Abstract
Across resource quality gradients, primary consumers must regulate homeostasis and release of nutrients to optimize growth and fitness. Based primarily on internal body composition, the ecological stoichiometry theory (EST) offers a framework to generalize interspecific patterns of these responses, yet the predictions and underlying assumptions of EST remain poorly tested across many species. We used controlled laboratory feeding experiments to measure homeostasis, nutrient release, and growth across seven field-collected aquatic invertebrate detritivore taxa fed wide resource carbon:nitrogen (C:N) and carbon:phosphorus (C:P) gradients. We found that most invertebrates exhibited strict stoichiometric homeostasis (average 1/H = − 0.018 and 0.026 for C:N and C:P, respectively), supporting assumptions of EST. However, the stoichiometry of new tissue production during growth intervals (growth stoichiometry) deviated − 30 to + 54% and − 145 to + 74% from initial body C:N and C:P, respectively, and across species, growth stoichiometry was not correlated with initial body stoichiometry. Notably, smaller non- and hemimetabolous invertebrates exhibited low, decreasing growth C:N and C:P, whereas larger holometabolous invertebrates exhibited high, often increasing growth C:N and C:P. Despite predictions of EST, interspecific sensitivity of egestion stoichiometry and growth rates to the resource gradient were weakly related to internal body composition across species. While the sensitivity of these patterns differed across taxa, such differences carried a weak phylogenetic signal and were not well predicted by EST. Our findings suggest that traits beyond internal body composition, such as feeding behavior, selective assimilation, and ontogeny, are needed to generalize interspecific patterns in consumer growth and nutrient release across resource quality gradients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homeostasis is a fundamental property of life which describes the ability of a system to maintain consistent internal conditions despite variation in external conditions (Cooper 2008). Characterizing the degree and consequences of homeostasis across living systems is an important step to understand responses to environmental change (Lovelock and Margulis 1974; Meunier et al. 2014). Ecological stoichiometry theory (EST) uses the homeostasis concept to infer organismal responses to varying resource elemental ratios; this “stoichiometric homeostasis” can predict organism feedbacks on the availability of resources through altered uptake, storage, and release of limiting nutrients like nitrogen (N) and phosphorus (P), as well as carbon (C), during metabolism (Sterner and Elser 2002; Frost et al. 2005; Small et al. 2009). For example, EST uses homeostasis to predict the ratios at which organisms selectively retain and release elements to maintain homeostasis of body C:N:P across resource gradients (Frost et al. 2005). Despite its central role in EST, stoichiometric homeostasis has been quantified among few consumer taxa, and EST models often assume consumers do not change body elemental composition across resource stoichiometry gradients (Sterner and Elser 2002). Yet, some consumer taxa can flexibly change their internal body composition (Persson et al. 2010) and the lack of empirical assessments of homeostasis, attributable to a historical focus on a few model organisms as well as methodological challenges among slow-growing and difficult-to-rear taxa with complex life histories (Persson et al. 2010; Halvorson and Small 2016), hampers understanding of species responses to resource stoichiometry, and consequent links between organismal stoichiometry and ecosystem processes (Welti et al. 2017; Atkinson et al. 2017).
The concept of strict homeostasis is focal to EST as the basis of predicting interspecific organismal stoichiometry and nutrient demands for regulation and growth (Fig. 1; Sterner and Elser 2002; Frost et al. 2005; Sperfeld et al. 2017). By definition, strictly homeostatic consumers maintain internal body stoichiometry independent of resource stoichiometry (Sterner and Elser 2002) and, given significant elemental imbalances with their resources, must adjust feeding, assimilation, and excretion to release excess and retain limiting elements (Frost et al. 2005; Liess 2014). Most predictions are based on simplifying assumptions of strict homeostasis and tight correlation of organism initial body stoichiometry with the stoichiometry of tissue production (growth) during an interval of time (Fig. 1a, b). Early EST models predicted that among organisms fed similar resource stoichiometry gradients, taxa of lower body C:P or C:N should subsequently release (egest or excrete and respire) at higher C:P and C:N ratios, and show lower resource sensitivity of release C:P and C:N, expressed as slope responses to resource stoichiometry, due to greater retention of N and P to support N- and P-rich growth (Fig. 1c, d; Sterner 1990; Elser and Urabe 1999). Because lower C:P and C:N taxa should also display greater growth sensitivity to C:P and C:N, these taxa are also expected to increase growth to a greater degree on lower resource C:P and C:N, including due to faster maximal growth rates of P-rich taxa (Fig. 1e, f; Elser et al. 2003; da Ferrão-Filho et al. 2007). Despite a robust theoretical basis offered from many of the above case studies and synthesis of these studies toward foundational concepts of EST (Sterner and Elser 2002), recent empirical tests show mixed support for these predictions and underlying assumptions across taxa (Torres and Vanni 2007; Alves et al. 2010; Hood and Sterner 2014). For example, body N and P contents only weakly predict interspecific animal N and P excretion, perhaps because some taxa deviate from strict homeostasis (Allgeier et al. 2015; Vanni and McIntyre 2016).
Conceptual summary of ecological stoichiometry theory regarding interspecific variation in homeostatic regulation and organismal stoichiometry of two elements, carbon (C) and nutrient X, under X-limited growth. Three taxa of increasing internal C:X composition (species 1, 2, and 3, respectively) are compared. Across C:X gradients, organisms are predicted to exhibit strict homeostasis of body C:X (a), minimal ontogenetic deviation of growth C:X from initial body C:X (b), flexible release C:X that is more sensitive to resource C:X among high C:X taxa (c, d), and increased growth rates on lower C:X resources, as well as greater growth sensitivity of low C:X taxa (e, f)
Organisms may deviate from the above assumptions and predictions of EST either due to non-strict homeostasis, or due to inaccurate characterization of elemental growth demands, diets, and assimilation. Such deviation is important to investigate across taxa, because it may relate to general, non-stoichiometric traits that can improve EST models (Prater et al. 2017; Meunier et al. 2017). First, some taxa may be weakly homeostatic, deviating from strict homeostasis and permitting flexible adjustment of body stoichiometry, leading to storage instead of release of excess elements, such as to increase nutrient use efficiency (Woods et al. 2002; Persson et al. 2010; Benstead et al. 2014). Second, many taxa show ontogenetic shifts during growth, which causes growth stoichiometry over an interval to differ from initial or final body stoichiometry, respectively, measured prior or after the interval (Back and King 2013; Hood and Sterner 2014; Richard and de Roos 2018). These ontogenetic shifts can change both P excretion and growth sensitivity during growth, such as among vertebrates investing in P-rich bone (El-Sabaawi et al. 2016; Stephens et al. 2017). Finally, taxa may deviate from EST predictions when individuals exhibit selective feeding or assimilation, which can weaken expected relationships between the stoichiometry of available resources and growth rates or nutrient release (Hood et al. 2014; Urabe et al. 2018).
Among the studies testing EST predictions across taxa, most either focus on a select few taxa, constraining broader inference, or use exclusively field-collected organisms, permitting less control of confounding variation (but see Hood and Sterner 2014). Additional comparisons across a suite of taxa, reared under controlled laboratory settings, would provide robust assessments of homeostasis and subsequent predictions of EST (Halvorson and Small 2016). Such comparisons are particularly needed from non-model organisms, to test the generality of EST predictions. An understudied trophic group with respect to EST (Martinson et al. 2008), detritivores are slow growing and face significant consumer–resource elemental imbalances compared to well-studied herbivorous zooplankton, and may therefore respond strongly to resource ratio gradients (Cross et al. 2003; Frost et al. 2006; Demi et al. 2018). Moreover, detritivores may deviate from EST predictions due to selective feeding and assimilation of N- and P-rich detrital microbial biomass, potentially confounding EST predictions (Hood et al. 2014; Dodds et al. 2014). Detritivores play crucial roles channeling detrital/microbial energy and nutrients into the food web, and in processing detrital nutrients via production of nutrient wastes (Moore et al. 2004; Zou et al. 2016; Parr et al. 2019). Detritivores thus represent a novel, potentially sensitive, and important trophic group in which to test assumptions and deviation from several predictions of EST.
Here, we tested general predictions of EST among seven field-collected aquatic invertebrate detritivore taxa (Table 1) reared in the laboratory on resource stoichiometry gradients of oak (Quercus stellate) and maple (Acer saccharum) leaf litter. In a recent analysis, we showed that growth, consumption, and assimilation by all taxa changed in response to resource N and P contents and leaf type (Halvorson et al. 2018). We sought to further investigate interspecific differences underlying these findings, presenting stoichiometric homeostasis and ontogenetic shifts of body stoichiometry to examine how general patterns across taxa are related to interspecific egestion stoichiometry and growth rates across the resource gradient. We predicted that (H1) consistent with Fig. 1a, invertebrates would exhibit strict homeostasis, indicating no response of body C:N or C:P to resource C:N or C:P, as is most common among other animals (Persson et al. 2010). However, we predicted (H2) C:P of new tissues (referred to as growth C:P) would exceed initial body C:P, because organisms reduce growth rates as they develop, causing newer tissues to contain less P-rich ribosomal RNA and resulting in lower P tissues during ontogenetic change (Elser et al. 2003; Gillooly et al. 2005; Hood and Sterner 2014), but we predicted that growth C:N would reflect initial body C:N during growth, because invertebrate tissue N contents tend to remain constant during ontogeny (Fig. 1b; Back and King 2013). As a reflection of strict homeostasis and minimal changes in assimilation C:N and C:P across resource gradients, we predicted (H3) egestion C:N and C:P would decrease on lower C:N and C:P diets, as shown previously among three taxa included in this study (Fig. 1c; Halvorson et al. 2015a), and we expected higher C:P and C:N taxa would show greater sensitivity (steeper, higher slopes) of egestion stoichiometry to resource stoichiometry (Fig. 1d). Finally, because growth rates increased on lower C:P and C:N resources across all seven taxa (Halvorson et al. 2018), we further predicted (H4) the slopes would be more sharply negative (greater growth sensitivity) among lower C:N and C:P taxa, compared to higher C:N and C:P taxa (Fig. 1e, f).
Materials and methods
Feeding experiments
We collected recently abscised sugar maple (Acer saccharum) and post oak (Quercus stellate) leaves in Washington County, Arkansas, USA in the fall of 2010, air-dried leaves, and stored them in a greenhouse. These two leaf species were chosen to provide contrasting recalcitrance and responses to increased dissolved nutrient concentrations in association with initial experiments (Scott et al. 2013). Before experiments, we cut maple and oak leaves into 13.5 mm diameter disks for feeding to Pycnopsyche and Tipula. We used whole leaves of each leaf species and tore them into large sections for feeding to Lirceus, Strophopteryx, Allocapnia, Amphinemura, and Lepidostoma. To establish gradients of litter resource stoichiometry, leaves were leached for 2–3 days and underwent microbial conditioning in coarse mesh bags for a minimum of 7 and maximum of 12 weeks in tubs containing 20 L aerated dechlorinated tap water under 1000 µg L−1 N–NO3 as KNO3 and one of four dissolved P amendments as Na2HPO4: 0, 50, 100, or 500 µg P L−1 at room temperature (~ 23 °C). Water was flushed and nutrients re-amended every 2–3 days during conditioning. Leaf litter incubation tubs were inoculated with microbes using mixed detrital slurry collected, homogenized, and subsampled (50 mL of slurry added to each tub) from Mullins and Scull Creeks in Fayetteville, Arkansas.
Conditioned litter was fed ad libitum to each of the seven field-collected shredder-detritivore invertebrate taxa in the laboratory (Table 1). We collected all taxa from Ozark Highlands streams between 2012 and 2014. Prior to each experiment, individuals were collected from the same stream reach, given mixed litter, and returned to the laboratory. We determined initial dry mass from a subset of gut clearance individuals using head capsule width (Pycnopsyche), blotted mass (Tipula), or dry mass across a random subset of individuals of a set range in body length. We cleared guts of subsampled gut clearance individuals by allowing them to sit without food for 24 h prior to freezing, reducing but not completely eliminating the amount of material within the gut. All other experimental individuals were randomly assigned growth chambers (1 or 5 individuals per chamber) containing 100 mL stream water and mesh inserts (0.5 or 1 mm, depending on body size). We used differing densities of individuals to ensure measurable accumulation of egesta (i.e., we needed more individuals from small-bodied taxa) within each growth chamber. Growth chambers were constantly aerated and were assigned litter from one of the two leaf species incubated under one of the four P amendments. We assigned individuals separate leaf species diets to investigate effects of leaf type on responses to nutrients (see Halvorson et al. 2018). Individuals were fed fresh litter every 2–3 days (leaf disks) or every week (whole leaf sections) and growth chamber water was changed every 5 days. On each feeding date, we collected and oven dried (50 °C) a subset of litter to determine C, N, and P contents (see below).
Growth experiments ranged in duration from 14 to 33 days and also differed in temperature, based on the temperatures at which we could successfully rear individuals (see Table 1). At the end of each experiment, individuals underwent 24-h gut clearance and were frozen. Individuals were thawed, subsequently oven dried (50 °C), and weighed to determine dry mass.
Growth and egestion measurements
We conducted trials to measure egestion rates once weekly during each experiment with each taxon. Egestion trials took place between feeding events. At the beginning of trials, growth chambers were provided with fresh filtered stream water, and egesta were allowed to collect below mesh inserts over a 2–3 days period. Egestion trial duration was similar across all taxa. At the end of each egestion trial, accumulated egesta were filtered onto pre-combusted (500 °C, 2 h) and pre-weighed glass fiber filters (1 µm, Pall, Inc.), and oven dried (50 °C) prior to desiccation and weighing. Our method of measuring egestion also captured small fragments of leaf litter derived from feeding activity, which would overestimate egestion rates, but we estimate leaf fragments contributed < 10% of dry mass to fine particulates measured as egesta.
We calculated growth rates based on the change in dry mass during each experiment. We calculated instantaneous growth rates using the equation
where DM represents final or initial dry mass, respectively, and time is experiment duration in days.
Elemental content analysis
Leaf litter samples, egestion filters, and initial and final individuals were measured for C, N and P contents. Leaf litter was ground using a Wig-L-Bug (Crescent Manufacturing, Elgin, IL). Egestion filters were cut in half, and each half was re-weighed and assigned analysis for either C/N or P content. Individual Tipula, Pycnopsyche, and Lepidostoma were homogenized into fine powder using a spatula and subsampled for C/N and P, whereas individuals from all other taxa were randomly assigned whole for either C/N or P analysis, due to insufficient mass to homogenize individuals into powder. All samples were analyzed for C/N contents using a Thermo Flash 2000 elemental analyzer (Thermo Scientific, Inc., Waltham, MA). Sample P contents were determined by combustion at 500 °C for a minimum of 2 h, digestion in hot hydrochloric acid (85 °C, 30 min), and analysis for soluble reactive phosphorus (APHA 2005). All elemental ratios are reported in molar units.
Calculations
We calculated per capita C-, N-, and P-specific egestion rates on each measurement date for each growth chamber. We then calculated average element-specific egestion rates across all egestion trials, providing a mean per capita rate for each chamber, and we used these element-specific rates to determine the average growth chamber C:N and C:P of egestion throughout each experiment.
For each growth chamber, we also calculated total per capita C-, N-, and P-specific growth during the growth interval using the equation
where DM is individual dry mass and QX is the proportion of element X in body tissues among initial or final individuals. We subsequently calculated the C:N and C:P ratios of growth among individuals that showed net positive growth in both C and N or C and P, respectively. Of 378 total growth chambers, 332 (88%) showed survival to experiment end; 257 and 244 growth chambers (65–68%) showed positive growth in C and N or C and P, respectively, permitting calculation of growth C:N and C:P. Some chambers exhibited negative element-specific growth, which indicates net loss of elements during the experiment, such as due to molting, excretion, and respiration. The majority of these growth chambers also exhibited negative instantaneous growth rates (mean − 0.0015 day−1) mostly on high C:N or C:P resources (Figs. S7, S8).
Data analysis
Previous analyses of datasets associated with these experiments showed that leaf species does not affect organismal growth, consumption, or assimilation responses to resource C:N or C:P (Halvorson et al. 2018). For this reason, because EST makes explicit predictions about resource C:N and C:P but not leaf species, and because our primary interest was testing EST across detritivore taxa, the present analysis did not include leaf species as a factor.
To assess H1 that organisms would exhibit strict stoichiometric homeostasis, we calculated C:P and C:N stoichiometric homeostasis coefficients (1/H) for each taxon using the equation
where body C:X and resource C:X represent final measured body C:N or C:P and litter C:N or C:P, respectively (Persson et al. 2010). We conducted this test using least-squares linear regression using a significance threshold of α = 0.10 (Persson et al. 2010). We adjusted α up to 0.10 to increase the sensitivity of detecting shifts of body stoichiometry in response to resource stoichiometry (e.g., deviation from strict homeostasis). All other analyses used a significance threshold of α = 0.05 or lower.
To test H2 that growth C:P but not C:N would deviate from initial body C:P and C:N, respectively, for each taxon we compared mean growth C:N and C:P across all individuals to initial body C:N or C:P using two-tailed, one-sample t tests. We subsequently used Pearson’s correlations to test whether body size, and initial body C:N or C:P, were related to growth C:N or C:P across taxa. Because we found growth stoichiometry diverged from initial body stoichiometry among most taxa (see “Results”), we considered growth C:N and C:P as the more appropriate indicator of elemental demands across taxa, and we therefore used these terms in tests of subsequent hypotheses.
We used general linear models preliminary to ANCOVA, with taxon as a factor and litter C:N or C:P as covariates, to investigate factor × covariate interactions associated with H3 that egestion C:N and C:P sensitivity (slopes) would be steeper and more positive among higher C:P and C:N taxa, and to test H4 that growth sensitivity (slopes) would be more sharply negative among lower C:N and C:P taxa. Prior to analysis, we adjusted growth rates of Strophopteryx and Tipula from 5 and 15 °C, respectively, to rates at 10 °C based on temperature scaling of invertebrate metabolic rates reported by Gillooly et al. (2001).
While conducting analyses preliminary to ANCOVA, we first tested factor × covariate interactions to indicate whether slope responses to resource stoichiometry differed across taxa; where significant, slopes were contrasted pairwise with Bonferroni correction to determine differences across taxa, and we subsequently conducted Pearson’s correlations to test whether slopes correlated with growth C:N or C:P, as well as body size (average individual dry mass). Where factor × covariate interactions were not significant, we removed the factor × covariate interaction term and used ANCOVA to determine whether ANCOVA-adjusted rates or ratios (e.g., growth rates adjusted for effects of resource C:P) differed, using Tukey’s honestly significant difference test to conduct pairwise comparisons across taxa. Response variables and covariates were log transformed as necessary to improve equality of variances and linearity for ANCOVA. All statistical analyses were conducted in R version 3.3.1.
Finally, we investigated the role of phylogeny in observed taxonomic differences in stoichiometric traits based on phylogenetically independent contrasts using the K statistic (Blomberg et al. 2003; González et al. 2018). We constructed a phylogenetic tree among the seven taxa used in our study following the most recently updated phylogeny of Arthropods (Thomas et al. 2000; Misof et al. 2014). Because Arthropod lineages are poorly dated at levels of family and genus divergence, we assumed branch lengths of log10 the number of tips originating from each source node in the phylogeny (Purvis 1995). We used the R packages ‘ape’ (Paradis et al. 2004) and ‘picante’ (Kembel et al. 2010) to calculate K statistics for the mean taxonomic traits growth C:N and C:P, egestion C:N and C:P slopes, growth C:N slopes, and C:P-adjusted growth rates. Statistical significance of phylogenetic signal was determined by comparing to a null model which randomly shuffled taxa across tips of the phylogeny using 1000 permutations.
Results
Resource stoichiometry gradients ranged widely, from overall minimum litter C:N = 26.5 and C:P = 663 to maximum litter C:N = 57.1 and C:P = 4727, with differences driven by changes of leaf litter %N and %P across P amendment levels (Fig. 2, Fig. S1 in Online Resource 1). The range of C:N and C:P gradients differed slightly for each taxon, due to natural variance of leaf litter stoichiometry across incubations prior to feeding. Across the resource stoichiometry gradients, most taxa exhibited strict homeostasis in C:N and C:P (Fig. 2, Table 2, Figs. S2,S3), with the exception of Strophopteryx in C:N (1/H = − 0.274; P = 0.010) and Tipula in C:P (1/H = 0.092; P = 0.055). Across all taxa, mean ± SE 1/H was − 0.018 ± 0.075 (C:N) and 0.026 ± 0.020 (C:P).
Summary linear regressions of final body C:N (a) and C:P (b) of seven invertebrate taxa fed gradients of litter C:N and C:P in the laboratory. Only least-squares regression fits are presented for each taxon. Gray lines indicate taxa exhibiting strict homeostasis (slope indistinguishable from zero, P > 0.10) and black lines indicate taxa exhibiting non-strict homeostasis (slope ≠ 0, P < 0.10). See Table 2 for statistics, Table 1 for abbreviated taxa names, and Figs. S2, S3 for associated scatterplots for each taxon
Growth stoichiometry deviated from initial body stoichiometry among the majority of taxa, often separating holometabolous vs. non- and hemimetabolous taxa and separating taxa by body size (Figs. 3, S4, S9). All six insect taxa exhibited growth C:N divergent from initial body C:N (t test, P < 0.05). Hemimetabolous Plecoptera exhibited lower, consistently decreasing growth C:N, whereas holometabolous Trichoptera and Diptera showed comparatively higher, usually increasing growth C:N (Fig. 3a). Similarly, all taxa except Amphinemura showed growth C:P divergent from initial C:P, with non- and hemimetabolous taxa showing significantly lower growth C:P and holometabolous taxa exhibiting significantly greater growth C:P compared to initial body C:P (Fig. 3b). Across taxa, growth C:N and C:P were not significantly correlated with initial C:N (r = 0.63, P = 0.132) or C:P (r = 0.32, P = 0.489), respectively. However, across taxa, growth C:N and C:P were both positively correlated with body size (P < 0.05), indicating lower growth C:N and C:P among smaller-bodied taxa (Fig. S9).
Scatterplot of mean ± SE initial body and growth C:N (a) and C:P (b) of seven invertebrate taxa fed gradients of litter stoichiometry in the laboratory. Taxa exhibiting significantly different growth vs. initial C:N or C:P are designated by asterisks (t test, P < 0.05). Solid black lines in each panel designate a 1:1 relationship between initial body stoichiometry and the stoichiometry of growth. Across taxa, neither growth C:N nor growth C:P were significantly correlated to initial body C:N (r = 0.63, P = 0.132) or C:P (r = 0.32, P = 0.489), respectively. See Fig. S4 for boxplots and scatterplots of growth C:N and C:P for each taxon
Egestion C:N declined on lower C:N resources among most taxa, with the exception of Strophopteryx (Fig. 4a), resulting in positive C:N egestion slopes (Figs. 4a, S5). Sensitivity (slopes) of egestion C:N to resource C:N differed across taxa (P < 0.001; Table 3) and was significantly different between Strophopteryx and all other taxa, but slopes were only weakly related to growth C:N (r = 0.73, P = 0.062; Fig. 4b). Egestion C:P consistently declined on lower C:P resources (Fig. 4c), signified by consistent positive egestion C:P slopes (Figs. 4c, S6). Similar to C:N, slopes of egestion responses to resource C:P differed across taxa (P < 0.001; Table 3). Lirceus exhibited a significantly lower egestion C:P slope compared to Lepidostoma and Pycnopsyche (Fig. 4d) and slopes were not significantly correlated with growth C:P (r = 0.44; P = 0.318 Fig. 4d). Egestion C:N and C:P slopes were not correlated with body size across taxa (Fig. S9).
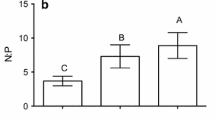
Relationships between egesta C:N and litter C:N (a) and egesta C:P and litter C:P (c) across seven invertebrate taxa. In a, c, least-squares regression fits are displayed as black lines for each taxon (abbreviations as in Table 1) relative to theoretical 1:1 relationships of no selective assimilation in C:N or C:P (light gray line). Slopes differed significantly across taxa (ANCOVA P < 0.05; Table 3) and mean ± SE slopes and growth C:N and C:P of each taxon are presented in b, d, respectively. Letters in (b) and (d) designate statistically different taxa based on Bonferroni-corrected pairwise comparisons (P < 0.0024). Across taxa, C:N and C:P egestion slopes were not significantly related to growth C:N (r = 0.73, P = 0.062) or C:P (r = 0.44, P = 0.318), respectively. See Figs. S5, S6 for associated scatterplots for each taxon
Temperature-corrected growth rates generally increased on low C:N litter, as indicated by negative slopes between growth rates and resource C:N among most taxa (Figs. 5a, b, S7). These growth slopes differed significantly across taxa (P = 0.002; Table 3) and growth of Tipula and Pycnopsyche responded more strongly than growth of Lepidostoma, with Pycnopsyche also responding more strongly than Lirceus (Fig. 5b). Growth sensitivity to resource C:N was not correlated with growth C:N (r = − 0.09, P = 0.857; Fig. 5b). Similarly, growth rates often increased on lower C:P litter (Fig. S8), but unlike growth slopes with resource C:N, growth slopes with resource C:P were statistically similar across taxa (P > 0.05; Table 3). After accounting for uniform effects (slopes) of litter C:P on growth across taxa, growth rates were significantly higher among Allocapnia compared to Tipula, which grew faster than Pycnopsyche, and Pycnopsyche grew faster than Lepidostoma and Strophopteryx (Fig. 5d). ANCOVA C:P-adjusted growth rates (growth rates adjusted for differences in litter C:P) were not correlated with growth C:P across taxa (r = 0.03, P = 0.949). Neither growth C:N slopes nor C:P-adjusted growth rates were correlated with body size across taxa (Fig. S9).
Relationships between growth rates and litter C:N (a) and litter C:P (c) across seven invertebrate taxa. In a, c, least-squares regression fits are displayed for each taxon (abbreviations as in Table 1). In a, slopes differed significantly across taxa (P < 0.001; Table 3) and mean ± SE slopes and growth C:N are presented in b. In c, taxa displayed statistically similar slopes (no factor × covariate interaction) and ANCOVA indicated significant differences in growth rates after adjusting for litter C:P ( P< 0.001), displayed in d. Letters in (b) and (d) designate statistically different taxa based on pairwise comparisons. Across taxa, growth C:N slopes and mean adjusted growth rates were not related to growth C:N (r = − 0.09, P = 0.857) or C:P (r = 0.03, P = 0.949), respectively. See Figs. S7, S8 for associated scatterplots for each taxon
The analysis of phylogenetic signal (K statistics) suggested that phylogeny plays the greatest role in growth C:N differences across taxa (K = 0.79), followed by growth C:P and C:N egestion slopes (K = 0.52 and 0.53, respectively; Fig. S10). Phylogenetic signal was minimal among all other traits, and K did not statistically differ from zero among any of the six traits analyzed across taxa (P > 0.05).
Discussion
Our study shows taxonomic variation in the stoichiometry of homeostasis, growth, and nutrient release of aquatic invertebrates across resource stoichiometry gradients, highlighting support of some principles tempered by weak support of several hypotheses based on EST predictions across species. Broadly, we found support for the assumption of strict stoichiometric homeostasis of most taxa across resource C:N and C:P gradients (H1). This finding may apply broadly across invertebrate detritivores given we used multiple species, and our study was conducted using robust methods including replication in a controlled laboratory setting among organisms at similar initial life stages, as recommended previously (Persson et al. 2010; Halvorson and Small 2016). Despite maintenance of homeostasis across resource gradients, we did not find support of H2 because most taxa exhibited growth C:N and C:P deviating from initial measured body C:N and C:P, indicating ontogenetic changes in body stoichiometry likely explained by species-specific ontogeny and body size. While growth C:N and C:P should provide relative indicators of organism demands for growth, we found these terms weakly predicted interspecific patterns in the sensitivity of organism nutrient release and growth rates across resource gradients (H3 and H4, respectively). Our tests of EST suggest an additional need for models that consider other components of species-level variation such as feeding behavior, selective assimilation, and ontogeny, to accurately predict interspecific variation in stoichiometric traits (Dodds et al. 2014; Allgeier et al. 2015; Vanni and McIntyre 2016; Meunier et al. 2017).
The degree of organism homeostasis indicates how body storage or depletion of excess or limiting elements, respectively, may change flexibly with variable resource C:N or C:P (Persson et al. 2010). In our study, the majority of seven taxa displayed strict homeostasis, because 1/H did not differ from zero (Fig. 2). Among taxa deviating from strict homeostasis, the 1/H values indicate that Tipula exhibited homeostasis in C:P and Strophopteryx exhibited weak homeostasis in C:N, based on classifications of Persson et al. (2010). The mean strength of homeostasis across all taxa (1/H = − 0.018 and 0.026 for C:N and C:P, respectively) was consistent with other animals’ strict homeostasis (Sterner and Elser 2002). Yet, compared to other aquatic macroinvertebrates (exclusively herbivorous Mollusca) showing mean unweighted 1/H = 0.21 and 0.11 in C:N and C:P, respectively, and zooplankton (Cladocerans) exhibiting 1/H = 0.17 and 0.12 in C:N and C:P, respectively, detritivores in our study were consistently more homeostatic (Persson et al. 2010). Because we assessed homeostasis over physiological and ecological timeframes of days to weeks, our results suggest short-term stoichiometric flexibility is an unlikely explanation for increased aquatic invertebrate nutrient contents associated with nutrient enrichment in the field (Cross et al. 2003; Small and Pringle 2010; Feijoó et al. 2014). Instead, longer-term processes, such as phenotypic plasticity (e.g., altered development from extended rearing) or evolutionary adaptation, may drive increased animal storage of excess N or P in nutrient-rich environments (Prater et al. 2017; Leal et al. 2017). Maintenance of homeostasis across wide resource gradients would require significant changes at other levels of regulation, such as flexible ingestion, egestion, respiration, and excretion (Frost et al. 2005; Moody et al. 2015; Halvorson et al. 2018).
While organisms may regulate internal body stoichiometry across resource gradients, the stoichiometry of tissue growth may differ from existing tissues due to dynamic ontogenetic changes that require specific allocation of elements to new tissues (Hood and Sterner 2014; Richard and de Roos 2018). These ontogenetic changes are well documented for many taxa including invertebrates, fish, and amphibians, in which investment in bone or lipid storage alters organismal stoichiometry (Pilati and Vanni 2007; Back and King 2013; Zhang et al. 2016; Stephens et al. 2017). Across our focal species, ontogenetic deviation of stoichiometry was not consistent, and we show that instantaneous measures of initial body stoichiometry are poor predictors of growth stoichiometry over an interval, both within and across species (Fig. 3). Growth limitation models, including classic models of EST (Sterner and Elser 2002), frequently assume organism growth stoichiometry matches tissue stoichiometry measured prior to a growth interval. Because growth C:N and C:P diverged − 30 to + 54% and − 145 to 74% from initial body C:N and C:P, these models may be sensitive to the assumption of no change in organismal stoichiometry during growth (Halvorson et al. 2015b). Stoichiometric models would therefore benefit from additional study and consideration of dynamic organismal stoichiometry during growth (Bullejos et al. 2014; Stephens et al. 2017; Richard and de Roos 2018). Specifically, our study suggests models applied across multiple species would benefit from considering body size, which across taxa was positively correlated with growth C:N and C:P.
Our findings did not support EST assumptions that growth stoichiometry would reflect initial body stoichiometry. However, our study does show interspecific patterns may reflect broad phylogenetic differences across invertebrates, attributable to life history or body size (González et al. 2018). Patterns in growth C:N and C:P differed notably between smaller, non- and hemimetabolous taxa (Isopoda, Plecoptera) versus larger holometabolous taxa (Trichoptera, Diptera; Fig. 3). For example, all Plecoptera exhibited low, declining C:N of growth, whereas Trichoptera and Diptera exhibited high, increasing (except Lepidostoma) C:N of growth during experiments. This pattern may reflect investment in high C:N fat body (lipids) during prepupal larval stages among holometabolous taxa (Sun et al. 2013; Nestel et al. 2016); in turn, Plecoptera may exhibit low C:N growth to invest in muscle tissues to support wing development. Indeed, both Allocapnia and Amphinemura larvae were in terminal instars with wingpads, or emerging as winged adults, at the end of their respective experiments. Likewise, Lepidostoma was closest to pupation among the holometabolous taxa, and may have exhibited growth C:N below initial C:N, because individuals were beginning lipid mobilization prior to pupation. We observed similar general patterns in growth C:P, suggesting major invertebrate phylogenetic groups could differ in the stoichiometry of growth limitation and nutrient release. These patterns were correlated with body size, indicating smaller-bodied taxa synthesize N- and P-rich tissues compared to larger-bodied taxa. Additionally, growth C:N exhibited the greatest phylogenetic signal across all traits, consistent with an evolutionary signal recently illustrated for %C, %N, and C:N across a broader interspecific study of invertebrates at global scales (González et al. 2018). As an implication, smaller hemi- and non-metabolous larvae may exhibit lower C:N and C:P growth demands compared to larger holometabolous larvae, because the latter group exhibits greater C demands for lipid storage (Sun et al. 2013; Nestel et al. 2016). Factors which affect aquatic invertebrate assemblage traits, such as stream intermittency selection for high adult mobility and shorter aquatic larval life spans (Townsend et al. 1997; Cañedo-Argüelles et al. 2015), could favor lower C:N and -C:P tissue investment, in turn affecting ecosystem nutrient dynamics by increasing nutrient limitation and enhancing animal nutrient retention relative to recycling (Meunier et al. 2017; Atkinson et al. 2017).
Interspecific variation in organismal stoichiometry should drive variable stoichiometry of nutrient release and growth rates across resource gradients (Fig. 1; Elser and Urabe 1999; Hood and Sterner 2014). In our study, growth C:N and C:P did not strongly relate to interspecific patterns in C:N or C:P egestion in response to resource C:N and C:P (Fig. 4). As predicted, most taxa exhibited lower C:N and C:P egestion on lower C:N and C:P diets (positive slopes), indicating flexibility in the stoichiometry of assimilation and nutrient release necessary to maintain homeostasis (Frost et al. 2005). Notably, Strophopteryx did not follow other interspecific trends in egestion stoichiometry, exhibiting a negative slope in C:N release and a high C:P egestion slope given its low growth C:P (Fig. 4b, d). Strophopteryx may be an outlier because individuals exhibited the slowest (often negative) growth rates and were likely N-limited, given low growth C:N. With exclusion of Strophopteryx, C:P egestion slopes were strongly positively related to growth C:P (r = 0.89, P = 0.018), indicating lower C:P taxa exhibit lower sensitivity of egestion C:P to resource C:P gradients, likely due to greater P assimilation to support P-rich growth, resulting in less efficient release of P (Elser et al. 2000). We note that our study was limited by considering only egestion stoichiometry, reflective of regulation during assimilation, when the stoichiometry of other forms of release, namely respiration, excretion, and exuviation, may also contribute to homeostatic regulation post-assimilation (Anderson et al. 2005). Such data would provide a more thorough test of EST predictions, offering complete elemental budgets and addressing the comparative importance and flexibility of pre- versus post-assimilatory pathways of homeostasis. Still, our findings affirm that diet and organism stoichiometry can both control the stoichiometry of egestion, the dominant pathway of nutrient release among our study organisms, and more work should investigate mechanisms of species-specific patterns (Vanni et al. 2002; Dalton et al. 2017; Halvorson et al. 2017).
Organism growth rates may also respond to resource stoichiometry gradients depending on taxonomic traits such as organism stoichiometry (da Ferrão-Filho et al. 2007; Hood and Sterner 2014). We found that most taxa increased growth rates on lower C:N and C:P resources, indicating N- and P-limited growth. Co-limitation by N and P may have contributed to these responses because N and P in litter increased simultaneously, rendering it difficult to determine which element was most limiting for growth. Further, growth rates may have also increased due to greater fungal or bacterial biomass (higher C quality) on low C:N and C:P litter (Manning et al. 2015), a response to be investigated further by controlling for microbial biomass or N content (see Danger et al. 2013). While taxa differed in their growth responses to resource C:N, growth C:N was not a strong predictor of these taxonomic differences. Growth responses to resource C:P were similar across taxa, but overall growth rates spanned a wide range across species and were not related to growth C:P nor body size, as would be expected by the growth rate hypothesis (Elser et al. 2003; Gillooly et al. 2005; Hood and Sterner 2014) (Fig. 5). This weak correlation may reflect different life stages or ability to assimilate nutrients from leaf litter across taxa. Overall, species-specific stoichiometry of assimilation or selective feeding, not growth stoichiometry, may be most important to determining taxonomic variation in growth rates and growth sensitivity to resource stoichiometry (Hood et al. 2014; Urabe et al. 2018). For example, Urabe et al. (2018) recently showed that measuring feeding and assimilation rates improves the prediction of Daphnia growth responses to diet C:P. Accounting for the stoichiometry of species-specific feeding and assimilation may therefore improve explanation of interspecific growth rates across taxa fed resource gradients.
While all focal species in our study are shredder detritivores, some of our results suggest the degree of selective feeding on N- and P-rich litter microbes, namely fungi, may differ across major phylogenetic groups and explain interspecific differences (Arsuffi and Suberkropp 1989). Fed a given resource C:N:P, Isopoda and Plecoptera species consistently egested at lower C:P and C:N ratios compared to Trichoptera and Diptera species (Fig. 4a, c), indicating N- and P-enrichment of egesta which may indicate greater selective feeding on microbes by the former two taxonomic groups (Hood et al. 2014) and/or greater assimilation of limiting N and P by the latter two groups (Clissold et al. 2010). Based on our observation of feeding behavior, certain taxa (Plecoptera) tend to “scrape” biofilm off leaves, leaving a skeleton of leaf matrix and ingesting lower C:N and C:P diets compared to bulk litter, in contrast to Pycnopsyche and Tipula that “chew” leaf litter in bulk and thus feed less selectively on leaf litter microbes. These taxa also differ in mobility, ranging from highly mobile feeders which may be able to selectively forage (Lirceus, Plecoptera) to less mobile case-making caddisflies (Lepidostoma and Pycnopsyche) and Tipula that cannot forage as selectively (Arsuffi and Suberkropp 1989). These strategies of greater selective feeding by Isopoda and Plecoptera may also support the demands of comparatively N- and P-rich growth. Still, our study indicates a weak phylogenetic signal among most of these stoichiometric traits, possibly due to the limited number of taxa we studied, as well as our restriction to specific life stages which prevents an integrated measure of whole lifespan taxonomic traits. Additional analysis of traits, such as of gut contents or mouthpart electron microscopy analysis, would resolve species-level differences, providing greater accuracy of ingestion stoichiometry, but gut contents would be difficult to extract from some small taxa used in this study (Lauridsen et al. 2012). Overall, the taxonomic differences in egestion and growth stoichiometry point to substantial diversity among shredder detritivores, to be investigated as a basis for divergent nutritional niches and responses to nutrient enrichment (Evans-White et al. 2009; Prater et al. 2015; González et al. 2017).
Conclusions
We quantified changes in the stoichiometry of growth, homeostasis, and nutrient release (egestion) across resource nutrient gradients among seven detritivorous invertebrate taxa. Our findings show support for strict homeostasis of most taxa, but indicate organismal stoichiometry can change during growth, with patterns differing across species and carrying weak phylogenetic signal, but coarsely related to major phylogenetic groups. Growth limitation models may therefore lose accuracy by assuming organism growth stoichiometry matches initial stoichiometry among species with complex ontogeny (see also Richard and de Roos 2018). Interestingly, growth stoichiometry was further related only weakly to interspecific patterns in growth and nutrient release, with the exception that lower C:P taxa tended to exhibit lower sensitivity of C:P egestion to resource C:P. Consideration of additional stoichiometric and non-stoichiometric traits (e.g., feeding, assimilation, and ontogeny) may improve prediction of interspecific patterns in growth and nutrient release. Notably, taxa in our study may be ranked along a continuum of overall sensitivity to resource stoichiometry; Lirceus and Allocapnia exhibited low whereas Tipula and Pycnopsyche exhibited high growth and egestion sensitivity across the resource gradients. These groupings in sensitivity may be associated with other traits, namely the degree of selective feeding, as the former two taxa also produced comparatively N- and P-rich egesta indicative of ingesting N- and P-rich microbes, which may “buffer” organismal sensitivity to resource nutrient composition (Meunier et al. 2016; Sperfeld et al. 2017). Because growth and nutrient acquisition are intimately linked through homeostasis (Sterner and Elser 2002), degrees of resource sensitivity of these organismal processes are likely to be interconnected and affect community- and ecosystem-level processes across wide resource nutrient gradients (Jochum et al. 2017; Farrell et al. 2018).
References
Allgeier JE, Wenger SJ, Rosemond AD, Schindler DE, Layman CA (2015) Metabolic theory and taxonomic identity predict nutrient recycling in a diverse food web. Proc Natl Acad Sci USA 112:E2640–E2647. https://doi.org/10.1073/pnas.1420819112
Alves JM, Caliman A, Guariento RD, Figueiredo-Barros MP, Carneiro LS, Farjalla VF, Bozelli RL, Esteves FA (2010) Stoichiometry of benthic invertebrate nutrient recycling: interspecific variation and the role of body mass. Aquat Ecol 44:421–430. https://doi.org/10.1007/s10452-009-9302-3
American Publish Health Assocation (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Anderson TR, Hessen DO, Elser JJ, Urabe J (2005) Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am Nat 165:1–15. https://doi.org/10.1086/426598
Arsuffi TL, Suberkropp K (1989) Selective feeding by shredders on leaf-colonizing stream fungi: comparison of macroinvertebrate taxa. Oecologia 79:30–37. https://doi.org/10.1007/BF00378236
Atkinson CL, Capps KA, Rugenski AT, Vanni MJ (2017) Consumer-driven nutrient dynamics in freshwater ecosystems: from individuals to ecosystems. Biol Rev 92:2003–2023. https://doi.org/10.1111/brv.12318
Back JA, King RS (2013) Sex and size matter: ontogenetic patterns of nutrient content of aquatic insects. Freshw Sci 32:837–848. https://doi.org/10.1899/12-181.1
Benstead JP, Hood JM, Whelan NV, Kendrick MR, Nelson D, Hanninen AF, Demi LM (2014) Coupling of dietary phosphorus and growth across diverse fish taxa: a meta-analysis of experimental aquaculture studies. Ecology 95:2757–2767. https://doi.org/10.1890/13-1859.1
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745. https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Bullejos FJ, Carrillo P, Gorokhova E, Medina-Sánchez JM, Balseiro EG, Villar-Argaiz M (2014) Shifts in food quality for herbivorous consumer growth: multiple golden means in the life history. Ecology 95:1272–1284. https://doi.org/10.1890/13-0410.1
Cañedo-Argüelles M, Boersma KS, Bogan MT, Olden JD, Phillipsen I, Schriever TA, Lytle DA (2015) Dispersal strength determines meta-community structure in a dendritic riverine network. J Biogeogr 42:778–790. https://doi.org/10.1111/jbi.12457
Clissold FJ, Tedder BJ, Conigrave AD, Simpson SJ (2010) The gastrointestinal tract as a nutrient-balancing organ. Proc R Soc Lond B Biol Sci 277:1751–1759. https://doi.org/10.1098/rspb.2009.2045
Cooper SJ (2008) From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite 51:419–427. https://doi.org/10.1016/j.appet.2008.06.005
Cross WF, Benstead JP, Rosemond AD, Wallace JB (2003) Consumer-resource stoichiometry in detritus-based streams. Ecol Lett 6:721–732. https://doi.org/10.1046/j.1461-0248.2003.00481.x
da Ferrão-Filho AS, Tessier AJ, DeMott WR (2007) Sensitivity of herbivorous zooplankton to phosphorus-deficient diets: testing stoichiometric theory and the growth rate hypothesis. Limnol Oceanogr 52:407–415. https://doi.org/10.4319/lo.2007.52.1.0407
Dalton CM, El-Sabaawi RW, Honeyfield DC, Auer SK, Reznick DN, Flecker AS (2017) The influence of dietary and whole-body nutrient content on the excretion of a vertebrate consumer. PLoS One 12:e0187931. https://doi.org/10.1371/journal.pone.0187931
Danger M, Arce Funck J, Devin S et al (2013) Phosphorus content in detritus controls life-history traits of a detritivore. Funct Ecol 27:807–815. https://doi.org/10.1111/1365-2435.12079
Demi LM, Benstead JP, Rosemond AD, Maerz JC (2018) Litter P content drives consumer production in detritus-based streams spanning an experimental N:P gradient. Ecology 99:347–359. https://doi.org/10.1002/ecy.2118
Dodds WK, Collins SM, Hamilton SK, Tank JL, Johnson S, Webster JR, Simon KS, Whiles MR, Rantala HM, McDowell WH, Peterson SD, Riis T, Crenshaw CL, Thomas SA, Kristensen PB, Cheever BM, Flecker AS, Griffiths NA, Crowl T, Rose-Marshall EJ, El-Sabaawi R, Martí E (2014) You are not always what we think you eat: selective assimilation across multiple whole-stream isotopic tracer studies. Ecology 95:2715–2722. https://doi.org/10.1890/13-2276.1
El-Sabaawi RW, Warbanski ML, Rudman SM, Hovel R, Matthew B (2016) Investment in boney defensive traits alters organismal stoichiometry and excretion in fish. Oecologia 181:1209–1220. https://doi.org/10.1007/s00442-016-3599-0
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80:735–751. https://doi.org/10.1890/0012-
Elser J, O’Brien W, Dobberfuhl D (2000) The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. J Evol Biol 13:845–853
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner RW (2003) Growth rate–stoichiometry couplings in diverse biota. Ecol Lett 6:936–943. https://doi.org/10.1046/j.1461-0248.2003.00518.x
Evans-White MA, Dodds WK, Huggins DG, Baker DS (2009) Thresholds in macroinvertebrate biodiversity and stoichiometry across water-quality gradients in Central Plains (USA) streams. J N Am Benthol Soc 28:855–868. https://doi.org/10.1899/08-113.1
Farrell KJ, Rosemond AD, Kominoski JS, Bonjour SM, Rüegg J, Koenig LE, Baker CL, Trentman MT, Harms TK, McDowell WH (2018) Variation in detrital resource stoichiometry signals differential carbon to nutrient limitation for stream consumers across biomes. Ecosystems. https://doi.org/10.1007/s10021-018-0247-z
Feijoó C, Leggieri L, Ocón C, Muñoz I, Rodrigues Capítulo A, Giorgi A, Colautti D, Ferreiro N, Licursi M, Gómez N, Sabater S (2014) Stoichiometric homeostasis in the food web of a chronically nutrient-rich stream. Freshw Sci 33:820–831. https://doi.org/10.1086/677056
Frost PC, Evans-White MA, Finkel ZV, Jensen TC, Matzek V (2005) Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 109:18–28. https://doi.org/10.1111/j.0030-1299.2005.14049.x
Frost PC, Benstead JP, Cross WF et al (2006) Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol Lett 9:774–779. https://doi.org/10.1111/j.1461-0248.2006.00919.x
Gillooly JF, Brown JH, West GB et al (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251. https://doi.org/10.1126/science.1061967
Gillooly JF, Allen AP, Brown JH et al (2005) The metabolic basis of whole-organism RNA and phosphorus content. Proc Natl Acad Sci USA 102:11923–11927. https://doi.org/10.1073/pnas.0504756102
González AL, Dézerald O, Marquet PA, Romero GQ, Srivastava DS (2017) The multidimensional stoichiometric niche. Front Ecol Evol 5:110. https://doi.org/10.3389/fevo.2017.00110
González AL, Céréghino R, Dézerald O, Farjalla VG, Leroy C, Richardson BA, Richardson MJ, Romero GQ, Srivastava DS (2018) Ecological mechanisms and phylogeny shape invertebrate stoichiometry: a test using detritus-based communities across Central and South America. Funct Ecol 32:2448–2463. https://doi.org/10.1111/1365-2435.13197
Halvorson HM, Small GE (2016) Observational field studies are not appropriate tests of consumer stoichiometric homeostasis. Freshw Sci 35:1103–1116. https://doi.org/10.1086/689212
Halvorson HM, Fuller C, Entrekin SA, Evans-White MA (2015a) Dietary influences on production, stoichiometry and decomposition of particulate wastes from shredders. Freshw Biol 60:466–478. https://doi.org/10.1111/fwb.12462
Halvorson HM, Scott JT, Sanders AJ, Evans-White MA (2015b) A stream insect detritivore violates common assumptions of threshold elemental ratio bioenergetics models. Freshw Sci 34:508–518. https://doi.org/10.1086/680724
Halvorson HM, Hall DJ, Evans-White MA (2017) Long-term stoichiometry and fates highlight animal egestion as nutrient repackaging, not recycling, in aquatic ecosystems. Funct Ecol. https://doi.org/10.1111/1365-2435.12875
Halvorson HM, Fuller CL, Entrekin SA, Scott JT, Evans-White MA (2018) Detrital nutrient content and leaf species differentially affect growth and nutritional regulation of detritivores. Oikos. https://doi.org/10.1111/oik.05201
Hood JM, Sterner RW (2014) Carbon and phosphorus linkages in Daphnia growth are determined by growth rate, not species or diet. Funct Ecol 28:1156–1165. https://doi.org/10.1111/1365-2435.12243
Hood JM, McNeely C, Finlay JC, Sterner RW (2014) Selective feeding determines patterns of nutrient release by stream invertebrates. Freshw Sci 33:1093–1107. https://doi.org/10.1086/678693
Jochum M, Barnes AD, Weigelt P, Ott D, Rembold K, Farajallah A, Brose U (2017) Resource stoichiometry and availability modulate species richness and biomass of tropical litter macro-invertebrates. J Anim Ecol 86:1114–1123. https://doi.org/10.1111/1365-2656.12695
Kembel SW, Cowan PD, Helmus MR et al (2010) Picante: r tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464. https://doi.org/10.1093/bioinformatics/btq166
Lauridsen RB, Edwards FK, Cross WF, Woodward G, Hildrew AG, Jones JI (2012) Consumer–resource elemental imbalances in a nutrient-rich stream. Freshw Sci 31:408–422. https://doi.org/10.1899/11-052
Leal MC, Seehausen O, Matthew B (2017) The ecology and evolution of stoichiometric phenotypes. Trends Ecol Evol 32:108–117. https://doi.org/10.1016/J.TREE.2016.11.006
Liess A (2014) Compensatory feeding and low nutrient assimilation efficiencies lead to high nutrient turnover in nitrogen-limited snails. Freshw Sci 33:425–434. https://doi.org/10.1086/675533
Lovelock JE, Margulis L (1974) Atmospheric homeostasis by and for the biosphere: The Gaia hypothesis. Tellus 26:2–10. https://doi.org/10.1111/j.2153-3490.1974.tb01946.x
Manning DWP, Rosemond AD, Kominoski JS et al (2015) Detrital stoichiometry as a critical nexus for the effects of streamwater nutrients on leaf litter breakdown rates. Ecology 96:2214–2224. https://doi.org/10.1890/14-1582.1
Martinson HM, Schneider K, Gilbert J, Hines JE, Hambäck Fagan WF (2008) Detritivory: stoichiometry of a neglected trophic level. Ecol Res 23:487–491. https://doi.org/10.1007/s11284-008-0471-7
Meunier CL, Malzahn AM, Boersma M (2014) A new approach to homeostatic regulation: towards a unified view of physiological and ecological concepts. PLoS One 9:e107737. https://doi.org/10.1371/journal.pone.0107737
Meunier CL, Boersma M, Wiltshire KH, Malzahn AM (2016) Zooplankton eat what they need: copepod selective feeding and potential consequences for marine systems. Oikos 125:50–58. https://doi.org/10.1111/oik.02072
Meunier CL, Boersma M, El-Sabaawi R, Halvorson HM, Herstoff EM, Van de Waal DB, Vogt RJ, Litchman E (2017) From elements to function: toward unifying ecological stoichiometry and trait-based ecology. Front Environ Sci 5:18. https://doi.org/10.3389/fenvs.2017.00018
Misof B, Liu S, Meusemann K et al (2014) Phylogenomics resolves the timing and pattern of insect evolution. Science 346:763–767. https://doi.org/10.1126/science.1257570
Moody EK, Corman JR, Elser JJ, Sabo JL (2015) Diet composition affects the rate and N: P ratio of fish excretion. Freshw Biol 60:456–465. https://doi.org/10.1111/fwb.12500
Moore JC, Berlow EL, Coleman DC et al (2004) Detritus, trophic dynamics and biodiversity. Ecol Lett 7:584–600. https://doi.org/10.1111/j.1461-0248.2004.00606.x
Nestel D, Papadopoulos NT, Pascacio-Villafán C, Righini N, Altuzar-Molina A, Aluja M (2016) Resource allocation and compensation during development in holometabolous insects. J Insect Physiol 95:78–88. https://doi.org/10.1016/j.jinsphys.2016.09.010
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. https://doi.org/10.1093/bioinformatics/btg412
Parr TB, Capps KA, Inamdar SP, Metcalf KA (2019) Animal-mediated organic matter transformation: aquatic insects as a source of microbially bioavailable organic nutrients and energy. Funct Ecol 33:524–535. https://doi.org/10.1111/1365-2435.13242
Persson J, Fink P, Goto A, Hood JM, Jonas J, Kato S (2010) To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119:741–751. https://doi.org/10.1111/j.1600-0706.2009.18545.x
Pilati A, Vanni MJ (2007) Ontogeny, diet shifts, and nutrient stoichiometry in fish. Oikos 116:1663–1674. https://doi.org/10.1111/j.0030-1299.2007.15970.x
Prater C, Norman EJ, Evans-White MA (2015) Relationships among nutrient enrichment, detritus quality and quantity, and large-bodied shredding insect community structure. Hydrobiologia 753:219–232. https://doi.org/10.1007/s10750-015-2208-2
Prater C, Wagner ND, Frost PC (2017) Interactive effects of genotype and food quality on consumer growth rate and elemental content. Ecology 98:1399–1408. https://doi.org/10.1002/ecy.1795
Purvis A (1995) A composite estimate of primate phylogeny. Philos Trans R Soc Lond Ser B Biol Sci 348:405–421. https://doi.org/10.1098/rstb.1995.0078
Richard R, de Roos AM (2018) The impact of development on patterns of nutrient limitation. Funct Ecol 32:1507–1519. https://doi.org/10.1111/1365-2435.13101
Scott EE, Prater C, Norman E, Baker BC, Evans-White MA, Scott JT (2013) Leaf-litter stoichiometry is affected by streamwater phosphorus concentrations and litter type. Freshw Sci 32:753–761. https://doi.org/10.1899/12-215.1
Small GE, Pringle CM (2010) Deviation from strict homeostasis across multiple trophic levels in an invertebrate consumer assemblage exposed to high chronic phosphorus enrichment in a neotropical stream. Oecologia 162:581–590. https://doi.org/10.1007/s00442-009-1489-4
Small GE, Helton AM, Kazanci C (2009) Can consumer stoichiometric regulation control nutrient spiraling in streams? J North Am Benthol Soc 28:747–765. https://doi.org/10.1899/08-099.1
Sperfeld E, Wagner ND, Halvorson HM, Malishev M, Raubenheimer D (2017) Bridging ecological stoichiometry and nutritional geometry with homeostasis concepts and integrative models of organism nutrition. Funct Ecol 31:286–296. https://doi.org/10.1111/1365-2435.12707
Stephens JP, Stoler AB, Sckrabulis JP, Fetzer AJ, Berven KA, Tiegs SD, Raffel TR (2017) Ontogenetic changes in sensitivity to nutrient limitation of tadpole growth. Oecologia 183:263–273. https://doi.org/10.1007/s00442-016-3746-7
Sterner RW (1990) The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. Am Nat 136:209–229. https://doi.org/10.1086/285092
Sterner RW, Elser JJ (2002) Ecological stoichiometry. Princeton University Press, Princeton
Sun X, Zhou X, Small GE, Sterner R, Kang HZ, Liu C (2013) Energy storage and C:N:P variation in a holometabolous insect (Curculio davidi Fairmaire) larva across a climate gradient. J Insect Physiol 59:408–415. https://doi.org/10.1016/j.jinsphys.2013.01.010
Thomas MA, Walsh KA, Wolf MR et al (2000) Molecular phylogenetic analysis of evolutionary trends in stonefly wing structure and locomotor behavior. Proc Natl Acad Sci USA 97:13178–13183. https://doi.org/10.1073/pnas.230296997
Torres LE, Vanni MJ (2007) Stoichiometry of nutrient excretion by fish: interspecific variation in a hypereutrophic lake. Oikos 116:259–270. https://doi.org/10.1111/j.0030-1299.2007.15268.x
Townsend CR, Scarsbrook MR, Dolédec S (1997) Species traits in relation to temporal and spatial heterogeneity in streams: a test of habitat templet theory. Freshw Biol 37:367–387. https://doi.org/10.1046/j.1365-2427.1997.00166.x
Urabe J, Shimizu Y, Yamaguchi T (2018) Understanding the stoichiometric limitation of herbivore growth: the importance of feeding and assimilation flexibilities. Ecol Lett 21:197–206. https://doi.org/10.1111/ele.12882
Vanni MJ, McIntyre PB (2016) Predicting nutrient excretion of aquatic animals with metabolic ecology and ecological stoichiometry: a global synthesis. Ecology. https://doi.org/10.1002/ecy.1582
Vanni MJ, Flecker AS, Hood JM, Headworth JL (2002) Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking biodiversity and ecosystem function. Ecol Lett 5:285–293. https://doi.org/10.1046/j.1461-0248.2002.00314.x
Welti N, Striebel M, Ulseth AJ, Cross WF, DeVilbiss S, Gilbert PM, Guo L, Hirst AG, Hood J, Kominoski JS, MacNeill KL, Mehring AS, Welter JR, Hillebrand H (2017) Bridging food webs, ecosystem metabolism, and biogeochemistry using ecological stoichiometry theory. Front Microbiol 8:1298. https://doi.org/10.3389/fmicb.2017.01298
Woods HA, Perkins MC, Elser JJ, Harrison JF (2002) Absorption and storage of phosphorus by larval Manduca sexta. J Insect Physiol 48:555–564. https://doi.org/10.1016/S0022-1910(02)00060-4
Zhang C, Jansen M, De Meester L, Stoks R (2016) Energy storage and fecundity explain deviations from ecological stoichiometry predictions under global warming and size-selective predation. J Anim Ecol 85:1431–1441. https://doi.org/10.1111/1365-2656.12531
Zou K, Thébault E, Lacroix G, Barot S (2016) Interactions between the green and brown food web determine ecosystem functioning. Funct Ecol 30:1454–1465. https://doi.org/10.1111/1365-2435.12626
Acknowledgements
We thank Brad Austin, Ayla Smartt, Erin Scott, Erin Grantz, Ben Thompson, Jasmine Gilbert, Andrew Sanders, Jason Ramey, Lindsey Abel, Delaney Hall, Amanda Eddy, Grant White, and Clay Prater for assistance in the field and laboratory. This study was funded by the US National Science Foundation (DEB 1020722) and a Doctoral Academy Fellowship from the University of Arkansas Graduate School.
Author information
Authors and Affiliations
Contributions
MAE-W, JTS, and SAE conceived and designed the experiments; HMH and CLF conducted the experiments and collected the data under mentorship of MAE-W, JTS, and SAE; HMH analyzed the data and led writing of the manuscript. All authors contributed to writing and gave approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Maarten Boersma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Halvorson, H.M., Fuller, C.L., Entrekin, S.A. et al. Interspecific homeostatic regulation and growth across aquatic invertebrate detritivores: a test of ecological stoichiometry theory. Oecologia 190, 229–242 (2019). https://doi.org/10.1007/s00442-019-04409-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04409-w