Abstract
Ecological stoichiometry (ES) and allometry offer frameworks for predicting how nutrient recycling varies within and among animal species. Despite the importance of benthic-derived nutrients in most aquatic systems, predictions based on ES and allometry have been poorly tested among benthic invertebrate consumers. Here, we show that the rates and ratios at which three freshwater benthic invertebrate species (a crustacean, an insect, and a polychaeta) recycled nitrogen (N) and phosphorus (P) can be partially predicted by ES and allometry depending on whether data are analyzed intra- or interspecifically. Mass-specific N and P excretion rates were negatively correlated with invertebrate body size both among and within taxa, supporting allometric predictions. However, mass-specific N and P excretion rates were positively and negatively correlated to invertebrate body N and P, respectively, but only when data were analyzed intraspecifically. As a corollary, the mass-specific N:P excretion ratio was positively related to body N:P ratio. Such a contrasting pattern on excretion-mediated N and P recycling suggests that stoichiometric constraints regarding consumer-resource imbalances for the three species utilized in this study may be stronger for P than for N. Our results indicate that the variation in nutrient recycling, which is mediated by taxonomic constraints on stoichiometry and allometry, may substantially help us to understand the importance of benthic detritivorous species to the functioning of aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A major goal of ecological research is to find general, integrative concepts that simplify our understanding of the structure and functioning of natural populations, communities, and ecosystems (Lawton 1999). Ecological stoichiometry (ES) is a conceptual framework that analyzes the constraints and consequences of the mass balance of multiple chemical elements in ecological interactions (Sterner 1995; Elser et al. 1996; Hessen 1997). Currently, ES is one of the most influential theories in ecology and has provided a mechanistic framework that aims to understand how imbalances between the elemental composition of an organism’s body and its food affect individual physiology, population dynamics, interspecific trophic interactions, and ecosystem-level processes (Sterner 1995; Elser et al. 2000a, b). One of the main predictions of ES relies on a model proposed by Sterner (1990), who suggested that the N:P ratio of nutrient release (excretion N:P) by a consumer will decrease with increasing consumer N:P body content. This prediction suggests that animals maintain homeostasis in body nutrient composition, and that consequently, the rates and ratios of nutrient recycling reflect the imbalance between their body and their diet with direct implications for ecosystem nutrient recycling (Elser and Urabe 1999).
Allometric theory predicts that physiological rates of organisms are related to their body size. Ecological processes, such as consumer-mediated nutrient recycling, should also be related to body size, and nutrient cycling rates (per unit body mass) should decline with body mass (Peters 1983; West et al. 1997). The growth rate hypothesis predicts that the body chemical composition of an organism may change with body size, and this should change nutrient demands. Consequently, the amount of nutrients released will also change (Elser and Urabe 1999; Vanni et al. 2002).
Despite the conceptual frameworks considered by ES and allometry and the growing recognition that animals can be important in nutrient recycling in aquatic and terrestrial ecosystems (McNaughton et al. 1997; Covich et al. 1999; Elser and Urabe 1999; Vanni 2002; Vanni et al. 2005), the stoichiometric and allometric mechanisms involved in nutrient recycling by detritivorous consumers have been largely neglected (Martinson et al. 2008). Additionally, there have been recent advances in the knowledge of consumer-driven nutrient recycling (Devine and Vanni 2002; Cross et al. 2005; Higgins et al. 2006), yet little effort has been directed to elucidate whether and how ontogenetic mechanisms (e.g., body size and/or developmental stage) and species identity quantitatively and qualitatively affect the way in which benthic invertebrates recycle nutrients in freshwater ecosystems (Frost et al. 2002, 2005a, b). Benthic invertebrates play important roles in aquatic ecosystem functions due to their importance in energy and nutrient fluxes. In particular, benthic environments are important sites for uptake, transformation, and recycling of essential elements (e.g., N and P), and thus are good model systems to investigate and expand the principles of ES (Cross et al. 2003; Frost and Tuchman 2005). In addition, identifying the mechanisms by which benthic taxa mediate nutrient recycling is especially important in shallow lake ecosystems where the biodiversity of benthic organisms and the relative importance of benthic-derived nutrient recycling are presumed to be greater than in deep lakes (Scheffer 1998; Schindler and Scheuerell 2002).
In this study, we tested the hypothesis that intra- and interspecific variation in nutrient recycling rates and ratios can be predicted by allometry and ES. We conducted laboratory experiments with three species of benthic invertebrates that differ in terms of function and phylogeny and have a cosmopolitan distribution in inland aquatic ecosystems. Our objectives were to determine the following within and among species: (1) whether nutrient content and nutrient excretion rates are functions of invertebrate body mass and (2) whether invertebrate elemental composition affects nutrient excretion rates and ratios.
Materials and methods
Study site and study species
Benthic invertebrates were collected in Imboassica Lagoon (lat 22°50′S, long 42°00′W), a tropical, shallow (mean depth of 1.1 m), coastal freshwater ecosystem located in Rio de Janeiro State, Brazil. Untreated domestic sewage input adds large loads of N and P to the lagoon and causes eutrophication. Sediment at the sampling site predominantly consists of silt and clay, with mean total P and N concentrations of 0.067 and 2.12 mg/g, respectively, with a N:P (mass) ratio of approximately 32 (Figueiredo-Barros et al. 2006).
We quantified N and P recycling (excretion) rates, body mass, and body nutrient composition of the following three benthic macroinvertebrate species: nymphs of the mayfly Campsurus melanocephalus (Pereira and Da-Silva, 1991) (Ephemeroptera: Polymitarcidae), the annelid Heteromastus similis (Southern, 1921; Polychaeta: Captellidae), and the shrimp Macrobrachium acanthurus (Wiegmann, 1836; Crustacea: Palaemonidae). These species were selected based on their high biomass in Imboassica Lagoon, their body size differences, and their phylogenetic and functional dissimilarities (Albertoni et al. 2003; Caliman et al. 2007; Henriques-de-Oliveira et al. 2007).
Campsurus melanocephalus’ nymphs are filter feeders and tube dwellers that promote a continuous flux of water through permanent U- or J-shaped burrows within lake sediments (Pereira and Da-Silva 1991). The nymphs feed mainly on fine particulate detritus and microbial organisms within sediments. H. similis is a head-down subsurface-deposit feeder that builds extensive semi-permanent galleries in sediments (Rao 1980). The species shows a selective feeding on organic matter patches within sediments and deposits large amounts of fecal pellets on sediment surfaces (Caliman et al. 2007). M. acanthurus is a freshwater shrimp and feeds mainly on freshly deposited organic detritus and benthic algae on sediment surfaces (Albertoni et al. 2003).
Organism sampling and experimental procedures
A few hours before the nutrient excretion experiments, individuals of various body sizes of the three macroinvertebrate species were collected near the littoral region of Imboassica Lagoon (~0.5 m depth). Individuals of H. similis and C. melanocephalus were sampled from the upper layer of sediment (0–5 cm) using a core sampler and then gently washed through a sieve (1-mm mesh) to remove sediment. Individuals for each species were gently picked up with forceps and immediately transferred to species-specific aquaria with sediment and water from the sampling site. Individuals of M. acanthurus were sampled with dragnets and also immediately transferred to an aerated container with sediment and lagoon water and kept in the dark inside a Styrofoam box. All trapped individuals from each species were considered; therefore, the range and the relative proportions of body size for each population reflected the natural patterns occurring at the sampling site.
In the laboratory, individuals were placed in enamel pans, gently separated from the sediment with forceps, and rinsed with pre-filtered (0.7-μm-pore filters, GF/F Whatman) lagoon water to remove any attached sediment and detritus, as these particles could contaminate the nutrient excretion experiment. Then, individuals of a given species were weighed to the nearest 0.1 mg (wet mass after blotting excess water), grouped into nine species-specific size classes (2 individuals for M. acanthurus and up to 6 individuals for H. similis and C. melanocephalus), and immediately distributed into nutrient-release chambers. Size classes for each species ranged from 11–53 mg DW for M. acanthurus, 1.6–6.7 mg DW for C. melanocephalus and 1.3–4.9 mg DW for H. similes. Size classes were initially determined through species wet weight, but were reassessed at the end of incubation using species dry weight. Nutrient-release chambers consisted of either 100-ml (for M. acanthurus) or 20-ml (for H. similis and C. melanocephalus) acid-washed Nalgene jars (Nalgene Labware, Rochester, New York) filled with 0.2-μm lagoon water filtered using VacuCaps sterile membranes (Gelman Sciences, East Hills, NY). Nutrient excretion rates by incubating animals in containers can conceivably lead to biased estimates if animals behave differently than they do in nature (Vanni et al. 2002). This experimental artifact could be especially important in the case of burrowing species. To minimize bias in nutrient-release rates of the two burrowing species used in this study, the nutrient-release chambers of H. similis and C. melanocephalus were also filled with a layer (2 cm thick) of pre-combusted (for 5 h at 700o C) and acid-washed sediment (mineral fraction, silt and clay) from the sampling site. A previous pilot experiment showed that this procedure is adequate to keep the natural behavior of burrowing species without nutrient contamination from the sediment. Control chambers containing 0.2-μm filtered lagoon water only (for M. acanthurus) or filtered lagoon water plus mineral sediment (for H. similis and C. melanocephalus) were used to account for ambient inorganic N and P in lagoon water. All nutrient-release chambers were incubated in the dark at the lagoon water temperature (23°C) for 1 h incubation time. This short incubation time was chosen because nutrient excretion rates decline quickly when animals stop feeding, such that longer incubations may underestimate nutrient excretion rates for benthic invertebrates (Devine and Vanni 2002).
At the end of each incubation, water from each chamber was immediately filtered through pre-combusted 0.7-μm-mesh filters (GF/F Whatman) to exclude feces. The samples of filtrate were stored in acid-washed vials and immediately frozen for later analysis of inorganic N and P. The animals were immediately collected, grouped into their respective size classes, dried for 2 days (70°C) and then re-weighed to the nearest 0.01 mg to obtain the dry mass of organisms. Then, organisms from the same species and size class were ground into a coarse powder with a mortar and pestle. The powders were stored in glass vials for subsequent analysis of tissue N and P.
From the excretion filtrate, we analyzed NH3-N and PO4-P as chemical inorganic forms of N and P. Ammonia-N determination was made manually using the phenol-hypochlorite technique (Solorzano 1969). We determined the amount of orthophosphate by using the molybdenum blue technique according to Golterman et al. (1978). Samples of ground animals were analyzed for total N through persulfate oxidation and nitrate reduction in a cadmium column with post-nitrite determination in a flow injection analyzer (model FIAlab—3500). Tissue total P was determined through HCl digestion followed by orthophosphate analysis according to Golterman et al. (1978).
The mass-specific excretion rates were estimated by first subtracting NH3-N and PO4-P mass concentrations in controls from the values in nutrient-release chambers and then dividing the result by the dry mass of organisms (mg dry weight) and the incubation time (hours).
Data analysis
We quantified the interspecific allometric relationships between body N and P content and NH3-N and PO4-P recycling rates by regressing these parameters as a function of body mass across species. To quantify interspecific stoichiometric constraints, we regressed nutrient excretion rates and ratios as a function of invertebrate N and P content and ratio, respectively, across species. In both analyses, all variables were log10-transformed to standardize variance among species. To compare stoichiometric and allometric trends in elements and ratios among benthic invertebrate species, we used an analysis of covariance (ANCOVA) after standardizing the body size and nutrient content/ratio variables. As the range of absolute body size and elemental composition is different for the three species used (M. acanthurus attain much larger adult size), we transformed body mass to percent maximum reported size and elemental composition or ratio to percent maximum observed value for each species, thereby bringing all variables to comparable scales (Pilati and Vanni 2007). We then ran ANCOVAs to compare individual and interactive effects of species, invertebrate body mass, and invertebrate body nutrient composition on response variables. In all cases, species was considered the categorical variable. When was the case, statistical differences among species were determined using Bonferroni adjustments (for slopes) accounting for multiple comparisons. To compare the allometric predictions of different species, we included nutrient excretion rates and invertebrate body nutrient contents as dependent variables in ANCOVAs with invertebrate body mass as a covariate. To compare the stoichiometric predictions of different species, we included nutrient excretion rates and ratios as dependent variables with body nutrient content and ratio as covariates. We also compared the means and 95% confidence intervals of body nutrient content among species, as well as excretion rates and ratios. Statistical significance was assessed at α = 0.05 for all analyses. All statistical analyses were performed using GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego California USA).
Results
Benthic invertebrate species in Imboassica Lagoon varied by more than two orders of magnitude in their body N and P content (Fig. 1a, b). N and P mass-specific body content correlated significantly with invertebrate body mass. The directions of the relationships were opposite, where N body content scaled negatively with body mass (Fig. 1a) and P body content scaled positively with body mass (Fig. 1b). However, data were somewhat clustered around species and variable across body mass. This was especially true for mass-specific P body content of C. melanocephalus, where data varied more than an order of magnitude (Fig. 1b). Intraspecific investigations showed significant individual and interactive effects of species and body mass on both N body content (ANCOVA, P < 0.0001 for species effect, P = 0.01 for body mass effect and P = 0.03 for interaction effect) and P body content (ANCOVA, P = 0.0007 for species effect, P = 0.028 for body mass effect and P = 0.007 for interaction effect). N body content was not a significant function of invertebrate body mass for any species (Table 1), but the average values of N body content were significantly different among species, with the highest N body content observed for H. similis (Fig. 1a). P body content was significantly negatively affected by invertebrate body mass only for H. similis and C. melanocephalus, and the slopes of P body content versus body mass for these two species differed significantly (Bonferroni post hoc test for slopes, Table 1). However, H. similis was statistically different from the two arthropod species with respect to the average values of P body content (Fig. 1b).
Cross-species linear regressions of mass-specific body nutrient content for N (a) and P (b) as a function of invertebrate body mass. Points represent a mean for a species size class. The right-hand panels show species’ average values and 95% confidence intervals for the mass-specific nutrient body content. Identical letters above symbols indicate that the species are not significantly different from one another because their confidence intervals do not overlap
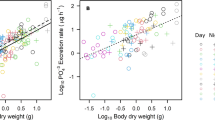
Across taxa, mass-specific NH3-N and PO4-P excretion rates decreased significantly with increasing invertebrate body mass (Fig. 2a, b). The invertebrate body mass explained more variation in the mass-specific PO4-P excretion rates (75% of variance) than the mass-specific NH3-N excretion rates (40% of variance). Interspecifically, we observed a significant effect of species on mass-specific NH3-N excretion rates (ANCOVA, P = 0.045) and significant effects of species and body mass on mass-specific PO4-P excretion rates (ANCOVA, P = 0.02 and P = 0.04, respectively). Heteromastus similis showed the highest average value of mass-specific NH4-N excretion rates, and M. acanthurus exhibited the lowest average value of mass-specific PO4-P excretion rates among species (Fig. 2). Mass-specific NH4-N and PO4-P excretion rates also decreased significantly with body mass for all three species, but the slopes for each species did not differ significantly (Bonferroni post hoc test for slopes, Table 1).
Cross-species linear regressions of mass-specific excretion rates for N-NH3 (a) and P-PO4 (b) as a function of invertebrate body mass. Points represent a mean for a species size class. The right-hand panels show species’ average values and 95% confidence intervals for the mass-specific nutrient excretion rates. Identical letters above symbols indicate that the species are not significantly different from one another because their confidence intervals do not overlap
Across species, the mass-specific NH4-N excretion rate was a positive function of invertebrate body N content, which is contrary to ES theory (Fig. 3a). However, mass-specific PO4-P excretion rate was a negative function of invertebrate body P content (Fig. 3b). As a result of such contrasting patterns, the NH4-N:PO4-P excretion ratio was positively affected by the invertebrate body N:P ratio across species (Fig. 3c). Considering each species individually, there were no significant effects of body nutrient content and ratio on the scaling of mass-specific nutrient excretion rates and ratio with body nutrient content and ratio, respectively (ANCOVA, Table 2). Furthermore, species had no effects on average values of mass-specific nutrient excretion ratios (ANCOVA, Fig. 3).
Cross-species linear regressions of mass-specific excretion rates for N-NH3 (a) and P-PO4 (b) as a function of mass-specific body N and P content, respectively. c Nutrient excretion ratios as a function of invertebrate body N:P (values represent mass ratios in both axes). Each point represents a mean for a species size class. The right-hand panel in c shows species’ average values and 95% confidence intervals for the nutrient excretion ratio. Identical letters above symbols indicate that the species are not significantly different from one another because their confidence intervals do not overlap
Discussion
Our results showed that body size, species, and stoichiometric constraints are all important in determining the rates and ratios by which nutrients are recycled by benthic detritivorous invertebrates in freshwater ecosystems. Body size showed a direct effect on benthic-derived nutrient recycling, corroborating both the principles of allometric theory (Peters 1983) and previous studies of aquatic vertebrate and invertebrate consumers (Vanni et al. 2002; Frost and Tuchman 2005). However, relationships between invertebrate body elemental compositions and nutrient excretion rates sometimes conflicted with ES predictions, which were observed within and between species (Sterner 1990; Schindler and Eby 1997; Vanni et al. 2002). This suggests that different mechanisms may operate when more than one taxa are involved, such as differences in physiological constraints or even diet specialization.
As predicted by allometric theory, body size was a predictor of mass-specific body N content across species, but it did not present any effect within species. Among the species studied, H. similis presented the highest mass-specific N content. This may be due to the presence of a N-rich cuticle that covers the entire body (Watson 1958) and to a system of longitudinal and circular muscles at each segment, which represents the major component of body structure (Brusca and Brusca 1990). Body size was also a significant predictor of mass-specific P body content at both the community and species level except for M. acanthurus. The proximate mechanism determining nutrient composition differences among animals may be related to physiological constraints and to differences among the constituent and biochemical forms that compose the bodies of these organisms (Anderson et al. 2004; Ferrao et al. 2007). Nevertheless, the ultimate mechanism driving such differences may result from evolutionary adaptations to “stoichiometric niches” (Torres and Vanni 2007), such as the development of different body demands for N or P among different species (Hessen et al. 2004). The phylogenetic component of body nutrient stoichiometry of freshwater invertebrates may partly explain the different patterns between community-level body mass and nutrient content relationships. The very low mass-specific P content in H. similis contrasts greatly with that observed for the other species. To our knowledge, this is the first study to provide quantitative information about annelid stoichiometry. The ways in which its nutrient stoichiometry is affected should be the subject of future research.
The species H. similis had the highest average N release rate among the species studied, while M. acanthurus had the lowest average P release rate among species. Such results suggest that these species recycle distinct nutrients at different rates. We speculate that these differences may be attributed to phylogenetic distances between species and functional group dissimilarities. They may also be due to differences in the stoichiometry of ingested food, which were demonstrated as important aspects determining nutrient recycling patterns in freshwater ecosystems (Vanni et al. 2002; Frost and Tuchman 2005). Body size was also an important predictor of N and P excretion rates at the species level, but the scaling relationships between nutrient excretion rates and body size were not different among the three species studied. Individuals with lower biomass had higher P and N excretion rates when normalized by their biomass. This is consistent with allometric theory and the prediction that smaller individuals have higher metabolic rates on a per biomass basis (Peters 1983; West et al. 1997).
With respect to community-level relationships between body nutrient content and nutrient excretion, we found partial support for stoichiometric predictions. A negative relationship was found between P excretion and P body content. The negative relationship between P excretion rates and invertebrates body P may be a result of physiological constraints across invertebrate body mass. In general, we observed that P content increased with increasing body mass, which may be due to the fact that organisms with a higher demand for P at the end of the mass gradient have low P-regeneration (i.e., low excretion rates). This finding is interesting because according to the growth rate hypothesis, small organisms are assumed to grow faster than large ones and should therefore exhibit a higher demand for (and accumulation of) P, which is ultimately related to the larger quantities of ribosomal RNA that are necessary for protein synthesis (Elser et al. 2000b). Furthermore, nitrogen excretion increased with increasing organism body N content across taxa, which conflicts with the predictions of ecological stoichiometry and organism homeostasis (Sterner 1990). Consequently, we observed a positive relationship between N:P excretion and N:P body content, which is contrary to ES theory. A possible explanation for this pattern may be an increase in food acquisition to supply energy (carbon) or another nutrient demand (e.g., phosphorus) throughout an organism’s development, which leads to high regeneration rates of the non-limiting nutrient present in the food (i.e., nitrogen), resulting in a high excretion N:P ratio. However, such a mechanism is difficult to test without knowledge of food stoichiometry and species’ growth efficiency for the limiting nutrient (Sterner 1990). We only have information about the bulk sediment N:P ratio, whereas knowledge about growth efficiency for the limiting nutrient is difficult to obtain for sediment detritivorous species. These factors complicate our ability to predict nutrient excretion ratios for the three species based on bioenergetic models. Future efforts should overcome the difficulties associated with measuring food stoichiometry for sediment dwelling invertebrate species, and thereby elucidate the mechanisms involved in the stoichiometry of benthic consumer’s nutrient recycling.
We did not observe a significant relationship between body nutrient content and nutrient excretion rate at the species level, but significant differences on average nutrient excretion rate were observed for P and N. Thus, body mass seemed to be more important in determining nutrient recycling patterns at the species level. Previous studies have highlighted that body stoichiometry alone poorly predicts excretion patterns. Body size and food stoichiometry must be considered to predict excretion N:P ratios (Torres and Vanni 2007), although other studies have found that feeding guild affiliation may be less important in lower levels of taxonomic affiliation (Vanni et al. 2002). Although we lack data on diet stoichiometry, we can presume that the observed differences and similarities of excretion stoichiometry among taxa involved in this study may be also a consequence of their functional similarities regarding resource use.
Understanding the extent to which species differ or are redundant in mediating ecosystem processes has been a central subject in ecology in recent years (see Hooper et al. 2005 for review). In this context, the knowledge of animal roles in nutrient cycling may be very important because animals may influence overall nutrient flux, primary production rates, and the extent to which primary producers are limited by N versus by P (Sterner 1990; Sterner et al. 1992; Elser and Urabe 1999; McIntyre et al. 2008). Our investigation showed that body mass and taxonomic identity of consumers may be important in determining the rates and ratios of nutrients recycling; thus, modifications of taxonomic composition, as well as size-structure, by natural environmental disturbances may have important effects on nutrient cycling and overall functioning of Imboassica Lagoon. These arguments are especially relevant for coastal environments because they are usually shallow, which intensifies the benthic–pelagic coupling and therefore the benthic contribution to overall ecosystem functioning (Schindler and Scheuerell 2002). Additionally, these systems would be particularly vulnerable to rising sea levels (Cabanes et al. 2001). Marine-driven disturbances are important regulating forces in coastal aquatic ecosystems because they modify physico-chemical environmental characteristics (e.g., salinity) and promote the transport of organisms’ propagules and juveniles across ecosystems. In the present study, all species have their natural history tightly linked to marine hydrologic regime. C. melanocephalus only lives at low salinity and experiences drastic population reduction or local extinction with increased salinity (Callisto et al. 1998). On the other hand, both M. acanthurus and H. similis disperse among coastal ecosystems through marine intrusions (Rao 1980; Callisto et al. 1998); therefore, their dispersion abilities may be altered by changes in marine hydrological regimes.
The factors influencing nutrient cycling in coastal lagoons are especially relevant because the high productivity of coastal lagoons encourages the reproduction and survival of commercial fish and migrating birds; thus, understanding the factors affecting nutrient cycling may ultimately be used to enhance the goods and services that coastal systems provide (Kjerfve 1994). The issue of whether biodiversity affects ecosystem function has focused largely on how the number and identity of species (or functional groups of species) affect ecosystem processes (Hooper et al. 2005). Our results showed that organism identity is an important aspect in determining nutrient recycling rates. Additionally, variation in body size may be significantly related to nutrient body content and excretion stoichiometry. These results highlight that both stoichiometric and allometric theory should be incorporated into studies predicting how benthic invertebrates can influence aquatic ecosystem nutrient recycling.
References
Albertoni EF, Palma-Silva C, Esteves FA (2003) Natural diet of three species of shrimp in a tropical coastal lagoon. Braz Arch Biol Technol 46:395–403
Anderson TR, Boersma M, Raubenheimer D (2004) Stoichiometry: linking elements to biochemicals. Ecology 85:1193–1202
Brusca RC, Brusca GJ (1990) Invertebrates. Sinauer Associates, Massachusetts
Cabanes C, Cazenave A, Le Provost C (2001) Sea level rise during past 40 years determined from satellite and situ observations. Science 294:840–842
Caliman A, Leal JJF, Esteves FA, Carneiro LS, Bozelli RL, Farjalla VF (2007) Functional bioturbator diversity enhances benthic-pelagic processes and properties in experimental microcosms. J North Am Benthol Soc 26:450–459
Callisto M, Gonçalves-Junior JF, Leal JJF, Petrucio MM (1998) Macroinvertebrados bentônicos nas lagoas Imboassica, Cabiúnas, e Comprida. In: Esteves FA (ed) Ecologia das Lagoas Costeiras do Parque Nacional da Restinga de Jurubatiba e do Município de Macaé (RJ). Nupem/UFRJ, Rio de Janeiro, pp 479–486
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems. Bioscience 49:119–127
Cross WF, Benstead JP, Rosemond AD, Wallace JB (2003) Consumer-resource stoichiometry in detritus-based streams. Ecol Lett 6:721–732
Cross WF, Benstead JP, Frost PC, Thomas SA (2005) Ecological stoichiometry in freshwater benthic systems: recent progress and perspectives. Freshw Biol 50:1895–1912
Devine JA, Vanni MJ (2002) Spatial and seasonal variation in nutrient excretion by benthic invertebrates in a eutrophic reservoir. Freshw Biol 47:1107–1121
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80:735–751
Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH (1996) Organism size, life history, and N:P stoichiometry. Bioscience 46:674–684
Elser JJ et al (2000a) Nutritional constraints in terrestrial and freshwater food webs. Nature 408:578–580
Elser JJ et al (2000b) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Ferrao AD, Tessier AJ, DeMott WR (2007) Sensitivity of herbivorous zooplankton to phosphorus-deficient diets: testing stoichiometric theory and the growth rate hypothesis. Limnol Oceanogr 52:407–415
Figueiredo-Barros MP, Leal JJF, Esteves FD, Rocha AD, Bozelli RL (2006) Life cycle, secondary production and nutrient stock in Heleobia australis (d’Orbigny 1835) (Gastropoda: Hydrobiidae) in a tropical coastal lagoon. Estuar Coast Shelf Sci 69:87–95
Frost PC, Tuchman NC (2005) Nutrient release rates and ratios by two stream detritivores fed leaf litter grown under elevated atmospheric CO2. Arch Hydrobiol 163:463–477
Frost PC, Stelzer RS, Lamberti GA, Elser JJ (2002) Ecological stoichiometry of trophic interactions in the benthos: understanding the role of C:N:P ratios in lentic and lotic habitats. J North Am Benthol Soc 21:515–528
Frost PC, Cross WF, Benstead JP (2005a) Ecological stoichiometry in freshwater benthic ecosystems: an introduction. Freshw Biol 50:1781–1785
Frost PC, Evans-White MA, Finkel ZV, Jensen TC, Matzek V (2005b) Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 109:18–28
Golterman HL, Clymo RS, Ohnstad MAM (1978) Methods for physical and chemical analysis of freshwater. Blackwell, Oxford
Henriques-de-Oliveira C, Baptista DF, Nessimian JL (2007) Sewage input effects on the macroinvertebrate community associated to Typha domingensis Pers in a coastal lagoon in southeastern Brazil. Braz J Biol 67:73–80
Hessen DO (1997) Stoichiometry in food webs—Lotka revisited. Oikos 79:195–200
Hessen DO, Agren GI, Anderson TR, Elser JJ, De Ruiter PC (2004) Carbon, sequestration in ecosystems: the role of stoichiometry. Ecology 85:1179–1192
Higgins KA, Vanni MJ, González MJ (2006) Detritivory and the stoichiometry of nutrient cycling by a dominant fish species in lakes of varying productivity. Oikos 114:419–430
Hooper DU et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Kjerfve B (1994) Coastal lagoon processes. 1. In: Kjerfve B (ed) Coastal lagoon processes, 60th edn. Elsevier Oceanography Series, New York, pp 1–8
Lawton JH (1999) Are there general laws in ecology? Oikos 84:177–192
Martinson HM, Schneider K, Gilbert J, Hines JE, Hamback PA, Fagan WF (2008) Detritivory: stoichiometry of a neglected trophic level. Ecol Res 23:487–491
McIntyre PB, Flecker AS, Vanni MJ, Hood JM, Taylor BW, Thomas SA (2008) Fish distributions and nutrient cycling in streams: can fish create biogeochemical hotspots? Ecology 89:2335–2346
McNaughton SJ, Banyikwa FF, McNaughton MM (1997) Promotion of the cycling of diet enhancing nutrients by African grazers. Science 278:1798–1800
Pereira S, Da-Silva E (1991) Descrição de uma nova espécie de Campsurus Eaton, 1868 do sudeste do Brasil, com notas biológicas (Ephemeroptera: Polymitarcyidae: Campsurinae). Rev Bras Biol 51:321–326
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Pilati A, Vanni MJ (2007) Ontogeny, diet shifts, and nutrient stoichiometry in fish. Oikos 116:1663–1674
Rao DS (1980) Ecology of Heteromastus-Similis Southern 1921 (Polychaeta, Capitellidae) in the Vasishta Godavari Estuary. Proc Ind Acad Sci (Anim Sci) 89:407–414
Scheffer M (1998) Ecology of shallow lakes. Kluwer, Dordrecht
Schindler DE, Eby LA (1997) Stoichiometry of fishes and their prey: implications for nutrient recycling. Ecology 78:1816–1831
Schindler DE, Scheuerell MD (2002) 1 Habitat coupling in lake ecosystems. Oikos 98:177–189
Solorzano L (1969) Determination of ammonia in natural waters by phenolhypochlorite method. Limnol Oceanogr 14:799–801
Sterner RW (1990) The ratio of nitrogen to phosphorus resupplied by herbivores–zooplankton and the algal competitive arena. Am Nat 136:209–229
Sterner RW (1995) Elemental stoichiometry of species in ecosystems. In: Jones CB, Lawton JH (eds) Linking species and ecosystems. Chapman & Hall, New York, p 387
Sterner RW, Elser JJ, Hessen DO (1992) Stoichiometric relationships among producers, consumers and nutrient cycling in pelagic ecosystems. Biogeochemistry 17:49–67
Torres LE, Vanni MJ (2007) Stoichiometry of nutrient excretion by fish: interspecific variation in a hypereutrophic lake. Oikos 116:259–270
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370
Vanni MJ, Flecker AS, Hood JM, Headworth JL (2002) Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol Lett 5:285–293
Vanni MJ et al (2005) Linking landscapes and food webs: effects of omnivorous fish and watersheds on reservoir ecosystems. Bioscience 55:155–167
Watson M (1958) The chemical composition of earthworm cuticle. Biochem J 68:416
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Acknowledgments
We are indebted to Claudio Cardoso Marinho for his help in conducting the laboratory nutrient analysis. This work was supported by grants from PETROBRAS. Scholarships were provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, J.M., Caliman, A., Guariento, R.D. et al. Stoichiometry of benthic invertebrate nutrient recycling: interspecific variation and the role of body mass. Aquat Ecol 44, 421–430 (2010). https://doi.org/10.1007/s10452-009-9302-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-009-9302-3