Abstract
Restoration plantings are frequently occupied by native wildlife, but little is known about how planting attributes influence breeding by, and persistence of, fauna populations. We monitored breeding success of woodland birds in restoration plantings in a fragmented agricultural landscape in south-eastern Australia. We documented nest fate and daily nest survival (DSR) in plantings and remnant woodland sites. We analysed the influence on breeding success of patch attributes (size, shape, type) compared to other potentially influential predictors such as nest-site and microhabitat variables. We found that, in general, patch attributes did not play a significant role in determining breeding success for woodland birds. However, we examined a subset of species of conservation concern, and found higher DSR for these species in restoration plantings than in similarly sized woodland remnants. We also found negative effects of patch size and linearity on DSR in species of conservation concern. The primary cause of nest failure was predation (91%). We used camera trap imagery to identify the most common nest predators in our study sites: native predatory bird species, and the introduced red fox (Vulpes vulpes). Our findings are further evidence of the value of restoration plantings and small habitat patches for bird populations in fragmented agricultural landscapes. We recommend controlling for foxes to maximise the likelihood that restoration plantings and other woodland patches in Australia support breeding populations of woodland birds. More broadly, our study highlights the importance of taking a detailed, population-oriented approach to understanding factors that influence habitat suitability for fauna of conservation concern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss due to agricultural expansion is a key threat to biodiversity in many parts of the world (Maxwell et al. 2016; Egli et al. 2018). Ongoing loss of habitat in fragmented agricultural landscapes is making it increasingly difficult for many organisms to persist and maintain viable populations (Bennett et al. 2015; Haddad et al. 2015; Stanton et al. 2018). In an attempt to address these problems, there are increasing efforts to replant native vegetation in agricultural landscapes in many parts of the world. Ecological tree plantings—hereafter referred to as “restoration plantings”—collectively comprise millions of hectares of planted vegetation, costing billions of dollars to establish and maintain (Kimball et al. 2015; Crouzeilles et al. 2016). They are often implemented as a specific conservation strategy to replace lost habitat for threatened and declining fauna (McAlpine et al. 2016; Catterall 2018; Lindenmayer et al. 2018a). For example, in south-eastern Australia, over 90% of box-gum grassy woodland habitat has been lost (Thiele and Prober 2000), and woodland birds in this region have suffered substantial population declines (Barrett et al. 2007; Rayner et al. 2014). Consequently, woodland birds are frequently considered among the key beneficiaries of restoration plantings in south-eastern Australia (Belder et al. 2018).
There is evidence suggesting that many species of woodland bird will readily occupy restoration plantings (Barrett et al. 2008; Lindenmayer et al. 2010; Debus et al. 2017). Studies examining woodland bird responses to restoration plantings typically use pattern data such as the presence and abundance to infer habitat quality. Previous research has offered insights into colonisation and extinction patterns (Barrett et al. 2008; Mortelliti and Lindenmayer 2015), changes in bird community composition in plantings over time (Mac Nally 2008; Lindenmayer et al. 2016, 2018c; Debus et al. 2017), and occupancy trends relating to site type, habitat structure, and composition (Martin et al. 2011; Munro et al. 2011; Ikin et al. 2018). However, do patch attributes have the same influence on breeding success as they do on site occupancy? Few studies have investigated breeding success in restoration plantings, and little is known about the role of restoration plantings in supporting successful breeding by woodland birds.
The presence of a species in a restoration planting does not necessarily mean that the site is supporting successful breeding of that species. Previous work has found that the relative abundance of woodland bird species is not necessarily correlated with their degree of breeding activity (Belder et al. 2019). For restoration plantings to support breeding populations of woodland birds, they must provide adequate resources and quality habitat to encourage persistence of individuals in a site, and to enable resident individuals to breed successfully (Arlt and Pärt 2007; Flockhart et al. 2016). This is an important outcome if restoration plantings are to be widely implemented as a conservation strategy (Ruiz-Jaen and Aide 2005).
In this study, we focus on breeding success as an indicator of habitat quality in restoration plantings and remnant woodland patches. Breeding success is a key measure of the productivity and quality of a habitat patch (Hinsley et al. 2008; Milligan and Dickinson 2016). By assessing whether successful breeding is occurring, we can begin to assess the extent to which a habitat patch is supporting the species that it is intended to help conserve. Moreover, identifying site attributes (e.g. size, shape) that best support successful breeding facilitates conservation planning, and has the potential to improve the cost-effectiveness of restoration plantings as a conservation strategy.
It is also important to identify the most common causes of breeding failure in restoration plantings. For example, low nesting success could be due to an introduced predator that thrives in fragmented agricultural landscapes, such as the red fox (Vulpes vulpes) in Australia (Braysher 2017). If this is the case, then an otherwise good-quality restoration planting may never support species that are vulnerable to fox predation. However, this type of threat, once identified, could be readily addressed in management plans. Conversely, if nest predation is low but birds are abandoning nests or failing to fledge their young, it may indicate that resource limitation is the primary factor influencing breeding success and survival (Zanette et al. 2000). In this case, more detailed studies might establish what is driving resource limitation. For example, a lack of suitable nesting sites (exposure, competition), food shortage, or perhaps inefficient foraging strategies due to home ranges that are constrained by patch geometry or landscape context.
Research objectives
The primary aim of this study was to determine whether restoration plantings are able to support breeding populations of woodland birds. We used two different indicators of breeding success: nest fate and daily nest survival. Specifically, we posed the following three questions:
Question 1. How does breeding success in restoration plantings compare to breeding success in remnant woodland patches?
We compared breeding success (nest fate and daily nest survival) in restoration plantings and similarly sized woodland remnants. We used larger woodland remnants, such as travelling stock reserves, as “reference” sites. Belder et al. (2019) found equal levels of breeding activity in restoration plantings and woodland remnants in the study area. We, therefore, predicted that breeding success in plantings would be similar to that in remnants.
Question 2. Are patch attributes such as size, shape and type important determinants of breeding success in plantings and remnant woodland patches?
We used a model-selection approach to compare the influence of patch attributes (size, shape, type) with other variables that may influence breeding success, including nest-site variables (distance to edge of patch, height off ground, concealment), and microhabitat variables (shrub cover, ground layer composition). A previous study by Belder et al. (2019) identified a negative relationship between patch size and breeding activity, and a positive relationship between planting age and breeding activity. We expected these findings to be reflected in our study of breeding success, and postulated that patch attributes would significantly influence breeding success.
Question 3. What are the primary causes of nest failure in restoration plantings and woodland remnants?
We sought to identify the reasons for nest failure in restoration plantings, and establish whether the same processes are responsible for nest failure in woodland remnants. We predicted that predation would be the leading cause of nest failure in all sites, as it is the primary driver of nest failure in most bird communities (Belder et al. 2018). We also sought to quantify whether major nest-predators differ between patch types. Based on research conducted in a similar study region (Okada et al. 2017), and a recent review of nest-predators in Australia (Fulton 2019), we expected the dominant predators of woodland bird nests to be predatory bird species, including ravens (Corvus spp.), butcherbirds (Cracticus spp.), and currawongs (Strepera spp.). We also expected the eastern brown snake (Pseudonaja textilis) to be a common nest-predator in restoration plantings, as they have been detected more frequently in plantings than in similarly sized woodland remnants in our study region (Cunningham et al. 2007). Snakes have been identified as important nest-predators in Australia (Fulton 2019) and internationally (Weatherhead and Blouin-Demers 2004).
Approach
Our study was conducted over two breeding seasons and used real, active bird nests to quantify breeding success and nest predation. The majority of previous studies in Australian landscapes, including in our study region, have used indicators of breeding activity as a proxy for breeding success (Barrett et al. 2008; Selwood et al. 2009; Mac Nally et al. 2010; Belder et al. 2019). While such indirect measures are an important step away from traditional diversity and abundance measures, they cannot accurately represent breeding success or identify reasons for breeding failure. We document, for the first time, nesting success, daily nest survival, and primary predators of woodland birds breeding in restoration plantings in a fragmented agricultural landscape.
Materials and methods
Study area
We conducted our study in the South-west Slopes bioregion of New South Wales, Australia. The region is part of Australia’s sheep-wheat belt and has been extensively cleared of native vegetation, with as little as 0.1% of the original temperate woodland remaining in intact condition (Thiele and Prober 2000). Remnant patches are predominantly white box (Eucalyptus albens)/yellow box (E. melliodora)/Blakely’s red gum (E. blakelyi) grassy woodland, a critically endangered ecological community (NSW OEH 2016). Patches of red stringybark (E. macrorhyncha) woodland and mugga ironbark (E. sideroxylon) woodland are also present.
Study sites
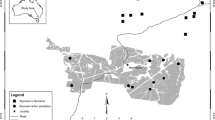
We used spring bird survey data collected over 12 years (see Lindenmayer et al. 2018c) to select a subset of 21 long-term monitoring sites: 12 plantings (1.3–7.7 ha), six similarly sized woodland remnants (2.1–5.8 ha), and 3 large, intact remnants (“reference” sites > 44 ha) (Fig. 1). Plantings were aged between 12 and 25 years. We attempted to control for the effects of competitive exclusion by selecting sites that did not have a history of occupancy by the noisy miner (Manorina melanocephala). Details regarding study site selection are described in Belder et al. (2019).
Map of study sites in the South-west Slopes bioregion of New South Wales, Australia. Map created using ggmap for R (Kahle and Wickham 2013)
Nest searches
We conducted fixed time-per-unit-area surveys (1 h per hectare) to locate nests in study sites over two breeding seasons. We completed two rounds of surveys (October and November) in 2015, and three rounds (September, October, November) in 2016. We searched sites systematically, with search areas designated by size and shape of sites. For sites with a total area less than 3 ha, we searched 1.3 ha within the site—this was equivalent to the size of the smallest site in the study. For sites with a total area greater than 3 ha, we searched 3 ha within the site. We surveyed block sites in a grid fashion, and linear sites along their length until we had searched the desired area (i.e. 1.3 ha or 3 ha). Due to the large geographic spread of sites, we were unable to completely randomise the order of site surveys during each round. However, we ensured that sites were not consistently surveyed at the same time of day. Sites were surveyed at any time of day from dawn to dusk, except during November 2016, when sites were surveyed only in the 4 h post-sunrise and pre-sunset.
Nest monitoring
Once a nest was located, we used flagging tape to mark its position (near to but not at the nest to avoid attracting the attention of predators) and recorded its location using a handheld GPS. Depending on accessibility, we determined the status (i.e. the stage of development) of the nest at discovery by either manual inspection or through observations of parental behaviour. Some nests required multiple visits on different days to ascertain status. We conducted regular checks in person to verify status—every 7–10 days in 2015 and every 3–5 days in 2016. We inspected nests manually or used a nest inspection tool (endoscopy-type camera for dome nests, and mirror on an extendible pole for open cup nests). For nests that were out of arm’s reach or could not be reached by extendible pole, we used behavioural observations to determine status. We observed nests for up to 30 min, or until we recorded activity at the nest and could verify the status. If we could not determine the status within the 30 min observation period, we repeated the observation at the next scheduled visit (3–5 days later in 2016). If we did not record activity in three consecutive visits, we assumed the nest was no longer active. In the later stages of nesting (i.e. when the nest was estimated to be within 5 days of fledging), we did not approach the nest, and used only behavioural observations to determine status. This was to minimise the risk of premature fledging. We considered a nesting attempt to have succeeded if at least one chick fledged.
Where possible, we used fixed motion-sensing wildlife cameras to monitor nests, with the primary aim of detecting nest predation. We used a combination of Bushnell Trophy HD, UOVision UV565HD, and HCO ScoutGuard SG560K black flash cameras. All cameras are triggered by motion within the field of view. To reduce the incidence of false triggers (e.g. by wind-blown foliage), we set camera sensitivity to “low”. We were able to use nest cameras for nests at heights of up to 6 m.
Nest site measurements and microhabitat surveys
For all nests, we recorded a GPS location (accurate to the nearest 2 m), the height of the nest above ground, and the substrate (foliage, branch, woody debris, etc.) in which the nest was built. For nests in the 2016 breeding season, we also recorded concealment (visually estimated at a distance of approximately 10 m, and to the nearest 5%). We used ArcMap (ESRI 2011) to calculate the distance of each nest to the nearest patch edge, where relevant, we also recorded the plant species in which nests were built.
For nests in the 2016 breeding season, we collected microhabitat data around the nest site. We conducted microhabitat surveys when the nest was no longer active (either fledged or failed). At each nest, we used a tape measure to mark out a quadrat measuring 25 m along its diagonal, with the nest at its centre. The two diagonals were aligned north–south and east–west. We visually estimated the proportion of ground cover (to the nearest 1%) and midstorey cover (to the nearest 5%). We chose these microhabitat variables as multiple studies have documented their influence on site occupancy by woodland birds (Seddon et al. 2003; Antos and Bennett 2006; Montague-Drake et al. 2009; Munro et al. 2011).
Statistical analyses
We used a model-selection approach (Burnham and Anderson 2004) to investigate the effects of patch-level, nest-level, and microhabitat variables on nest fate and daily nest survival (Table 1). For clarity, and to address inconsistencies with data collection between years, we modelled data only from nests monitored in 2016. We used generalised linear mixed effects regression models with study site as a random effect. Our response variables were nest fate (binary, where success = 0 and fail = 1), and daily nest survival (DSR). For nest fate and DSR analyses, we excluded nests for which the failure date was uncertain (to the nearest 5 days), most of which were classified as “abandoned”. We included these nests, along with those monitored in 2015, when calculating the total proportion of successful nests, and we report these results in the General findings section of our Results. Due to inherent differences in nest survival, we analysed dome-nesters and cup-nesters separately. We had sufficient data to individually examine one dome-nesting species: the superb fairywren (Malurus cyaneus), and one cup-nesting species: the willie wagtail (Rhipidura leucophrys). We also examined a subset of dome-nesting species of conservation concern (Appendix 1). We did not include nests of introduced species in our study.
We used a comparative model-selection approach, in which we modelled combinations (sets) of variables and used Akaike’s Information Criterion to determine which variables best predicted nest fate and DSR:
-
1.
Patch attributes: type, size (ha), shape (calculated as perimeter/width).
-
2.
Nest site attributes: height above ground, distance to edge of patch, concealment.
-
3.
Microhabitat variables: shrub cover and ground layer composition within 20 m of the nest.
We included date of nest discovery (DATE) as an explanatory variable in all models, as preliminary analyses indicated that date within the breeding season was a significant influence on breeding success. For the first two sets of variables, we fitted models with the variables of interest plus interaction terms. For models including microhabitat variables, we did not include interaction terms. For daily nest survival model selection, we included a model that assumed constant nest survival (null model). We ranked candidate models using Akaike’s Information Criterion corrected for small sample sizes (AICc). We considered models with ΔAICc ≤ 2 as top-ranked models (Burnham and Anderson 2004).
When reporting DSR results, we provide both the sample size (n) and effective sample size (ness) (Rotella et al. 2004; Shaffer and Thompson 2007). Effective sample size is equal to the number of known days survived for each nest plus the number of intervals in which a nest failed (Rotella et al. 2004). For example, a nest that survived for 10 days and then failed between day 10 and day 13 contributes 11 to the study’s effective sample size.
We used the packages ‘lme4’ (Bates et al. 2015) and ‘MuMIn’ (Bartoń 2018) in R version 3.5.2 (R Core Team 2019) to fit and select models for FATE. For DSR calculation and model selection, we used Program MARK (White and Burnham 1999) via the R package ‘RMark’ (Laake 2003). To calculate model estimates and confidence intervals, we used the R packages ‘dplyr’ (Wickham et al. 2019) for FATE and ‘RMark’ for DSR.
Prior to fitting models, we checked all explanatory variables for multi-collinearity using variance inflation factors. We corrected for multi-collinearity by removing large reference sites from models that included both size and type (site type was significantly correlated with site size due to the comparatively large size of reference sites). That is, we included data only from plantings and similarly sized woodland remnants when modelling our response variable against site size and shape. We also scaled and centred our continuous predictor variables for generalised linear mixed modelling.
Results
General
We located 324 woodland bird nests over the course of the 2 years of field study: 89 in 2015, and 235 in 2016. Of these, we were able to successfully track the fate of 222 nests, or 69% of the total number of nests. Of the nests that were tracked successfully, 129 were in plantings (12 sites), 45 were in remnants (six sites), and 48 were in large reference sites (three sites). We analysed nests from 24 different woodland bird species: 11 dome-nesters and 13 cup-nesters (Appendix 1).
Nests were predominately in the lower strata. Mean nest height was 2.2 m (SE = 0.16 m). Cup nests in large reference sites were significantly higher on average than in restoration plantings and similarly sized woodland remnants (p < 0.0001). Site type did not influence nest height for dome nests.
Mean nest success (succeed vs. fail) across all nest types was 33.8%. Success rates were 29.6% for cup-nesters and 38.1% for dome-nesters (Fig. 2).
Proportion of failed woodland bird nests according to nest type. Shaded areas indicate upper and lower 95% confidence intervals. Clustered points indicate frequency of success (0) and failure (1) for each nest type. Data from both 2015 and 2016 were modelled to produce estimates. Plot created using ggplot2 for R (Wickham 2016)
We calculated daily nest survival for 107 dome nests (ness = 2134) and 50 cup nests (ness = 599) (Table 2). As the breeding season progressed, DSR decreased for dome-nesters but increased for cup-nesters (Fig. 3).
Daily nest survival of cup-nesting species (a) and dome-nesting species (b) over the course of the 2016 spring breeding season in the South-west Slopes bioregion, NSW. Probability refers to the likelihood of the nest surviving to the end of the study. Day 1 represents the first day of the study (the first day on which a nest could be discovered). Shaded areas indicate upper and lower 95% confidence intervals. Plot created using ggplot2 for R (Wickham 2016)
Dome-nesters frequently nested in kangaroo thorn (Acacia paradoxa), red box (Eucalyptus polyanthemos), Blakely’s red gum, and Phalaris aquatica (an introduced grass species). Plant species used frequently by cup-nesters included Blakely’s red gum, white box, and kangaroo thorn. Both cup-nesters and dome-nesters nested most often in trees. Dome-nesters also frequently nested in shrubs and woody debris. Cup-nesters rarely built nests in shrubs or woody debris.
We found that in linear sites that were oriented north–south, it was common for nests to be located on the eastern side of the site. This meant that nests were exposed to the warmth of the early morning sun but protected from overheating in the afternoon.
Model selection results
When analysing nest fate, we found that the null model was retained for every assemblage and species of interest, and in every iteration of our analyses (Appendix 2–4). That is, none of the predictors in our candidate models explained the variation in nest fate. We found that analysing daily nest survival produced more conclusive results. When all sites were included (plantings, remnants, large reference sites), the null model was again retained in every instance (Appendix 5). We found that candidate models performed better against the null model when large reference sites were excluded (Table 3). We did not find any conclusive results when analyses were restricted to restoration plantings (Appendix 6). Note that we had sufficient data to examine only dome-nesters and the superb fairywren in restoration plantings.
Effects of patch type
Daily nest survival for species of conservation concern was higher in plantings than in similarly sized woodland remnants (Table 4). Patch type did not influence daily nest survival for any other groups of interest. We did not identify any effect of patch type on nest fate for woodland birds in our study (Appendix 2, Appendix 3).
Importance of patch attributes
For species of conservation concern, daily nest survival in plantings and similarly sized woodland remnants was better predicted by patch attributes than by nest-site or microhabitat variables (Table 3). This was the only instance in which patch attributes outperformed the null model. In addition to the aforementioned effect of patch type, we found that daily nest survival for species of conservation concern decreased with increasing patch size (Table 4). We also found a negative effect of linearity, with lower daily nest survival in more linear sites.
Daily nest survival for cup-nesting species in plantings and similarly sized remnants was best predicted by nest-site variables (Table 3). We found a negative effect of nest height—nests situated higher above the ground were associated with lower survival probabilities (Table 5). Effects estimates for other variables in the model had large standard errors, and were, therefore, not interpretable.
Microhabitat variables were of little importance in determining breeding success of woodland birds in our study (Table 3, Appendix 2–7). Likewise, the age of restoration plantings did not contribute to predicting either nest fate or daily nest survival (Appendix 4, 7).
Causes of nest failure
The primary cause of nest failure was predation, which we identified as the cause of 91% of failed nests. This did not differ significantly between plantings, remnants, or large reference sites. Most other nest failures were attributed to abandonment, usually during the egg stage.
Nest-predators
We monitored 85 nests with cameras, and analysed a total of 308,249 camera trap images. Predation events recorded during our study were most often perpetrated by generalist avian predators, including ravens, the pied currawong (Strepera graculina), and pied butcherbird (Cracticus torquatus) (Table 6). The next most common nest-predator (and the most damaging individual species) identified in restoration plantings and woodland remnants was the red fox (Table 6; Appendix 7). Foxes targeted nests close to the ground, including those of the superb fairywren (Table 6). We also recorded some unexpected nest-predators, including the white-browed babbler (Pomatostomus superciliosus) (see Belder 2018), and common ringtail possum (Pseudocheirus peregrinus). The eastern brown snake was recorded as a nest-predator in a restoration planting on one occasion (Table 6).
Discussion
Our results are empirical evidence that restoration plantings provide suitable breeding habitat for woodland birds, and may eclipse remnant patches in supporting successful breeding of woodland birds. We found that woodland birds bred at least as successfully in restoration plantings as they did in remnant woodland patches and large reference sites. Indeed, species of conservation concern were more likely to breed successfully in restoration plantings than in remnant woodland patches. Other notable findings included negative effects of both patch size and linearity on daily nest survival for species of conservation concern.
Nest survival as measured in our study was somewhat lower than expected, particularly for cup-nesting species (29.6% for cup-nesting species and 38.1% for dome-nesting species). Nest survival for Australian songbirds of the families included in our study average 42.2% for dome-nesting species and 37.7% for cup-nesting species (Remeš et al. 2012). This may indicate that habitat suitability of restoration plantings and remnant patches in our study region is lower for cup-nesters than it is for dome-nesters. Many cup-nesting species are perch-and-pounce ground-foraging species, including the willie wagtail and various robins (Petroicidae). Species in the latter family have been identified as susceptible to population decline, and careful management of the ground layer has been recommended to improve habitat suitability for these species (Recher et al. 2002; Antos and Bennett 2006; Montague-Drake et al. 2009).
A decline in breeding success over the course of the breeding season, as documented for the dome-nester assemblage, is consistent with patterns observed for many bird species worldwide (Arnold et al. 2004). The positive effect of date on DSR that we recorded for cup-nesters was unexpected. Potential explanations include more stable weather conditions later in the season, lower predation risk (particularly by avian predators), or changes in microhabitat variables such as grass cover over the course of the breeding season.
Belder et al. (2019) documented equivalent levels of breeding activity in restoration plantings and woodland remnants, including for species of conservation concern. Our findings regarding breeding success are quantitative evidence that restoration plantings provide valuable habitat in which threatened and declining bird species can persist and breed. They also potentially highlight a need to improve the quality of woodland remnants through restorative actions such as excluding stock or replanting the shrub layer. Some species of conservation concern, such as the brown treecreeper (Climacteris picumnus), rely on habitat features that are present in woodland remnants but take decades to develop in restoration plantings (Vesk et al. 2008). It is for this reason that restoration plantings should be considered complementary to, and not a replacement for, remnant woodland (Cunningham et al. 2008; Lindenmayer et al. 2018d; Ikin et al. 2018).
Previous studies have documented a positive relationship between patch size and reproductive output in birds (e.g. Burke and Nol 2000; Zanette et al. 2000; Zanette and Jenkins 2000; Zanette 2001). This has led to the prevalent view that larger patches are more valuable for woodland birds in fragmented agricultural landscapes. However, Belder et al. (2019) found that breeding activity in the South-west Slopes bioregion decreased with increasing patch size. The results of the present study substantiate this finding. Previous research has described the value of small patches for sustaining wildlife populations (Tulloch et al. 2016; Lindenmayer 2019; Wintle et al. 2019). Our study provides direct evidence that woodland birds are able to breed successfully in small habitat patches. Possible reasons for greater success in small patches include lower abundances of predators and brood parasites in small patches, the dominance of edge-specialists and habitat generalists, and concentration effects (Belder et al. 2019).
While linear patches may provide suitable habitat for some species (as evidenced by our general finding of little influence of linearity on breeding success), our results indicate that species of conservation concern may benefit more from block-shaped sites. This may be one reason why linear sites have previously been found to contain a less diverse species assemblage than block-shaped sites (Kinross 2004; Lindenmayer et al. 2010, 2018b). This is of interest for conservation planning, as it highlights the need to take into account the habitat requirements of different species and assemblages when designing revegetation programs.
The presence of nest height as an explanatory variable in top models for cup-nesters may be a reflection of the dominant predators in the study region—open cup-nests are frequently targeted by avian predators (Okada et al. 2019), which may more easily locate these nests higher up in the canopy. We did not find any evidence that the distance of a nest to the nearest patch edge influenced breeding success. This is suggestive of a lack of edge-effects, which have been thought to decrease the value of small and/or linear habitat patches for birds in fragmented agricultural landscapes (Ewers and Didham 2007; King et al. 2009). However, as discussed earlier, our results showed that species of conservation concern bred more successfully in sites of decreasing linearity. One potential explanation is that linear sites do not facilitate optimal central place foraging, since nesting birds must expend more energy traversing a linear home range than one that is more uniform in shape (Andersson 1978; Bovet and Benhamou 1991; Rosenberg and McKelvey 2016).
It is somewhat surprising that microhabitat variables and planting age contributed little to explaining breeding success in our study. Previous research has documented the influence of variables such as shrub cover and ground layer complexity on site occupancy by woodland birds (Seddon et al. 2003; Antos and Bennett 2006; Montague-Drake et al. 2009; Munro et al. 2011). Belder et al. (2019) also reported increased breeding activity of woodland birds in younger restoration plantings, which the authors postulated was due to a diversity of nest-site choices and foraging opportunities associated with the presence of an intact shrub layer. It is possible that microhabitat variables other than the ones included in this study may have had a greater influence on breeding success.
The high nest-predation rate we recorded during our study is not unprecedented (see Zanette and Jenkins 2000; Guppy et al. 2017), but it is nonetheless of concern for the persistence of woodland bird populations in our study region. Generalist avian predators, including corvids, are often considered among species that have benefited from land clearing and habitat fragmentation in agricultural landscapes worldwide (Andrén 1992; Ford et al. 2001; Fuller et al. 2005). Invasive predators, including foxes, also benefit from increasing agricultural land-use (Graham et al. 2012). Zanette and Jenkins (2000) suggest that decreasing forest cover at the landscape scale is a key factor that has led to increased incidence of nest predation. Measuring landscape-scale vegetation cover was outside the scope of our study, but more than five million hectares of white box/yellow box/Blakely’s red gum grassy woodland has been cleared since European settlement, and less than 10% of this ecological community remains across its historic range (Manning et al. 2011). The low levels of landscape vegetation cover in our study region may be a significant influence on woodland bird population dynamics.
Prior to commencing this study, we predicted that predatory bird species and the eastern brown snake would be the dominant nest-predators in our study sites. While avian predators such as ravens and butcherbirds were indeed responsible for the majority of predation events captured during our study, we also identified another common predator—the introduced red fox. The prevalence of foxes as nest-predators in restoration plantings is cause for concern, and may limit the habitat suitability of plantings for woodland birds that nest in the lower strata or on the ground. These include several threatened and declining species, such as the speckled warbler (Pyrrholaemus sagittatus).
Inferential limitations
Our study has revealed previously undocumented trends in woodland bird breeding success, and provided insights into the capacity of restoration plantings and small habitat patches to support woodland birds. We acknowledge a number of limitations pertaining to the present study, and communicate these here to assist with interpretation.
First, this study was conducted over a short duration. The first field season was a pilot study that enabled collection of nest fate data only, leaving one field season in which we could collect sufficiently detailed data to calculate daily nest survival. Caution is advised when extrapolating from studies of only a year duration (Maron et al. 2005). The field season on which a majority of the data in this paper are based coincided with a year of above average rainfall. Since the productivity of southern temperate woodlands is strongly linked to soil moisture (Watson 2011), it is possible that breeding success in our study region may ordinarily be lower than documented in our study.
Second, the presence of the noisy miner, a hyperaggressive native honeyeater, in fragmented agricultural landscapes is a key threatening process for many woodland bird species (Montague-Drake et al. 2011; Bennett et al. 2015; Maron et al. 2011). The noisy miner harasses small woodland birds, is a known nest-predator, and has been directly implicated in reduced breeding success of woodland bird species (Maron 2007; Maron et al. 2013; Bennett et al. 2015; Beggs et al. 2019). Our study was conducted in the absence of the noisy miner, but it is important to recognise that this species may be present and adversely affect breeding success of woodland birds in restoration plantings or other woodland patches.
Future research directions and management implications
We suggest that future research should focus on identifying the reasons for low breeding success in linear patches, particularly given the popularity of such configured habitat patches in agricultural landscapes. We also recommend more detailed studies on breeding success of individual species. In particular, it would be worth focusing on robins and other declining cup-nesting species to identify reasons for low nest survival and more frequent failure earlier in the breeding season (sensu Zanette et al. 2000). More extensive habitat surveys (as opposed to a focus on the immediate vicinity of the nest) might prove useful in assessing the influence of habitat variables on breeding success. In terms of management, we recommend controlling for foxes to maximise the likelihood that restoration plantings and other woodland patches support breeding populations of woodland birds.
There is still much to be learned about woodland bird population dynamics in restored landscapes, and in fragmented agricultural landscapes generally. We suggest that future studies on the responses of woodland birds (and other fauna) to conservation strategies move beyond pattern data and adopt more detailed, population-oriented approaches such as the one presented in our study. Future research should focus on aspects of habitat quality that are likely to influence population persistence, such as identifying the major threats to woodland bird breeding. We also suggest that future studies be undertaken over longer time periods, to capture inter-annual variation in breeding success and reproductive output. This is particularly relevant in large parts of Australia, where animal populations fluctuate in response to extreme interannual variations in climate and rainfall (Letnic and Dickman 2006; Burbidge and Fuller 2007). Basing management outcomes on multiple years of study is a crucial component of ongoing successful biodiversity conservation. It would be highly beneficial to include studies such as ours in long-term monitoring projects, so that community responses to environmental change can be documented. However, we acknowledge that monitoring breeding success is labour intensive, time-consuming, and costly.
With the emergence of new wildlife-monitoring technologies, including improvements in camera trap technology, we are hopeful that nest-monitoring will become easier and, therefore, more commonplace in bird breeding studies. A camera-trapping method and/or software that could accurately and reliably determine key events in the nesting cycle (completion of building, egg-laying, hatching, nest predation, fledging, and abandonment) could revolutionise our ability to assess breeding success in studies worldwide. This would facilitate the incorporation of breeding studies into long-term monitoring projects, and importantly, in projects that aim to evaluate the success of conservation strategies.
References
Andersson M (1978) Optimal foraging area: size and allocation of search effort. Theor Popul Biol 13:397–409. https://doi.org/10.1016/0040-5809(78)90054-0
Andrén H (1992) Corvid density and nest predation in relation to forest fragmentation: a landscape perspective. Ecology 73:794–804. https://doi.org/10.2307/1940158
Antos MJ, Bennett AF (2006) Foraging ecology of ground-feeding woodland birds in temperate woodlands of southern Australia. Emu 106:29–40
Arlt D, Pärt T (2007) Nonideal breeding habitat selection: a mismatch between preference and fitness. Ecology 88:792–801. https://doi.org/10.1890/06-0574
Arnold JM, Hatch JJ, Nisbet ICT (2004) Seasonal declines in reproductive success of the common tern Sterna hirundo: timing or parental quality? J Avian Biol 35:33–45. https://doi.org/10.1111/j.0908-8857.2004.03059.x
Barrett GW, Freudenberger D, Drew A et al (2008) Colonisation of native tree and shrub plantings by woodland birds in an agricultural landscape. Wildl Res 35:19–32. https://doi.org/10.1071/Wr07100
Barrett GW, Silcocks AF, Cunningham RB et al (2007) Comparison of atlas data to determine the conservation status of bird species in New South Wales, with an emphasis on woodland-dependent species. Aust Zool 34:37–77. https://doi.org/10.7882/AZ.2007.003
Bartoń K (2018) MuMIn: Multi-Model Inference. R package version 1.40.4. https://cran.r-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48
Beggs R, Tulloch AIT, Pierson J et al (2019) Patch-scale culls of an overabundant bird defeated by immediate recolonization. Ecol Appl 29:e01846. https://doi.org/10.1002/eap.1846
Belder DJ (2018) Likely depredation of a superb Fairy-wren Malurus cyaneus nest by an uncommon predator, the White-browed Babbler Pomatostomus superciliosus. Aust F Ornithol 35:146–148. https://doi.org/10.20938/afo35146148
Belder DJ, Pierson JC, Ikin K et al (2019) Is bigger always better? Influence of patch attributes on breeding activity of birds in box-gum grassy woodland restoration plantings. Biol Conserv 236:134–152
Belder DJ, Pierson JC, Ikin K, Lindenmayer DB (2018) Beyond pattern to process: current themes and future directions for the conservation of woodland birds through restoration plantings. Wildl Res 45:473–489. https://doi.org/10.1071/WR17156
Bennett JM, Clarke RH, Thomson JR, Mac Nally R (2015) Fragmentation, vegetation change and irruptive competitors affect recruitment of woodland birds. Ecography 38:163–171. https://doi.org/10.1111/ecog.00936
Bovet P, Benhamou S (1991) Optimal sinuosity in central place foraging movements. Anim Behav 42:57–62. https://doi.org/10.1016/S0003-3472(05)80605-0
Braysher ML (2017) Managing Australia’s pest animals: a guide to strategic planning and effective management. CSIRO Publishing, Clayton South
Burbidge AA, Fuller PJ (2007) Gibson desert birds: responses to drought and plenty. Emu 107:126–134
Burke DM, Nol E (2000) Landscape and fragment size effects on reproductive success of forest-breeding birds in Ontario. Ecol Appl 10:1749–1761
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304. https://doi.org/10.1177/0049124104268644
Catterall CP (2018) Fauna as passengers and drivers in vegetation restoration: a synthesis of processes and evidence. Ecol Manag Restor 19:54–62. https://doi.org/10.1111/emr.12306
Crouzeilles R, Curran M, Ferreira MS et al (2016) A global meta-analysis on the ecological drivers of forest restoration success. Nat Commun 7:11666. https://doi.org/10.1038/ncomms11666
Cunningham RB, Lindenmayer DB, Crane MJ et al (2007) Reptile and arboreal marsupial response to replanted vegetation in agricultural landscapes. Ecol Appl 17:609–619. https://doi.org/10.1890/05-1892
Cunningham RB, Lindenmayer DB, Crane MJ et al (2008) The combined effects of remnant vegetation and tree planting on farmland birds. Conserv Biol 22:742–752
Debus SJS, Martin WK, Lemon JM (2017) Changes in woodland bird communities as replanted woodland matures. Pac Conserv Biol 23:359–371. https://doi.org/10.1071/PC16028
Egli L, Meyer C, Scherber C et al (2018) Winners and losers of national and global efforts to reconcile agricultural intensification and biodiversity conservation. Glob Chang Biol 24:2212–2228. https://doi.org/10.1111/gcb.14076
ESRI (2011) ArcGIS Desktop: Release 10
Ewers RM, Didham RK (2007) The effect of fragment shape and species’ sensitivity to habitat edges on animal population size. Conserv Biol 21:926–936. https://doi.org/10.1111/j.1523-1739.2007.00720.x
Flockhart DTT, Mitchell GW, Krikun RG, Bayne EM (2016) Factors driving territory size and breeding success in a threatened migratory songbird, the Canada Warbler. Avian Conserv Ecol 11:4. https://doi.org/10.5751/ACE-00876-110204
Ford HA, Barrett GW, Saunders DA, Recher HF (2001) Why have birds in the woodlands of Southern Australia declined? Biol Conserv 97:71–88. https://doi.org/10.1016/S0006-3207(00)00101-4
Fuller RJ, Noble DG, Smith KW, Vanhinsbergh D (2005) Recent declines in populations of woodland birds in Britain: a review of possible causes. Br Birds 98:116–143
Fulton G (2019) Meta-analyses of nest predation in temperate Australian forests and woodlands. Austral Ecol 44:389–396. https://doi.org/10.1111/aec.12698
Graham CA, Maron M, McAlpine CA (2012) Influence of landscape structure on invasive predators: feral cats and red foxes in the brigalow landscapes, Queensland Australia. Wildl Res 39:661. https://doi.org/10.1071/WR12008
Guppy M, Guppy S, Marchant R et al (2017) Nest predation of woodland birds in south-east Australia: importance of unexpected predators. Emu 117:92–96. https://doi.org/10.1080/01584197.2016.1258997
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052. https://doi.org/10.1126/sciadv.1500052
Hinsley SA, Hill RA, Bellamy PE et al (2008) Effects of structural and functional habitat gaps on breeding woodland birds: working harder for less. Landsc Ecol 23:615–626
Ikin K, Tulloch AIT, Ansell D, Lindenmayer DB (2018) Old growth, regrowth, and planted woodland provide complementary habitat for threatened woodland birds on farms. Biol Conserv 223:120–128. https://doi.org/10.1016/j.biocon.2018.04.025
Kahle D, Wickham H (2013) ggmap: spatial visualization with ggplot2. R J 5:144–161
Kimball S, Lulow M, Sorenson Q et al (2015) Cost-effective ecological restoration. Restor Ecol 23:800–810. https://doi.org/10.1111/rec.12261
King DI, Chandler RB, Collins JM et al (2009) Effects of width, edge and habitat on the abundance and nesting success of scrub–shrub birds in powerline corridors. Biol Conserv 142:2672–2680. https://doi.org/10.1016/j.biocon.2009.06.016
Kinross CM (2004) Avian use of farm habitats, including windbreaks, on the New South Wales tablelands. Pac Conserv Biol 10:180–192
Laake JL (2003) RMark: an R interface for analysis of capture–recapture data with MARK. R package version 2.2.6. https://CRAN.R-project.org/package=RMark
Letnic M, Dickman CR (2006) Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodivers Conserv 15:3847–3880. https://doi.org/10.1007/s10531-005-0601-2
Lindenmayer D (2019) Small patches make critical contributions to biodiversity conservation. Proc Natl Acad Sci USA 116:717–719. https://doi.org/10.1073/pnas.1820169116
Lindenmayer DB, Blanchard W, Crane M et al (2018a) Biodiversity benefits of vegetation restoration are undermined by livestock grazing. Restor Ecol 26:1157–1164. https://doi.org/10.1111/rec.12676
Lindenmayer DB, Blanchard W, Crane M et al (2018b) Size or quality. What matters in vegetation restoration for bird biodiversity in endangered temperate woodlands? Austral Ecol 43:798–806. https://doi.org/10.1111/aec.12622
Lindenmayer DB, Knight EJ, Crane MJ et al (2010) What makes an effective restoration planting for woodland birds? Biol Conserv 143:289–301
Lindenmayer DB, Lane P, Crane M et al (2018c) Weather effects on birds of different size are mediated by long-term climate and vegetation type in endangered temperate woodlands. Glob Chang Biol 25:675–685. https://doi.org/10.1111/gcb.14524
Lindenmayer DB, Lane PW, Barton PS et al (2016) Long-term bird colonization and turnover in restored woodlands. Biodivers Conserv 25:1587–1603. https://doi.org/10.1007/s10531-016-1140-8
Lindenmayer DB, Michael D, Crane M, Florance D (2018d) Ten lessons in 20 years: insights from monitoring fauna and temperate woodland revegetation. Ecol Manag Restor 19:36–43. https://doi.org/10.1111/emr.12303
Mac Nally R (2008) The lag dæmon: hysteresis in rebuilding landscapes and implications for biodiversity futures. J Environ Manag 88:1202–1211
Mac Nally R, De Vries L, Thomson JR (2010) Are replanted floodplain forests in southeastern Australia providing bird biodiversity benefits? Restor Ecol 18:85–94
Manning A, Wood J, Cunningham R et al (2011) Integrating research and restoration: the establishment of a long-term woodland experiment in south-eastern Australia. Aust Zool 35:633–648. https://doi.org/10.7882/AZ.2011.016
Maron M (2007) Threshold effect of eucalypt density on an aggressive avian competitor. Biol Cons 136:100–107. https://doi.org/10.1016/j.biocon.2006.11.007
Maron M, Grey MJ, Catterall CP et al (2013) Avifaunal disarray due to a single despotic species. Divers Distrib 19:1468–1479. https://doi.org/10.1111/ddi.12128
Maron M, Lill A, Watson DM, Mac Nally R (2005) Temporal variation in bird assemblages: How representative is a one-year snapshot? Austral Ecol 30:383–394. https://doi.org/10.1111/j.1442-9993.2005.01480.x
Maron M, Main A, Bowen M et al (2011) Relative influence of habitat modification and interspecific competition on woodland bird assemblages in eastern Australia. Emu 111:40–51. https://doi.org/10.1071/MU09108
Martin WK, Eldridge D, Murray PA (2011) Bird assemblages in remnant and revegetated habitats in an extensively cleared landscape, Wagga Wagga, New South Wales. Pac Conserv Biol 17:110–120
Maxwell SL, Fuller RA, Brooks TM, Watson JEM (2016) Biodiversity: the ravages of guns, nets and bulldozers. Nature 536:143–145. https://doi.org/10.1038/536143a
McAlpine C, Catterall CP, Mac Nally R et al (2016) Integrating plant- and animal-based perspectives for more effective restoration of biodiversity. Front Ecol Environ 14:37–45
Milligan MC, Dickinson JL (2016) Habitat quality and nest-box occupancy by five species of oak woodland birds. Auk 133:429–438. https://doi.org/10.1642/AUK-15-187.1
Montague-Drake RM, Lindenmayer DB, Cunningham RB (2009) Factors affecting site occupancy by woodland bird species of conservation concern. Biol Conserv 142:2896–2903
Montague-Drake RM, Lindenmayer DB, Cunningham RB, Stein JAR (2011) A reverse keystone species affects the landscape distribution of woodland avifauna: a case study using the Noisy Miner (Manorina melanocephala) and other Australian birds. Landsc Ecol 26:1383–1394. https://doi.org/10.1007/s10980-011-9665-4
Mortelliti A, Lindenmayer DB (2015) Effects of landscape transformation on bird colonization and extinction patterns in a large-scale, long-term natural experiment. Conserv Biol 29:1314–1326. https://doi.org/10.1111/cobi.12523
Munro NT, Fischer J, Barrett GW et al (2011) Bird’s response to revegetation of different structure and floristics—Are “restoration plantings” restoring bird communities? Restor Ecol 19:223–235
NSW OEH (2016) White box yellow box Blakely’s red gum woodland—endangered ecological community listing. https://www.environment.nsw.gov.au/determinations/BoxgumWoodlandEndComListing.htm
Okada S, Lindenmayer DB, Wood JT (2019) Does land use change influence predation of bird nests? Austral Ecol 44:768–776. https://doi.org/10.1111/aec.12747
Okada S, Lindenmayer DB, Wood JT et al (2017) How does a transforming landscape influence bird breeding success? Landsc Ecol 32:1039–1048. https://doi.org/10.1007/s10980-017-0507-x
R Core Team (2019) R: A language and environment for statistical computing
Rayner L, Lindenmayer DB, Gibbons P, Manning AD (2014) Evaluating empirical evidence for decline in temperate woodland birds: a nationally threatened assemblage of species. Biol Conserv 171:145–155. https://doi.org/10.1016/j.biocon.2014.01.029
Recher HF, Davis WE Jr, Calver MC (2002) Comparative foraging ecology of five species of ground-pouncing birds in western Australian woodlands with comments on species decline. Ornithol Sci 1:29–40
Remeš V, Matysioková B, Cockburn A (2012) Data from: Long-term and large-scale analyses of nest predation patterns in Australian songbirds and a global comparison of nest predation rates. Dryad Digit Repos. https://doi.org/10.5061/dryad.0ct6s
Rosenberg DK, McKelvey KS (2016) Estimation of habitat selection for central-place foraging animals. J Wildl Manage 63:1028–1038
Rotella JJ, Dinsmore SJ, Shaffer TL (2004) Modeling nest-survival data: a comparison of recently developed methods that can be implemented in MARK and SAS. Anim Biodivers Conserv 27:187–205
Ruiz-Jaen MC, Aide TM (2005) Restoration success: How is it being measured? Restor Ecol 13:569–577. https://doi.org/10.1111/j.1526-100X.2005.00072.x
Seddon JA, Briggs SV, Doyle SJ (2003) Relationships between bird species and characteristics of woodland remnants in central New South Wales. Pac Conserv Biol 9:95–119
Selwood K, Mac Nally R, Thomson JR (2009) Native bird breeding in a chronosequence of revegetated sites. Oecologia 159:435–446
Shaffer TL, Thompson FR III (2007) Making meaningful estimates of nest survival with model-based methods. Stud Avian Biol 34:84–95
Stanton RL, Morrissey CA, Clark RG (2018) Analysis of trends and agricultural drivers of farmland bird declines in North America: a review. Agric Ecosyst Environ 254:244–254. https://doi.org/10.1016/j.agee.2017.11.028
Thiele KR, Prober SM (2000) Reserve concepts and conceptual reserves: options for the protection of fragmented ecosystems. In: Hobbs RJ, Yates CJ (eds) Temperate eucalypt woodlands in australia: biology, conservation, management and restoration. Surrey Beatty and Sons, Chipping Norton, pp 351–358
Tulloch AIT, Barnes MD, Ringma J et al (2016) Understanding the importance of small patches of habitat for conservation. J Appl Ecol 53:418–429. https://doi.org/10.1111/1365-2664.12547
Vesk PA, Nolan R, Thomson JR et al (2008) Time lags in provision of habitat resources through revegetation. Biol Conserv 141:174–186. https://doi.org/10.1016/j.biocon.2007.09.010
Watson DM (2011) A productivity-based explanation for woodland bird declines: poorer soils yield less food. Emu 111:10–18. https://doi.org/10.1071/MU09109
Weatherhead PJ, Blouin-Demers G (2004) Understanding avian nest predation: Why ornithologists should study snakes. J Avian Biol 35:185–190. https://doi.org/10.1111/j.0908-8857.2004.03336.x
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:S120–S139. https://doi.org/10.1080/00063659909477239
Wickham H, François R, Henry L, Müller K (2019) dplyr: A Grammar of Data Manipulation. R package version 0.8.0. https://CRAN.R-project.org/package=dplyr
Wintle BA, Kujala H, Whitehead A et al (2019) Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc Natl Acad Sci USA 116:909–914. https://doi.org/10.1073/pnas.1813051115
Zanette L (2001) Indicators of habitat quality and the reproductive output of a forest songbird in small and large fragments. J Avian Biol 32:38–46. https://doi.org/10.1034/j.1600-048X.2001.320106.x
Zanette L, Doyle P, Trémont SM (2000) Food shortage in small fragments: evidence from an area-sensitive passerine. Ecology 81:1654–1666
Zanette L, Jenkins B (2000) Nesting success and nest predators in forest fragments: a study using real and artificial nests. Auk 117:445–454
Acknowledgements
We are grateful for the feedback of the reviewers, which greatly improved an earlier version of our manuscript. We acknowledge the contributions of several field assistants, especially Ashwin Rudder, Matt Gale, and Ira Dudley-Bestow. Additional field assistance was provided by Lachlan Duncan, Gary Belder, and Nick Shore. Hayden Stevens assisted with processing camera trap imagery. We extend our thanks and appreciation to the landowners who permitted access to their properties for the study, and to the Riverina Local Land Services for permission to conduct research on land under their jurisdiction. We are grateful to Phil Gibbons and the Riverina Local Land Services for the loan of wildlife cameras. A Stuart Leslie Research Award to DJB enabled the purchase of additional wildlife cameras. Further funding was provided by the Riverina Local Land Services (research grant no. MBG-0315 to DBL), the Australian Federal Government via the National Environmental Science Program’s Threatened Species Recovery Hub, a Margaret Middleton Fund Award (to DJB), and a Lesslie Scholarship (to DJB). DJB was personally supported by an Australian Postgraduate Award. DBL is supported by a range of key donors under the Sustainable Farms project.
Author information
Authors and Affiliations
Contributions
DJB, JCP and DBL conceptualised the study and designed the experiment. DJB conducted the fieldwork, analysed the data, and wrote the manuscript. JCP, KI and DBL provided advice on data analysis and helped edit the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This research was conducted with approval from the Australian National University's Animal Ethics and Experimentation Committee.
Additional information
Communicated by Ola Olsson.
Our research is novel in its use of a mechanistic approach to assess the effectiveness of restoration plantings as a conservation strategy. We highlight the value of small habitat patches for threatened and declining avifauna.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Belder, D.J., Pierson, J.C., Ikin, K. et al. Revegetation and reproduction: do restoration plantings in agricultural landscapes support breeding populations of woodland birds?. Oecologia 192, 865–878 (2020). https://doi.org/10.1007/s00442-020-04611-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04611-1