira et al. 2012), to build a resemblance matrix based on Bray–Curtis distances. The relationships among samples were visually represented by a Non-metric Multidimensional Scaling (nMDS) plot (Clarke 1993), and compared considering the effects of ‘founder identity’ and ‘predation’ using PERMANOVA (Anderson 2001), following the same model above for univariate analyses. Pairwise tests for multiple comparisons were undertaken for significant sources of variation, and the SIMPER procedure was used to identify the taxa that contributed the most to differences (Clarke 1993). All multivariate procedures were performed in the PRIMER 6 software (Clarke and Warwick 2001). Replication at the beginning was equal among all treatment combinations (n = 6), but a few panels were lost and sometimes sample size dropped to 4 or 5, as indicated in figures.
Results
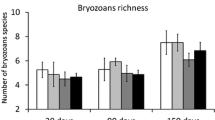
Founder identity was overall important for most of the community metrics investigated, including the modulation of predation effects on some of them (Table 1). Total richness was affected only by predation, with less species in the presence of predators regardless founder identity (Fig. 1a). Shannon diversity and dominance, in the other hand, differed between predation treatments only when bryozoans were founders (Table S1 in Online Resource 2), with a higher diversity and lower dominance when predators were absent (Fig. 1b, c). Regarding the richness of specific groups, only the number of bryozoan species was not affected by founder identity, with a higher number of bryozoan species in communities exposed to predators regardless of the founder identity (Fig. 2a; Table 1). However, founder identity was important for modulating the effect of predators on ascidian richness, with more ascidian species on communities protected from predators only when founded by bryozoans (Fig. 2b; Table S1 in Online Resource 2). For the number of other species, we observed isolated effects of the main factors (Table S1). Founder identity effect led to more species on communities founded by ascidians when compared to the ones founded by bryozoans (Fig. 4c). Additionally, predation also played a role, with communities protected from predators showing a lower number of other species, regardless founder identity (Fig. 4c).
Average total richness (± SE) of total (a), Shannon diversity (b), and dominance (c) on communities founded by colonial (COL, blue) and solitary (SOL, green) ascidians, and flat-encrusting (FLAT, orange) and arborescent (ARB, red) bryozoans, under the presence (+ P, dark shades) or absence (− P, light shades) of predators after 3 months of community development. The lines over the bars represent the significance of main effect of predation (continuous line in panel a) or the post hoc analyses for the interaction between founder identity and predation (separated lines in panels b and c)
Average richness (± SE) of bryozoans (a), ascidians (b), and other species (c) on communities founded by colonial (COL, blue) and solitary (SOL, green) ascidians, and flat-encrusting (FLAT, orange) and arborescent (ARB, red) bryozoans, under the presence (+ P, dark shades) or absence (− P, light shades) of predators after 3 months of community development. The lines over the bars represent the significance of the main effect of predation and/or founder identity (continuous line in panels a and c) or the post hoc analyses for the interaction between founder identity and predation (separated lines in panel b)
Founder identity was also important for modulating how predation affected community structure (Table 1; Fig. 3). While pairwise comparisons between predated and protected communities were significant for all founder identity treatments, the dispersion was different when colonial ascidians (PERMDISP: p = 0.039) and flat-encrusting bryozoans (PERMDISP: p = 0.008) were founders, and the groups important for differences between predation treatments varied (Table 2). For all founder identity treatments, the colonial ascidian D. perlucidum dominated when predators were absent, while the flat-encrusting bryozoan S. errata monopolized space in the presence of predators. However, founder identity determined how predation affected the abundance of non-dominant species. When colonial ascidians founded the community, predation reduced the abundance of arborescent bryozoans, but increased it when the founders were solitary ascidians, and this outcome was mainly related to effects on Amathia brasiliensis (Fig. 3b; Table 2). When flat-encrusting bryozoans were the community founders, predation promoted a drastic reduction in the abundance of arborescent bryozoans, mainly guided by effects on A. brasiliensis. However, when arborescent bryozoans were founders, predation also resulted in a decrease of arborescent bryozoans themselves, mainly A. brasiliensis, but not as drastic as when flat-encrusting bryozoans were founders, with Bugula neritina accounting for some remaining arborescent bryozoan cover on communities where predators had access. (Fig. 3b; Table 2).
nMDS plot comparing community structure 3 months after foundation by colonial (blue circles) and solitary (green squares) ascidians, and by flat-encrusting (orange diamonds) and arborescent (red triangles) bryozoans, both under the absence (− P, dark shades) or presence (+ P, light shades) of predators (a). Average cover of major taxonomic groups and bare space for all the above treatment combinations (b). The lines over the bars represent the post hoc analyses for the interaction between founder identity and predation
Discussion
We report in this study that the functional identity of early colonizers can affect how a niche process, predation, affects community assembly, structure and diversity at subsequent development stages. Our simulation putting ascidians as founders allowed them to achieve a size-refuge from predation even being less resistant (Russ 1980; Osman and Whitlatch 2004; Hiebert et al. 2019), reducing or dampening the importance of predators for community structuring and diversity. However, contrary to expectations, predation affected community diversity when bryozoans were founders, especially in the case of flat-encrusting forms. Those founders persisted by their higher resistance against predation and rapidly monopolized space and reduced the chances of colonization of ascidian species in the presence of predators. Additionally, the exposure to predators further reduced the cover, survival and consequently diversity of any new ascidian recruit, directly impacting overall community diversity.
Ascidians are known to be good competitors and efficient space monopolizers (Kay and Keough 1981; Nandakumar et al. 1993; Oricchio and Dias 2020), but their soft-body constitution make them preferential targets to consumers (Dias et al. 2020). Any chemical defenses against predators (Stoecker 1980a, b; Pisut and Pawlik 2002) are apparently insufficient to nullify predator effects (Osman and Whitlatch 2004; Freestone et al. 2011), including at this study site (Vieira et al. 2012; Oricchio et al. 2016a, b; Dias et al. 2020). We thus expected strong predation effects on ascidian-founded communities since ascidian competitive ability would not compensate for their low resistance against predators. However, we observed no effects of predation on neither community diversity and dominance, nor the number of ascidian species, in ascidian-founded communities. The size advantage imposed by our simulated foundation decreased the risk of a given ascidian species to be completely removed by predators, therefore, maintaining high diversity and low dominance even in predated communities. This is valid for both solitary species, that may attain a size-refuge from predation (Osman and Whitlatch 2004; Hiebert et al. 2019), and for colonial species that cannot be totally removed and are capable to regenerate from remaining colony tissue (Hiebert et al. 2019). Still, the effects of predation on overall ascidian dominance are evident, with virtual full removal in bryozoan-founded treatments (Fig. 4b). Therefore, any size advantage for ascidians in nature will rise only for those organisms that either find protection by settling away from the reach of a predator, as in crevices or as understory of sheltering species (Buss 1979; Marfenin 1997), or those that find an opportunity window for settlement when predation pressure is very low or absent (Sebens and Lewis 1985; Berlow 1997).
The competitive hierarchy between two or more dominant functional groups (a) may be altered to a transient state if they differ in terms of resistance against predators, or any other environmental condition (b). Therefore, taking together the colonization history (variable founder identity) and its consequences (e.g., positive or negative effects), and considering the competitive hierarchy and differential resistance that generates transience, several community structure/dominance scenarios are possible along the assembly process (c). In this study group A represents ascidians and group B bryozoans
Flat-encrusting bryozoan species are not only good competitors (Kay and Keough 1981; Nandakumar et al. 1993) but also greatly resistant against fish predators owing to their mineralized skeletons (Lidgard 2008; Oricchio and Dias 2020). Predator effects on bryozoan-founded treatments are largely due to removals of more palatable recently-colonized ascidians and arborescent bryozoan species (Fig. 3b). Predatory impacts on bryozoans are restricted to arborescent and less mineralized species, namely Bugula neritina and Crisia pseudosolena, or species lacking any calcification, such as Amathia brasiliensis, as observed in other studies (e.g., Dias et al. 2020). The heavily armored flat-encrusting bryozoan Schizoporella errata was actually favored by the exposure to predators. Any species removals by predation makes the spread of growing S. errata colonies easier, opening little room for other species to colonize and persist (Vieira et al. 2018a). If predators are excluded, S. errata cannot outcompete ascidians, as already shown by Oricchio and Dias (2020). The natural spatial variation of predation pressure and colonization history by functionally different founders may thus lead to variable community structure, with high species turnover, even over small spatial scales.
The hierarchy of competition abilities among the groups manipulated in this study is fully understood when considering resistance against predators. Colonial ascidians are better competitors than encrusting bryozoans, but differences in their resistance against predators impose some transience to this hierarchy (Buss and Jackson 1979), with bryozoans dominating communities when consumptive interactions reduce the capacity of ascidians to monopolize space (Oricchio and Dias 2020). Predation-driven transient competition, combined to variable colonization history related to founder identity, increase the potential outcomes of species interactions and make possible additional community states and larger fluctuations of overall diversity (Fig. 4). Careful trait-based inference on group-specific competition ability and resistance to predators, and a more probabilistic approach when accounting for the effects of historical contingencies (i.e., the timing of competitive hierarchy and escape windows from predation pressure), will ultimately allow better predictions of community shifts over space and time under given environmental conditions. The variable community assembly scenarios we report here are a result of the pathways produced by different colonization histories imposed by variable founder functional identities (Fukami 2015), which changed species abilities to compete and escape predation through the different stages of developing assemblages.
We also show that colonization contingencies related to founder identity delivers direct effects of inhibition and facilitation of subsequent species, and also indirect effects by modulating later effects of predation on fouling organisms settling at advanced stages of community assembly. We highlight that colonization history and species interactions must be equally considered for a better understanding of the mechanisms underlying the patterns of community assembly, not only in an additive way, but rather as interactive drivers. Although our study was conducted with marine organisms growing over artificial habitats, we believe that our results may also apply to natural systems where potential founders are functionally variable (Airoldi 2000; Antoniadou et al. 2011) and predation is an equally or even more important driver shaping communities (Rodemann and Brandl 2017; Freestone et al. 2020; Janiak et al. 2020; Janiak and Branson 2021). For instance, algae and sponges are the most likely founding species in other marine hard-bottom habitats, while markedly varying on their capacity to monopolize space (Aued et al. 2018) and to produce defenses against predation (Hay 1996; Rohde et al. 2015). Seemingly, founder terrestrial plants may either facilitate or inhibit the establishment of other species (Callaway and Walker 1997), and their ability to deter herbivores may also vary (Cárdenas et al. 2014; Hanley et al. 2007).
We conclude by suggesting critical questions to be considered in any attempt to understand the mechanisms underlying community dynamics: (I) can colonization contingencies related to functional variability in founder identity take place? (II) Is there any competitive hierarchy among the dominant species? (III) Do dominant species show any differential resistance against predators or other environmental conditions that may impose transience to the system? (IV) How may founder identity affect later-stage species interactions (e.g., competition and predation) over community assembly? A detailed and combined appraisal of these issues may greatly contribute to a more integrated understanding of community dynamics over space and time.
Data availability
Data are available on the Figshare repository—https://doi.org/10.6084/m9.figshare.13335332.
Code availability
Not applicable.
References
Adler PB, HilleRisLambers J, Levine JM (2007) A niche for neutrality. Ecol Lett 10:95–104. https://doi.org/10.1111/j.1461-0248.2006.00996.x
Airoldi L (2000) Responses of algae with different life histories to temporal and spatial variability of disturbance in subtidal reefs. Mar Ecol Prog Ser 195:81–92
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Antoniadou C, Voultsiadou E, Chintiroglou C (2011) Seasonal patterns of colonization and early succession on sublittoral rocky cliffs. J Exp Mar Biol Ecol 403:21–30
Aued AW, Smith F, Quimbayo JP, Cândido DV, Longo GO, Ferreira CEL, Witman JD, Floeter SR, Segal B (2018) Large-scale patterns of benthic marine communities in the Brazilian Province. PLoS ONE 13:e0198452
Berlow EL (1997) From canalization to contingency: historical effects in a successional rocky intertidal community. Ecol Monogr 67:435–460. https://doi.org/10.2307/2963465
Bonnici L, Evans J, Borg JA, Schembri PJ (2012) Biological aspects and ecological effects of a bed of the invasive non-indigenous mussel Brachidontes pharaonis (Fischer P., 1870) in Malta. Mediterr Mar Sci 13:153–161. https://doi.org/10.12681/mms.32
Breitburg DL (1985) Development of a subtidal epibenthic community: factors affecting species composition and the mechanisms of succession. Oecologia 65:173–184. https://doi.org/10.1007/BF00379215
Buss LW (1979) Habitat selection, directional growth and spatial refuges: why colonial animals have more hiding places. In: Larwood G, Rosen B (eds) Biology and systematics of colonial organisms. Academic Press, London, pp 459–497
Buss LW, Jackson JBC (1979) Competitive networks: nontransitive competitive relationships in cryptical coral reef environments. Am Nat 113:223–234
Cadotte MW, Arnillas CA, Livingstone SW, Yasui AE (2015) Predicting communities from functional traits. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2015.07.001
Callaway RM, Walker LR (1997) Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78:1958–1965
Cárdenas RE, Valencia R, Kraft NJB, Argoti A, Dangles O (2014) Plant traits predict inter- and intraspecific variation in susceptibility to herbivory in a hyperdiverse Neotropical rain forest tree community. J Ecol 102:939–952
Chang C, Marshall D (2016) Quantifying the role of colonization history and biotic interactions in shaping communities—a community transplant approach. Oikos 126:204–211. https://doi.org/10.5061/dryad.nt6bq
Chase JM (2007) Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci USA 104:17430–17434
Chase JM (2010) Stochastic community assembly causes higher biodiversity in more productive environments. Science 328:1388–1391
Chase JM, Myers JA (2011) Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B 366:2351–2363. https://doi.org/10.1073/pnas.0704350104
Cifuentes MA, Krueger I, Dumont CP, Lenz M, Thiel M (2010) Does primary colonization or community structure determine the succession of fouling communities? J Exp Mar Biol Ecol 395:10–20. https://doi.org/10.1016/j.jembe.2010.08.019
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E Ltd., Plymouth
Cleland EE, Esch E, McKinney J (2015) Priority effects vary with species identity and origin in an experiment varying the timing of seed arrival. Oikos 124:33–40. https://doi.org/10.1111/oik.01433
Coles AL, Brown EK (2007) Twenty-five years of change in coral coverage on a hurricane impacted reef in Hawaii: the importance of recruitment. Coral Reefs 26:705–717. https://doi.org/10.1007/s00338-007-0257-3
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 11:1119–1144
Crean AJ, Marshall DJ (2008) Gamete plasticity in a broadcast spawning marine invertebrate. Proc Natl Acad Sci USA 105:13508–13513. https://doi.org/10.1073/pnas.0806590105
Dean TA, Hurd LE (1980) Development in an estuarine fouling community: the influence of early colonists on later arrivals. Oecologia 46:295–301. https://doi.org/10.1007/BF00346255
Dias GM, Vieira EA, Pestana L, Marques AC, Karythis S, Jenkins SR, Griffith K (2020) Calcareous defense structures of prey mediate the effects of predation and biotic resistance towards the tropics. Divers Distrib 26:1198–1210. https://doi.org/10.1111/ddi.13020
Díaz S, Lavorel S, McIntyre S, Falczuk V, Casanoves F, Milchunas DG, Skarpe C, Rusch G, Sternberg M, Noy-Meir I, Landsberg J, Zhang W, Clark H, Campbell BD (2007) Plant trait response to grazing—a global synthesis. Glob Change Biol 13:313–341
Dini-Andreote F, Stegen JC, Elsas JD, Salles JF (2015) Disentangling mechanisms that mediate the balance between stochastic and deterministic process in microbial succession. Proc Natl Acad Sci USA 112:E1326–E1332. https://doi.org/10.1073/pnas.1414261112
Elwood MDF, Manica A, Foster WA (2009) Stochastic and deterministic processes jointly structure tropical arthropod communities. Ecol Lett 12:277–284. https://doi.org/10.1111/j.1461-0248.2009.01284.x
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks CA
Freestone AL, Osman RW, Ruiz GM, Torchin M (2011) Stronger predation in the tropics shapes species richness patterns in marine communities. Ecology 92:983–993. https://doi.org/10.1890/09-2379.1
Freestone AL, Carroll EW, Papacostas K, Ruiz GM, Torchin ME, Sewall BJ (2020) Predation shapes invertebrate diversity in tropical but not temperate seagrass communities. J Anim Ecol. https://doi.org/10.1111/1365-2656.13133
Fukami T (2015) Historical contingency in community assembling: integrative niches, species pools, and priority effects. Annu Rev Ecol Evol Syst 46:1–23. https://doi.org/10.1146/annurev-ecolsys-110411-160340
Greene CH, Shoener A (1982) Succession on marine hard substrata: a fixed lottery. Oecologia 55:289–297. https://doi.org/10.1007/BF00376914
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8:157–178
Hay ME (1996) Marine chemical ecology: what’s known and what’s next. J Exp Mar Biol Ecol 200:103–134
Hiebert LS, Vieira EA, Dias GM, Tiozzo S, Brown FD (2019) Colonial ascidians strongly preyed upon, yet dominate the substrate in a subtropical fouling community. Proc R Soc Lond B 286:20190396. https://doi.org/10.1098/rspb.2019.0396
Hu W, Zhang Q, Tian T, Li D, Cheng G, Mu J, Wu Q, Niu F, Stegen JC, An L, Feng H (2015) Relative roles of deterministic and stochastic processes in driving the vertical distribution of bacterial communities in a permafrost core from the Winghai-Tibet Plateau, China. PLoS ONE 10:e0145747. https://doi.org/10.1371/journal.pone.0145747
Jackson JBC (1977) Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Am Nat 111:743–768
Jackson JBC, Buss LW (1975) Allelopathy and spatial competition among coral reef invertebrates. Proc Natl Acad Sci USA 72:5160–5163. https://doi.org/10.1073/pnas.72.12.5160
Jackson JBC, Hughes TP (1985) Adaptive strategies of coral-reef invertebrates. Am Sci 73:265–274
Janiak DS, Freeman CJ, Seemann J, Campbell JE, Paul VJ, Duffy JE (2020) Spatial variation in the effects of predator exclusion on epifaunal community development in seagrass beds. Mar Ecol Prog Ser 649:21–33
Janiak DS, Branson D (2021) Impacts of habitat and predation on epifaunal communities from seagrass beds and artificial structures. Mar Environ Res. https://doi.org/10.1016/j.marenvres.2020.105225
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Jurgens L, Gaylord B (2016) Edge effects reverse facilitation by a widespread foundation species. Sci Rep 6:37573. https://doi.org/10.1038/srep37573
Kay AM, Keough MJ (1981) Occupation of patches in the epifaunal community on pier pilings and the bivalve Pinna bicolor at Edithburgh, South Australia. Oecologia 48:123–130. https://doi.org/10.1007/BF00346998
Koehl MAR (1982) The interaction of moving water and sessile organisms. Sci Am 274:124–134
Koehl MAR (1984) How do benthic organisms withstand moving water? Am Zool 24:57–70. https://doi.org/10.1093/icb/24.1.57
Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269. https://doi.org/10.1016/j.cageo.2005.11.009
Lidgard S (2008) Predation on marine bryozoan colonies: taxa, traits and trophic groups. Mar Ecol Prog Ser 359:117–131. https://doi.org/10.3354/meps07322
López DP, Freestone AL (2021) History of co-occurrence shapes predation effects on functional diversity and structure at low latitudes. Funct Ecol. https://doi.org/10.1111/1365-2435.13725
Maren IE, Kapfer J, Aarrestad PA, Grytnes J, Vandvik V (2018) Changing contributions of stochastic and deterministic processes in community assembly over a successional gradient. Ecology 99:148–157. https://doi.org/10.1002/ecy.2052
Marfenin NN (1997) Adaptation capabilities of marine modular organisms. Hydrobiologia 355:153–158. https://doi.org/10.1023/A:1003069831832
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185
Mittelbach GG, Schemske DW (2015) Ecological and evolutionary perspectives on community assembly. Trends Ecol Evol 30:241–247. https://doi.org/10.1016/j.tree.2015.02.008
Nandakumar K, Tanaka M, Kikuchi T (1993) Interspecific competition among fouling organisms in Tomioka Bay, Japan. Mar Ecol Prog Ser 94:43–50. https://doi.org/10.3354/meps094043
Oricchio FT, Dias GM (2020) Predation and competition interact to determine space monopolization by non-indigenous species in a sessile community from the southwestern Atlantic Ocean. Aquat Invasions 15:127–139. https://doi.org/10.3391/ai.2020.15.1.09
Oricchio FT, Flores AAV, Dias GM (2016a) The importance of predation and predator size on the development and structure of a subtropical fouling community. Hydrobiology 776:209–219
Oricchio FT, Pastro G, Vieira EA, Flores AAV, Gibran FZ, Dias GM (2016b) Distinct community dynamics at two artificial habitats in a recreational marina. Mar Environ Res. https://doi.org/10.1016/j.marenvres.2016.09.010
Osman RW, Whitlatch RB (2004) The control of the development of a marine benthic community by predation on recruits. J Exp Mar Biol Ecol 311:117–145. https://doi.org/10.1016/j.jembe.2004.05.001
Perea R, Gil L (2014) Shrubs facilitating seedling performance on ungulate-dominated systems: biotic versus abiotic mechanisms of plant facilitation. Eur J for Res 133:525–534. https://doi.org/10.1007/s10342-014-0782-x
Pisut DP, Pawlik JR (2002) Anti-predatory chemical defenses of ascidians: secondary metabolites or inorganic acids? J Exp Mar Biol Ecol 270:203–214. https://doi.org/10.1016/S0022-0981(02)00023-0
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Viena
Rodemann JR, Brandl SJ (2017) Consumption pressure in coastal marine environments decreases with latitude and in artificial vs. natural habitats. Mar Ecol Prog Ser 574:167–179
Rohde S, Nietzer S, Schupp PJ (2015) Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE 10:e0132236
Romme WH, Whitby TG, Tinker DB, Turner MG (2016) Deterministic and stochastic processes lead to divergence in plant community 25 years after the 1988 Yellowstone fires. Ecol Monog 86:327–351. https://doi.org/10.1002/ecm.1220
Russ GR (1980) Effects of predation by fishes, competition, and structural complexity of the substratum on the establishment of a marine epifaunal community. J Exp Mar Biol Ecol 42:55–69. https://doi.org/10.1016/0022-0981(80)90166-5
Sammarco PW, Coll JC, Barre SL, Willis B (1983) Competitive strategies of soft corals (Coelenterata: Octocorallia): allelopathic effects on selected scleractinian corals. Coral Reefs 1:173–178. https://doi.org/10.1007/BF00571194
Sebens KP, Lewis JR (1985) Rare events and population structure of the barnacle Semibalanus cariosus (Pallas, 1778). J Exp Mar Biol Ecol 87:55–65
Smith RS, Blaze JA, Byers JE (2020) Negative indirect effects of hurricanes on recruitment of range-expanding mangroves. Mar Ecol Prog Ser 644:65–74. https://doi.org/10.3354/meps13351
Sousa WP (1984) Intertidal mosaics: patch size, propagule availability, and spatially variable patterns of succession. Ecology 65:1918–1935. https://doi.org/10.2307/1937789
Stoecker D (1980a) Chemical defenses of ascidians against predation. Ecology 61:1327–1334. https://doi.org/10.2307/1939041
Stoecker D (1980b) Relationships between chemical defense and ecology in benthic ascidians. Mar Ecol Prog Ser 3:257–265. https://doi.org/10.3354/meps003257
Sutherland JP (1978) Functional roles of Schizoporella and Styela in the fouling community at Beaufort North Carolina. Ecology 59:257–264. https://doi.org/10.2307/1936371
Sutherland JP, Karlson RH (1977) Development and stability of the fouling community at Beaufort, North Carolina. Ecol Monog 47:425–446. https://doi.org/10.2307/1942176
Urban MC, De Meester L (2009) Community monopolization: local adaptation enhances priority effects in an evolving metacommunity. Proc Royal Soc Lond B 276:4129–4138. https://doi.org/10.1098/rspb.2009.1382
Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85:183–206. https://doi.org/10.1086/652373
Vieira EA, Duarte LFL, Dias GM (2012) How the timing of predation affects composition and diversity of species in a marine sessile community? J Exp Mar Biol Ecol 412:126–133. https://doi.org/10.1016/j.jembe.2011.11.011
Vieira EA, Dias GM, Flores AAV (2016) Effects of predation depend on successional stage and recruitment rate in shallow benthic assemblages of the Southwestern Atlantic. Mar Biol 163:1–12. https://doi.org/10.1007/s00227-016-2872-4
Vieira EA, Flores AAV, Dias GM (2017) Current conditions and colonization history asymmetrically shape the organization of shallow sessile communities after simulated state shift. Mar Environ Res 133:24–31. https://doi.org/10.1016/j.marenvres.2017.11.005
Vieira EA, Flores AAV, Dias GM (2018a) Persistence and space preemption explain species-specific founder effects on the organization of marine sessile communities. Ecol Evol 8:3430–3442. https://doi.org/10.1002/ece3.3853
Vieira EA, Dias GM, Flores AAV (2018b) Adding early-stage engineering species affects advanced-stage organization of shallow-water fouling assemblages. Hydrobiologia 818:211–222. https://doi.org/10.1007/s10750-018-3612-1
Vogt J, Lin Y, Pranchai A, Frohberg P, Mehlig U, Berger U (2014) The importance of conspecific facilitation during recruitment and regeneration: a case study in degraded mangroves. Basic Appl Ecol 15:651–660. https://doi.org/10.1016/j.baae.2014.09.005
Walters LJ, Wethey DS (1996) Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: the important of refuge dimensions and adult morphology. Mar Ecol Prog Ser 137:161–171. https://doi.org/10.3354/meps137161
Wernberg T, Smale DA, Tuya F, Thomsen MS, Langlois TJ, Bettignies T, Bennet S, Rousseaux CS (2012) An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat Clim Change. https://doi.org/10.1038/NCLIMATE1627
Willig MR, Moulton MP (1989) The role of stochastic and deterministic processes in structuring neotropical bat communities. J Mammal 70:323–329. https://doi.org/10.2307/1381514
Yodzis P (1986) Competition, mortality and community structure. In: Diamond J, Case TJ (eds) Community ecology. Harper & Row, New York, pp 480–549
Acknowledgements
The authors thank the staff at Centro de Biologia Marinha (CEBIMar-USP) and Yacht Club Ilhabela (YCI) for field assistance. Marcel O. Tanaka, Guilherme H. Pereira Filho, Rafael S. Oliveira, José R. Trigo, and Fabiane Gallucci provided helpful suggestions on early versions of this manuscript. EAV thanks São Paulo Research Foundation-FAPESP for the award of a PhD scholarship (#2012/18432-1). GMD and AAVF are supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, (308268/2019‐9, 301601/2018-6) and by the São Paulo Research Foundation-FAPESP (#2016/17647-5 and #2019/15628-1). This is a contribution of the Research Centre for Marine Biodiversity of the University of São Paulo (NP-Biomar/USP).
Funding
This study was funded by São Paulo Research Foundation with a PhD scholarship awarded to Edson A. Vieira (#2012/18432-1). GMD is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, (308268/2019‐9) and by São Paulo Research Foundation-FAPESP (#2016/17647-5 and #2019/15628-1).
Author information
Authors and Affiliations
Contributions
EAV, AAVF, and GMD formulated the idea, designed the experiments, conceived statistical analyses, and wrote the manuscript. EAV performed the experiments, field work, and data analyses.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Peter S Petraitis .
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vieira, E.A., Flores, A.A.V. & Dias, G.M. Colonization history meets further niche processes: how the identity of founders modulates the way predation structure fouling communities. Oecologia 196, 1167–1178 (2021). https://doi.org/10.1007/s00442-021-04996-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-04996-7