Abstract
Hibernation is a period of water deficit for some small mammals, and humidity strongly influences hibernation patterns. Dry conditions reduce length of torpor bouts, stimulate arousals, and decrease overwinter survival. To mitigate these effects, many small mammals hibernate in near saturated (100% RH) conditions. However, big brown bats (Eptesicus fuscus) hibernate in a wider variety of conditions and tolerate lower humidity than most other bats. To assess arid tolerance in this species, we compared torpid metabolic rates (TMR) and rates of total evaporative water loss (TEWL) between two populations of E. fuscus with differing winter ecologies: one that hibernates in humid karst caves and one that hibernates in relatively dry rock crevices. We used flow-through respirometry to measure TMR and TEWL of bats in humid and dry conditions. Torpid metabolic rates did not differ between populations or with humidity treatments. Rates of TEWL were similar between populations in humid conditions, but higher for cave-hibernating bats than crevice-hibernating bats in dry conditions. Our results suggest that E. fuscus hibernating in arid environments have mechanisms to decrease evaporative water loss that are not evident at more humid sites. Drought tolerance may facilitate the sedentary nature of the species, allowing them to tolerate more variable microclimates during hibernation and thus increasing the availability of overwintering habitat. The ability to survive arid conditions may also lessen the susceptibility of E. fuscus to diseases that affect water balance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Torpor allows individuals to lower their energetic demands during times of resource scarcity (Ruf and Geiser 2015). Metabolic rate (MR), and consequently body temperature (T b), are reduced, resulting in suppression of biological processes and considerable energy savings (Ruf and Geiser 2015). Water loss is also lower during torpor relative to euthermy (e.g., Studier 1970; Muñoz-Garcia et al. 2012a), and short-term torpor can be used as an adaptive strategy to avoid dehydration (Carpenter 1969). Rates of evaporative water loss (EWL) during torpor are reduced by over 90% from euthermic rates in some species (Morris et al. 1994; Webb et al. 1995). However, EWL persists and metabolically produced water often does not compensate for water lost through evaporation over the long term (Thomas and Cloutier 1992). Thus, torpor bouts are a period of negative water balance, and humidity is an important factor influencing patterns of small-mammal hibernation.
As humidity within or between hibernacula drops, the duration of torpor bouts shorten (Thomas and Geiser 1997) and arousal frequency increases (Ben-Hamo et al. 2013). In a similar vein, arousal rates also increase when hibernating animals are experimentally administered a diuretic (Németh et al. 2010). The implications of the effects of EWL are increased energy use throughout hibernation and ultimately decreased survival (Thomas and Cloutier 1992; Thomas and Geiser 1997; Willis et al. 2011; Ehlman et al. 2013). All small mammals periodically arouse during hibernation; although the exact causes are unclear (Willis 1982), dehydration may stimulate the need to drink (Fisher and Manery 1967; Speakman and Racey 1989; Thomas and Geiser 1997). Given sufficient energy stores (i.e., fat deposits), dehydration may be more likely to be a cause of death during hibernation than starvation (Speakman and Racey 1989).
To mitigate the effects of low humidity and avoid dehydration, many small mammals hibernate in humid roosts, dens, or burrows (e.g., Webb et al. 1996; Speakman and Thomas 2003). The rate of evaporation is determined by difference in absolute water vapor pressure (WVPabs) between a surface and the surrounding air (Δe v; Schmidt-Nielsen 1997). Convection and the temperature gradient between surface and air affect water vapor pressure and the rate of evaporation (Phillips 1966), but are likely negligible for animals thermoconforming in cool, still air typical of hibernacula (Webb et al. 1996). Thus, Δe v and the rate of EWL are minimized in near saturated conditions (~100% RH). Hibernators that experience lower rates of EWL arouse less often and use less energy (i.e., fat) throughout winter, which increases their chance of survival (Thomas and Cloutier 1992; Thomas and Geiser 1997; Ben-Hamo et al. 2013).
Bats present a particularly interesting model for water balance during hibernation. Although ventilation is reduced and completely arrested for periods of time (e.g., Thomas et al. 1990; Hays et al. 1991), bats have large lungs compared to birds and terrestrial mammals of similar size (Maina 2000) and likely experience higher rates of respiratory evaporative water loss (REWL). Additionally, the large surface area of the wings is a major avenue of cutaneous evaporative water loss (CEWL) in resting bats (Hosken and Withers 1997, 1999). On a wet mass basis, lipid oxidation produces more water than other fuel sources (Schmidt-Nielsen 1964), enough to keep larger, fully furred hibernators, such as bears, hydrated throughout the winter (Nelson 1980). However, metabolically produced water does not compensate for water lost through evaporation in bats (Thomas and Cloutier 1992). Thus, bats likely face challenges in maintaining water balance not incurred by other hibernators. Even at a humidity slightly below that typical of cave hibernacula used by vespertilionid bats in North America (~100% RH, WVPabs = 0.9 kPa at 6 °C; Webb et al. 1996), energy savings are diminished and survival is impacted (Thomas and Cloutier 1992; Geiser and Broome 1993).
Despite the profound influence of humidity on hibernating bats, North American big brown bats (Eptesicus fuscus) hibernate in a wider variety of conditions and tolerate lower humidity during hibernation than most other bat hibernators (e.g., Beer and Richards 1956; Kurta and Baker 1990). Populations of E. fuscus in the Canadian prairies hibernate in rock crevices that are drier and less thermally stable than most cave hibernacula (Klüg-Baerwald unpublished data). Water balance and the energy budgets of bats hibernating in the arid prairies appear different than those in other habitats and the risk of dehydration may be elevated. Factors that increase arousal frequency ultimately decrease overwintering survival (Ehlman et al. 2013). Given that water balance is critical during hibernation, some bat populations that overwinter in arid, “suboptimal” conditions may have mechanisms to mitigate water loss that are not evident in populations that hibernate in more typical cavernous hibernacula with high humidity. Our aim was to determine if E. fuscus that hibernate in prairie habitats show evidence of acclimatization or adaptation to dry conditions.
We compared torpid metabolic rate (TMR) and total evaporative water loss (TEWL) between two populations of bats with differing winter ecologies: one that overwinters in humid caves, and another that hibernates in arid rock crevices. Given that both populations hibernate in similar thermal conditions, we hypothesized that there would be no difference in TMR between populations under similar conditions. However, given the differences in humidity in which each population typically hibernates, we hypothesized that crevice-hibernating bats would be particularly well adapted to an arid environment, and thus have lower rates of TEWL in dry conditions than those of cave-hibernating bats.
Methods
Study species
Eptesicus fuscus is a medium-sized (mean mass = 18 g; van Zyll de Jong 1985), insectivorous bat (Family: Vespertilionidae) found throughout most of Canada and the U.S., through Central America and parts of the Caribbean, and into northern South America (Kurta and Baker 1990). Its distribution includes a variety of habitats, including urban, desert, forest, and prairie. Its roosting ecology is highly variable, using buildings (Barbour and Davis 1969), trees (Kalcounis and Brigham 1998), and rock crevices (Lausen and Barclay 2002) during summer, and buildings (Whitaker and Gummer 1992; Halsall et al. 2012), tree cavities (Rainey et al. 1992), rock crevices (Lausen and Barclay 2006), and caves (Mills et al. 1975; Reimer et al. 2014) as hibernacula in the winter. The wide range and varied roosting ecology of E. fuscus make it an ideal species to assess differences in physiology among multiple populations across heterogeneous environments.
Study sites
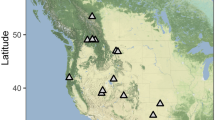
We sampled bats in Wood Buffalo National Park (WBNP) and Dinosaur Provincial Park (DPP), Alberta, Canada. Wood Buffalo National Park is located in northern Alberta and is comprised primarily of boreal forest with scattered wetlands and streams and some extensive karst formations (e.g., sinkholes and caves). Dinosaur Provincial Park is located along the Red Deer River in southern Alberta in a mixed landscape of prairie, badlands, and riparian habitat. It has a semiarid climate and contains an extensive network of creeks and drainages where E. fuscus overwinter in deep rock crevices (Lausen and Barclay 2006). We captured bats at Walk In Cave in WBNP (exact location withheld for confidentiality), a cavernous limestone hibernaculum used by E. fuscus and other species (Reimer et al. 2014). Conditions within this cave during winter are more humid (ca. 100% RH, mean WVPabs (±SD) = 0.61 ± 0.03 kPa at a mean T a (±SD) of 0.0 ± 0.63 °C; Klüg-Baerwald unpublished data) than those of rock-crevice hibernacula in DPP (ca. 52% RH, mean WVPabs (±SD) = 0.33 ± 0.09 kPa at a mean T a (±SD) of 0.6 ± 0.91 °C; Klüg-Baerwald, unpublished data).
Respirometry
Our study took place in mid- to late-September 2015, just prior to the hibernation season of E. fuscus, which typically begins mid-October in the WBNP region (Reimer et al. 2014) and late-October in DPP (Klüg-Baerwald et al. 2016). We captured bats within 3 h of sunset in mist nets set across the entrance to Walk In Cave in WBNP, and over the Little Sandhill Creek in DPP. We held captured bats in cloth bags and took morphometric measurements (e.g., forearm length, mass). We recorded body mass (m b) before and after metabolic trials and assumed a linear decrease in m b during metabolic measurements for use in mass-specific calculations. We then placed bats in closed metabolic chambers and allowed them to acclimate to experimental conditions for at least 8 h to ensure they were torpid and post-absorptive before measurements. During this time, we set chamber temperature to 6 °C and provided them with humidified air at a rate of 100 ml min−1. This prevented disturbance of bats prior to trials, and acclimated them in a microclimate resembling that typically experienced by hibernating bats in North America (Webb et al. 1996), thus increasing the likelihood of inducing torpor. We trimmed a small patch of hair from the interscapular region to within 1 mm of the skin and attached temperature sensitive dataloggers (iButton® model DS1921G; Maxim Semiconductors, Dallas, TX) to each bat using surgical adhesive (Skinbond®, Smith and Nephew United Inc., Largo, FL) to record skin temperature (T sk) at 1-min intervals. Skin temperature provides a reliable estimate of T b in small-bodied insectivorous bats (Willis and Brigham 2003). We considered an individual to be in steady-state torpor if T sk was within 3 °C of the chamber temperature and whole animal oxygen consumption (V O2) was stable for ≥1 h prior to metabolic measurements (Willis and Brigham 2003).
For metabolic chambers, we used 250 ml glass jars lined with metal mesh to allow bats to hang comfortably during trials, and covered the bottom of each chamber with a layer of mineral oil to prevent evaporation of water from feces and urine. We used thermocouple probes (TC-2000; Sable Systems International, Las Vegas, Nevada, USA) in each metabolic chamber to monitor temperature and placed chambers in a temperature-controlled cabinet (12 V portable cooler–warmer; Koolatron Refrigeration, Brantford, ON) set to 6 °C. We used Tygon tubing (Cole-Parmer, Montreal, QC) on all incurrent lines and Bev-A-Line tubing (Cole-Parmer) on all excurrent lines (Lighton 2008).
We used flow-through respirometry to measure oxygen consumption and determined total evaporative water loss (TEWL; cutaneous and evaporative) based on the difference in water vapor density between the incurrent and excurrent airstreams. We first measured MR and TEWL of bats exposed to relatively high humidity. To humidify incurrent air, we passed air at room temperature (~20 °C) through a bubbler made from a 500 ml glass jar filled with distilled water with an aquarium stone mounted on the inlet tubing. We then used a dew point generator and RH controller (DG-3; Sable Systems International) set to provide a saturated incurrent airstream at a dew point of 2 °C (~0.7 kPa). This level of humidity was not high enough for water vapor to condense inside the tubing and chambers. For low-humidity trials, we bypassed the bubbler and dew point generator, and instead used desiccant (Drierite; W. A. Hammond Drierite Co. Ltd., Xenia, OH) to remove water vapor from incurrent air. From there, we used a subsampler (SS-4; Sable Systems International) and factory calibrated flow controllers (MFC-2; Sable Systems International) to push air at a precisely controlled rate (100 ml min−1) into each of four metabolic chambers.
We used a water vapor analyzer (RH-300; Sable Systems International) to measure water vapor density (WVD; mg ml−1) of baseline air and excurrent air from the animal chambers. We then passed air through soda lime (to remove carbon dioxide) and Drierite (W. A. Hammond Drierite Co. Ltd.) before measuring oxygen concentration (%) with a factory calibrated oxygen analyzer (FC-10A; Sable Systems International). We sampled each animal for 15 min, with 5-min baselines before and after each chamber, and ran three consecutive sets of high-humidity trials. Following high-humidity trials, we allowed animals to acclimate to dry air conditions for 3 h before beginning measurements for low-humidity trials. Again, we sampled each animal for 15 min, with 5-min baselines before and after each chamber, and ran three consecutive sets of low-humidity trials. We recorded data at a rate of 1 Hz, discarded the first 2.5 min of data of each trial to account for system washout between samples, and later used ExpeData (ver. 1.8.5; Sable Systems International) to correct for drift and lag. We gave bats water ad libitum immediately after completion of the experiment and before they were released at the site of capture.
To calculate torpid metabolic rates (TMR; mW) of bats in steady-state torpor, we first calculated whole animal oxygen consumption (V O2; ml O2 min−1) of each trial for each individual using the equation of Withers (2001):
where V I is incurrent air flow (ml min−1) and F IO2 and F EO2 are fractional O2 composition for incurrent and excurrent air, respectively. We then multiplied these values by 60 to calculate hourly metabolic rates and used a conversion factor of 0.179 ml O2 h−1 per mW (Willis et al. 2005). We used the trial with the lowest mean TMR for each individual at each humidity treatment in our analyses.
We calculated whole animal total evaporative water loss (TEWL; mg H2O min−1), which includes respiratory and cutaneous EWL, as the difference in water vapor density between incurrent and excurrent air streams following Eq. (10.9) of Lighton (2008):
where FR i is incurrent air flow (ml min−1 STP), and F eH2O and F i H2O are fractional water vapor concentration (mg ml−1) in excurrent and incurrent air, respectively. We then multiplied these values by 60 to derive hourly rates of TEWL. For analyses, we used rate of TEWL recorded during the trial that corresponded with the lowest mean TMR trial for each individual at each humidity treatment, as described above.
Statistical analyses
We tested all data for normality using Shapiro–Wilk tests, as well as for homogeneity of variance between statistically compared groups using Bartlett tests. We used a Wilcoxon rank sum test to compare m b between populations because these data did not fit a normal distribution. We used Welch’s two sample t tests to compare excurrent WVPabs between populations within humidity treatments. The rate of EWL is negatively correlated with humidity of the microclimate surrounding the animal (i.e., WVP within the respirometry chambers; Thomas and Cloutier 1992). We found no difference in excurrent WVP between populations during high- and low-humidity treatments so we treated it as a categorical variable in further analyses. An analysis of variance (ANOVA) showed no significant variation in T sk or chamber temperature across trials, so we excluded this variable from further analyses. To compare TMR and TEWL between populations (WBNP and DPP) in humid and dry air, we used generalized linear mixed models (GLMM) with m b as a covariate, population and humidity as fixed effects, and individual as a random effect to account for the repeated measures design of our experiment. We did not remove any terms from our models given the importance of all independent variables and interactions to the hypotheses being tested. We conducted all statistical analyses using R (R Development Core Team 2016) and used an α value of 0.05 to assess significance. We present all data as mean ± SD.
The University of Regina President’s Committee on Animal Care All approved all methods and procedures (Animal Use Protocol #12-12). We conducted field captures under research and collection permits issued by Parks Canada (#WB-2015-19777), Alberta Sustainable Resource Development (#56512), and Alberta Tourism, Parks and Recreation Division (#15-096).
Results
We captured 15 male E. fuscus in WBNP and 12 males in DPP. Of these, 20 individuals (N WBNP = 10, N DPP = 10) entered steady-state torpor within 6 h of being placed in the metabolic chambers and remained torpid during all metabolic measurements; we included only these individuals in our analyses. Body masses differed between populations (W = 78, P = 0.035); bats from DPP had higher mb (\(\bar{X}\) = 20.2 ± 0.85 g) than those from WBNP (\(\bar{X}\) = 19.1 ± 1.25 g). Excurrent WVP did not vary between populations during high humidity (t 17.7 = 0.22, P = 0.828) or low humidity (t 17.8 = 0.04, P = 0.9705) treatments. Mean excurrent WVP was 0.78 ± 0.043 kPa for the high-humidity trials and 0.07 ± 0.012 kPa for low-humidity trials. Mean temperature inside metabolic chambers did not vary between treatments or populations (F 3,36 = 0.23, P = 0.854) and was 6.3 ± 0.15 °C during trials used in TMR and TEWL analyses. Mean T sk of bats recorded during analyzed trials was 7.4 ± 0.92 °C and did not vary with treatment and population (F 3,36 = 2.11, P = 0.105).
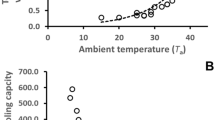
Torpid metabolic rate (TMR) did not differ between bats measured in humid or dry air (t 18 = 1.08, P = 0.297) or between populations (t 17 = 1.03, P = 0.319; Table 1; Fig. 1). Mean whole animal TMR of DPP bats was 4.2 ± 1.70 mW in humid air and 5.2 ± 1.58 mW in dry air. Mean whole animal TMR of WBNP bats was 4.9 ± 2.22 mW in humid air and 5.6 ± 2.91 mW in dry air. Conversely, rates of total evaporative water loss (TEWL) differed with humidity (t 18 = 3.33, P = 0.004), and the interaction between humidity and population was significant (t 18 = 6.55, P < 0.001; Table 2; Fig. 2). For both WBNP and DPP populations, whole animal TEWL was higher in dry air (WBNP \(\bar{X}\) = 15.9 ± 4.74 mg H2O h−1, DPP \(\bar{X}\) = 4.8 ± 1.27 mg H2O h−1) than in humid air (WBNP \(\bar{X}\) = 1.4 ± 0.72 mg H2O h−1, DPP \(\bar{X}\) = 1. 0 ± 1.03 mg H2O h−1). Mean whole animal TEWL of WBNP bats was approximately 3.3-fold higher than that of DPP bats in low humidity.
Discussion
Our data support the hypothesis that bats hibernating in the prairies are particularly well adapted to an arid environment, and thus have lower rates of evaporative water loss in dry conditions than bats from a more humid habitat. As expected, we found that the rate of total evaporative water loss (TEWL) of bats measured in dry air was higher than that of bats measured in humid conditions and, more importantly, the rate of TEWL did not differ between populations in humid air, but was approximately 3.3-fold higher in bats from the more mesic environment of Wood Buffalo National Park than in bats from the arid habitat of Dinosaur Provincial Park. Furthermore, we did not find differences in torpid metabolic rate between populations or between humidity treatments. This suggests that cutaneous evaporative water loss (CEWL) is important for the relationship between humidity and TEWL, with a lesser influence of variation in metabolism and respiratory evaporative water loss (REWL).
Small mammals use behavioral strategies to decrease EWL during hibernation. Clustering decreases the energetic cost of arousals (Roverud and Chappell 1991; Boyles et al. 2008) and reduces EWL (e.g., Boratyński et al. 2015; but see Studier 1970; Proctor and Studier 1970), presumably because it reduces exposed surface area (McNab 1969). Selection of humid hibernacula also mitigates water loss by minimizing the difference in water vapor pressure between air and skin (Schmidt-Nielsen 1997). Although many species of bats huddle in large groups during hibernation, E. fuscus is more commonly found solitarily or in small groups of less than 20 individuals (e.g., Phillips 1966), which likely limits the importance of huddling for water conservation. Furthermore, as evident in the population from DPP, E. fuscus commonly chooses hibernacula with variable and relatively low humidity compared to many other species of hibernating bat (Webb et al. 1996).
Small mammals also have physiological mechanisms to decrease EWL during hibernation. Although determining the specific mechanism of water retention is beyond the scope of this study, our data suggest a reduction in the rate of CEWL has a primary role. The rate of CEWL is largely determined by the composition of the Stratum corneum (SC), the outermost layer of the dermis (Bouwstra et al. 2003). Higher proportions of waxy lipids (cerebrosides and ceramides), and fewer free fatty acids and triacylglycerols, result in lower rates of CEWL (Haugen et al. 2003b). Indeed, greater amounts of cerebrosides and ceramides are found in birds (Muñoz-Garcia and Williams 2005; Clement et al. 2012; Champagne et al. 2012) and bats (Muñoz-Garcia et al. 2012b) from arid environments than are found in conspecifics from more mesic habitats. The role of SC composition in reducing water loss during hibernation remains largely unexplored.
The lipid profile of bat integument is not well described, but recent evidence suggests that the SC of epidermal wing tissue in some species of bat is comprised mostly of sphingomyelin (SM; Pannkuk et al. 2015). Breakdown of SM produces ceramide (van Smeden et al. 2014), thus regulation of this process may be involved in altering ceramide concentration of the SC and water permeability of the skin. There may also be a link between polyunsaturated fatty acids (PUFA) and CEWL. Increased consumption of dietary PUFA and a shift to higher proportions of n-6 fatty acids, particularly linoleic acid, facilitates entrance into torpor and lengthens torpor bouts during hibernation (Geiser and Kenagy 1987; Geiser 1991; Frank 1992). Although maintenance of cell membrane function at low temperature and reduction of metabolism are the ostensible benefits of high n-6 to n-3 PUFA ratios (Ruf and Arnold 2008), linoleic acid is also associated with ceramides and skin barrier function (Bowser et al. 1985). Deficiencies in ceramides or fatty acids within the SC result in increased transepidermal water loss (Menon et al. 2012). Determining the specific roles and interactions of lipids and fatty acids in permeability of wing membranes is an important area of future research.
Ventilation patterns during hibernation may also contribute to water conservation. Evaporative water loss from pulmonary structures (e.g., tracheal and alveolar surfaces) is reduced with decreased ventilation rates (Milsom and Jackson 2011). Additionally, episodic breathing allows some small hibernators to completely close the epiglottis or glottis during apneic periods, further reducing REWL while passively allowing sufficient gas exchange (e.g., Wilz et al. 2000). In some species, the respiratory tract remains open during hibernation only in high humidity (Thomas et al. 1990; Hays et al. 1991; Szewczak and Jackson 1992), which suggests an influence of REWL on the closing of the epiglottis or glottis to conserve water. Apneic periods are common in small hibernators (Milsom and Jackson 2011) and have been observed in hibernating E. fuscus (Szewczak and Jackson 1992).
Given that torpid big brown bats breathe approximately every 6.5 min at 5 °C (Szewczak and Jackson 1992), we expected to observe some sign of episodic breathing. However, we did not observe any evidence of this phenomenon (excurrent O2 and water vapor pressure were constant within trials, as well as between the 3 trails taken for each individual). Further, sampling of individuals during apneic periods, thus measuring passive diffusion of O2 into the lungs, would underestimate metabolic rates by ~35–54% (Szewczak and Jackson 1992). Our values for TMR recorded at ~6 °C (4.9 mW) are realistic given those recorded for this species at a temperature of 0–5 °C (2.3 mW; Willis et al. 2005). Other studies on hibernating E. fuscus also do not report apneic periods during sampling (Willis et al. 2005; Dunbar and Brigham 2010). Given the conflicting evidence for episodic breathing of hibernating E. fuscus despite the obvious benefit of apnea in reducing REWL, the potential for intraspecific variation in ventilation patterns and physiology to contribute to drought tolerance warrants further study.
Efficient mechanisms for water conservation are not unique to the population of E. fuscus from DPP. In fact, our measurements of TEWL are similar to those measured in desert conspecifics from populations in Arizona and California (Carpenter 1969). Thus, drought tolerance may be widespread in this species and may facilitate the sedentary nature of E. fuscus. Limited availability of appropriate overwintering habitat likely imposes constraints on the range of many temperate-zone species of bat. Seasonal, long distance movements from summer breeding grounds to humid, thermally stable subterranean (i.e., cave or mine) hibernacula (e.g., Norquay et al. 2013) or locations where winters are milder (e.g., Bisson et al. 2009; Cryan et al. 2014) are common. However, E. fuscus is generally thought to move only tens of kilometers between summering and wintering grounds (e.g., Beer 1955; Goehring 1972; Mills et al. 1975). Much of the habitat within the range of E. fuscus is non-mountainous, thus extensive cave systems are unavailable and roosts occur mostly in rock crevices, trees, and buildings (Kurta and Baker 1990). That E. fuscus is able to use such ubiquitous features as hibernacula, despite drier conditions within, may explain how it persists as one of the most common, widely distributed species of bat in North America without the need for seasonal, long distance movements.
Resilience of a population to changes in its environment depends on the physiological responses of individuals (Canale and Henry 2010). Acclimatization to dry conditions occurs within weeks of exposure in some species of bird, such as hoopoe larks (Alaemon alaudipes; Haugen et al. 2003a) and house sparrows (Passer domesticus; Muñoz-Garcia et al. 2008). Thus, individuals in these populations are likely to tolerate more variable conditions of humidity than those with less plastic physiological responses. Although we show E. fuscus can adapt to hibernate in arid environments, whether this is a result of acclimatization (i.e., phenotypic plasticity) or evolutionary adaptation (i.e., natural selection) remains unanswered. If the low rates of EWL we observed in E. fuscus are associated with phenotypic plasticity, individuals of this species may be less susceptible to novel threats or challenges that disrupt water balance during hibernation, such as increasingly arid conditions associated with climate change, or pathophysiologies associated with disease.
Dehydration may play a significant role in mortality from white-nose syndrome (WNS), an invasive fungal disease that has caused the deaths of over 6 million bats in North America (US Fish and Wildlife Service 2016). Bats die during winter while hibernating in cold, damp caves and mines, conditions under which the causative agent of WNS, Pseudogymnoascus destructans (Pd), grows best (Verant et al. 2012). Disrupted wing physiology caused by Pd infection leads to electrolyte imbalance and possible hypotonic dehydration (Cryan et al. 2013; Warnecke et al. 2013). Any mechanism to mitigate water loss during hibernation is likely to be advantageous given the pathology of WNS. Recent research even suggests the unique fatty acid composition of E. fuscus wing epidermis may even inhibit fungal growth (Frank et al. 2016). In addition, microclimates of hibernacula in the prairies are drier and colder than those of known cave hibernacula (Lausen and Barclay 2006; Klüg-Baerwald unpublished data) and outside the optimal growing conditions of Pd (Langwig et al. 2012). Bat populations that are able to hibernate in these colder, drier conditions may experience decreased WNS-related mortality.
In summary, our data support the hypothesis that bats overwintering in the prairies are well adapted to survive dry conditions, and experience lower rates of evaporative water loss than conspecific from more mesic environments. Drought tolerance is likely key in determining the range of conditions and habitats a species can inhabit successfully and may help predict the ability of a species to adapt to changes in climate or pathological threats that may alter their hibernation physiology. We also provide clear evidence of intraspecific differences in physiology between populations. Most energetic models based on physiological parameters sample individuals from a single population and do not account for the physiological differences associated with habitat or latitude (e.g., Humphries et al. 2002; but see Dunbar and Brigham 2010). Given that latitude, habitat, and possibly even microclimate can influence physiology, conclusions based on geographically restricted samples may not accurately represent the entire species. In general, more research is needed on intraspecific differences in physiology across heterogeneous habitats, and attempts to model energetics or survivorship based on physiological parameters should consider plasticity in these metrics.
References
Barbour RW, Davis WH (1969) Bats of America. University Press, Lexington
Beer JR (1955) Survival and movements of banded big brown bats. J Mammal 36:242–248. doi:10.2307/1375883
Beer JR, Richards AG (1956) Hibernation of the big brown bat. J Mammal 37:31–41. doi:10.2307/1375523
Ben-Hamo M, Muñoz-Garcia A, Williams JB et al (2013) Waking to drink: rates of evaporative water loss determine arousal frequency in hibernating bats. J Exp Biol 216:573–577. doi:10.1242/jeb.078790
Bisson I-A, Safi K, Holland RA (2009) Evidence for repeated independent evolution of migration in the largest family of bats. PLoS One 4:e7504. doi:10.1371/journal.pone.0007504
Boratyński JS, Willis CKR, Jefimow M, Wojciechowski MS (2015) Huddling reduces evaporative water loss in torpid Natterer’s bats, Myotis nattereri. Comp Biochem Physiol A 179:125–132. doi:10.1016/j.cbpa.2014.09.035
Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M (2003) Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res 42:1–36. doi:10.1016/S0163-7827(02)00028-0
Bowser PA, Nugteren DH, White RJ et al (1985) Identification, isolation and characterization of epidermal lipids containing linoleic acid. BBA Lipid Lipid Met 834:419–428. doi:10.1016/0005-2760(85)90016-5
Boyles JG, Storm JJ, Brack V (2008) Thermal benefits of clustering during hibernation: a field test of competing hypotheses on Myotis sodalis. Funct Ecol 22:632–636. doi:10.1111/j.1365-2435.2008.01423.x
Canale CI, Henry PY (2010) Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim Res 43:135–147. doi:10.3354/cr00897
Carpenter RE (1969) Structure and function of the kidney and the water balance of desert bats. Physiol Zool 42:288–302. doi:10.2307/30155492
Champagne AM, Muñoz-Garcia A, Shtayyeh T et al (2012) Lipid composition of the stratum corneum and cutaneous water loss in birds along an aridity gradient. J Exp Biol 215:4299–4307. doi:10.1242/jeb.077016
Clement ME, Muñoz-Garcia A, Williams JB (2012) Cutaneous water loss and covalently bound lipids of the stratum corneum in nestling house sparrows (Passer domesticus L.) from desert and mesic habitats. J Exp Biol 215:1170–1177. doi:10.1242/jeb.064972
Cryan PM, Meteyer CU, Blehert DS et al (2013) Electrolyte depletion in white-nose syndrome bats. J Wildl Dis 49:398–402. doi:10.7589/2012-04-121
Cryan PM, Stricker CA, Wunder MB (2014) Continental-scale, seasonal movements of a heterothermic migratory tree bat. Ecol Appl 24:602–616. doi:10.1890/13-0752.1
Dunbar MB, Brigham RM (2010) Thermoregulatory variation among populations of bats along a latitudinal gradient. J Comp Physiol B 180:885–893. doi:10.1007/s00360-010-0457-y
Ehlman SM, Cox JJ, Crowley PH (2013) Evaporative water loss, spatial distributions, and survival in white-nose-syndrome-affected little brown myotis: a model. J Mammal 94:572–583. doi:10.1644/12-MAMM-A-111.1
Fisher KC, Manery JF (1967) Water and electrolyte metabolism in heterotherms. In: Fisher KC, Dawe AR, Lyman CP, Schonbaum E (eds) Mammalian hibernation. Edinburgh, Scotland, pp 235–279
Frank CL (1992) The influence of dietary fatty acids on hibernation by golden-mantled ground squirrels (Spermophilus lateralis). Physiol Zool 65:906–920. doi:10.2307/30158549
Frank CL, Ingala MR, Ravenelle RE et al (2016) The effects of cutaneous fatty acids on the growth of Pseudogymnoascus destructans, the etiological agent of white-nose syndrome (WNS). PLoS One 11:e0153535. doi:10.1371/journal.pone.0153535
Geiser F (1991) The effect of unsaturated and saturated dietary lipids on the pattern of daily torpor and the fatty acid composition of tissues and membranes of the deer mouse Peromyscus maniculatus. J Comp Physiol B 161:590–597. doi:10.1007/BF00260749
Geiser F, Broome LS (1993) The effect of temperature on the pattern of torpor in a marsupial hibernator. J Comp Physiol B 163:133–137. doi:10.1007/BF00263598
Geiser F, Kenagy GJ (1987) Polyunsaturated lipid diet lengthens torpor and reduces body temperature in a hibernator. Am J Physiol Regul I 252:897–901
Goehring HH (1972) Twenty-year study of Eptesicus fuscus in Minnesota. J Mammal 53:201–207. doi:10.2307/1378850
Halsall AL, Boyles JG, Whitaker JO (2012) Body temperature patterns of big brown bats during winter in a building hibernaculum. J Mammal 93:497–503. doi:10.1111/brv.12137/full
Haugen MJ, Tieleman BI, Williams JB (2003a) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum. J Exp Biol 206:3581–3588. doi:10.1242/jeb.00596
Haugen MJ, Williams JB, Wertz PW, Tieleman BI (2003b) Lipids of the stratum corneum vary with cutaneous water loss among larks along a temperature-moisture gradient. Physiol Biochem Zool 76:907–917. doi:10.1086/380213
Hays GC, Webb PI, Speakman JR (1991) Arrhythmic breathing in torpid pipistrelle bats, Pipistrellus pipistrellus. Respir Physiol 85:185–192. doi:10.1016/0034-5687(91)90060-V
Hosken DJ, Withers PC (1997) Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. J Comp Physiol B 167:71–80. doi:10.1007/s003600050049
Hosken DJ, Withers PC (1999) Metabolic physiology of euthermic and torpid lesser long-eared bats, Nyctophilus geoffroyi (Chiroptera: Vespertilionidae). J Mammal 80:42–52. doi:10.2307/1383206
Humphries MM, Thomas DW, Speakman JR (2002) Climate-mediated energetic constraints on the distribution of hibernating mammals. Lett Nat 418:313–316. doi:10.1038/nature00828
Kalcounis MC, Brigham RM (1998) Secondary use of aspen cavities by tree-roosting big brown bats. J Wildl Manag 62:603–611. doi:10.2307/3802336
Klüg-Baerwald BJ, Gower LE, Lausen C, Brigham RM (2016) Environmental correlates and energetics of winter flight by bats in Southern Alberta. Can J Zool, Canada. doi:10.1139/cjz-2016-0055
Kurta A, Baker RH (1990) Eptesicus fuscus. Mammal Species 356:1–10. doi:10.2307/3504258
Langwig KE, Frick WF, Bried JT et al (2012) Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol Lett 15:1050–1057. doi:10.1111/j.1461-0248.2012.01829.x
Lausen CL, Barclay RMR (2002) Roosting behaviour and roost selection of female big brown bats (Eptesicus fuscus) roosting in rock crevices in southeastern Alberta. Can J Zool 80:1069–1076. doi:10.1139/z02-086
Lausen CL, Barclay RMR (2006) Winter bat activity in the Canadian prairies. Can J Zool 84:1079–1086. doi:10.1139/z06-093
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists. Oxford University Press, New York
Maina JN (2000) What it takes to fly: the novel respiratory structural and functional adaptations in birds and bats. J Exp Biol 203:3045–3064
McNab BK (1969) The economics of temperature regulation in neutropical bats. Comp Biochem Physiol 31:227–268. doi:10.1016/0010-406X(69)91651-X
Menon GK, Cleary GW, Lane ME (2012) The structure and function of the stratum corneum. Int J Pharm 435:3–9. doi:10.1016/j.ijpharm.2012.06.005
Mills RS, Barrett GW, Farrell MP (1975) Population dynamics of the big brown bat (Eptesicus fuscus) in southwestern Ohio. J Mammal 56:591–604. doi:10.2307/1379471
Milsom WK, Jackson DC (2011) Hibernation and gas exchange. Comprehen Physiol 1:397–420. doi:10.1002/cphy.c090018
Morris S, Curtin AL, Thompson MB (1994) Heterothermy, torpor, respiratory gas exchange, water balance and the effect of feeding in Gould’s long-eared bat Nyctophilus gouldi. J Exp Biol 197:309–335
Muñoz-Garcia A, Williams JB (2005) Cutaneous water loss and lipids of the stratum corneum in house sparrows Passer domesticus from arid and mesic environments. J Exp Biol 208:3689–3700. doi:10.1242/jeb.01811
Muñoz-Garcia A, Cox RM, Williams JB (2008) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum in house sparrows (Passer domesticus) following acclimation to high and low humidity. Physiol Biochem Zool 81:87–96. doi:10.1086/522651
Muñoz-Garcia A, Ben-Hamo M, Pinshow B et al (2012a) The relationship between cutaneous water loss and thermoregulatory state in Kuhl’s pipistrelle Pipistrellus kuhlii, a Vespertillionid bat. Physiol Biochem Zool 85:516–525. doi:10.1086/666989
Muñoz-Garcia A, Ro J, Reichard JD et al (2012b) Cutaneous water loss and lipids of the stratum corneum in two syntopic species of bats. Comp Biochem Physiol A 161:208–215. doi:10.1016/j.cbpa.2011.10.025
Nelson RA (1980) Protein and fat metabolism in hibernating bears. Fed Proc 39:2955–2958
Németh I, Nyitrai V, Németh A, Altbäcker V (2010) Diuretic treatment affects the length of torpor bouts in hibernating European ground squirrels (Spermophilus citellus). J Comp Physiol B 180:457–464. doi:10.1007/s00360-009-0426-5
Norquay KJO, Martinez-Nuñez F, Dubois JE et al (2013) Long-distance movements of little brown bats (Myotis lucifugus). J Mammal 94:506–515. doi:10.1644/12-MAMM-A-065.1
Pannkuk EL, McGuire LP, Warnecke L et al (2015) Glycerophospholipid profiles of bats with white-nose syndrome. Physiol Biochem Zool 88:425–432. doi:10.1086/681931
Phillips GL (1966) Ecology of the big brown bat (Chiroptera: Vespertilionidae) in northeastern Kansas. Am Midl Nat 75:168–198. doi:10.2307/2423489
Proctor JW, Studier EH (1970) Effects of ambient temperature and water vapor pressure on evaporative water loss in Myotis lucifugus. J Mammal 51:799–804. doi:10.2307/1378307
Rainey WE, Pierson ED, Colberg M, Barclay JH (1992) Bats in hollow redwoods: seasonal use and role in nutrient transfer into old growth communities. Bat Res News 33:71
Reimer JP, Lausen CL, Barclay RMR et al (2014) Bat activity and use of hibernacula in Wood Buffalo National Park, Alberta. Northwest Nat 95:277–288. doi:10.1898/13-30.1
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Roverud RC, Chappell MA (1991) Energetic and thermoregulatory aspects of clustering behavior in the neotropical bat Noctilio albiventris. Physiol Zool 64:1527–1541. doi:10.2307/30158228
Ruf T, Arnold W (2008) Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. Am J Physiol Regul Integr 294:1044–1052. doi:10.1152/ajpregu.00688.2007
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926. doi:10.1111/brv.12137
Schmidt-Nielsen K (1964) Desert animals: Physiological problems of heat and water. Oxford University Press, London
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment. Cambridge University Press, Cambridge
Speakman JR, Racey PA (1989) Hibernal ecology of the pipistrelle bat: energy expenditure, water requirements and mass loss, implications for survival and the function of winter emergence flights. J Anim Ecol 58:797. doi:10.2307/5125
Speakman JR, Thomas DW (2003) Physiological ecology and energetics of bats. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, pp 430–492
Studier EH (1970) Evaporative water loss in bats. Comp Biochem Physiol 35:935–943. doi:10.1016/0010-406X(70)90087-3
Szewczak JM, Jackson DC (1992) Apneic oxygen uptake in the torpid bat, Eptesicus fuscus. J Exp Biol 173:217–227
Thomas DW, Cloutier D (1992) Evaporative water loss by hibernating little brown bats, Myotis lucifugus. Physiol Zool 65:443–456. doi:10.2307/30158262
Thomas DW, Geiser F (1997) Periodic arousals in hibernating mammals: is evaporative water loss involved? Funct Ecol 11:585–591. doi:10.1046/j.1365-2435.1997.00129.x
Thomas DW, Cloutier D, Gagné D (1990) Arrhythmic breathing, apnea and non-steady state oxygen uptake in hibernating little brown bats (Myotis lucifugus). J Exp Biol 149:395–406
van Smeden J, Janssens M, Gooris GS, Bouwstra JA (2014) The important role of stratum corneum lipids for the cutaneous barrier function. BBA Mol Cell Biol L 1841:295–313. doi:10.1016/j.bbalip.2013.11.006
van Zyll de Jong CG (1985) Handbook of Canadian mammals. National Museums of Canada, Ottawa, ON
Verant ML, Boyles JG, Waldrep W Jr et al (2012) Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS One 7:e46280. doi:10.1371/journal.pone.0046280
Warnecke L, Turner JM, Bollinger TK et al (2013) Pathophysiology of white-nose syndrome in bats: a mechanistic model linking wing damage to mortality. Biol Lett 9:20130177. doi:10.1098/rsbl.2013.0177
Webb PI, Speakman JR, Racey PA (1995) Evaporative water loss in two sympatric species of vespertilionid bat, Plecotus auritus and Myotis daubentonii: relation to foraging mode and implications for roost site selection. J Zool 235:269–278. doi:10.1111/j.1469-7998.1995.tb05143.x
Webb PI, Speakman JR, Racey PA (1996) How hot is a hibernaculum? A review of the temperatures at which bats hibernate. Can J Zool 74:761–765. doi:10.1139/z96-087
Whitaker JO, Gummer SL (1992) Hibernation of the big brown bat, Eptesicus fuscus, in buildings. J Mammal 73:312–316. doi:10.2307/1382062
Willis JS (1982) The mystery of the periodic arousal. In: Lyman CP, Willis JS, Malan A, Wang LCH (eds) Hibernation and torpor in mammals and birds. Academic Press, New York, pp 92–103
Willis CKR, Brigham RM (2003) Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J Comp Physiol B 173:379–389. doi:10.1007/s00360-003-0343-y
Willis CKR, Lane JE, Liknes ET et al (2005) Thermal energetics of female big brown bats (Eptesicus fuscus). Can J Zool 83:871–879. doi:10.1139/z05-074
Willis CKR, Menzies AK, Boyles JG, Wojciechowski MS (2011) Evaporative water loss is a plausible explanation for mortality of bats from white-nose syndrome. Integr Comp Biol 51:1–10. doi:10.1093/icb/icr076
Wilz M, Milsom WK, Heldmaier G (2000) Intermittent ventilation in hibernating dormice—is ventilation always necessary to meet metabolic demands? In: Heldmaier G, Klingenspor M (eds) Life in the cold. Springer, Berlin, pp 169–178
Withers PC (2001) Design, calibration and calculation for flow-through respirometry systems. Aust J Zool 49:445–461. doi:10.1071/ZO00057
Acknowledgements
We thank: S. Bohn for valuable help in the field and for titling this paper; E. Baerwald and two anonymous reviewers for comments that improved this manuscript; Alberta Environment and Parks (Dinosaur Provincial Park) and Parks Canada (Wood Buffalo National Park) for logistics and lodging; and the American Society of Mammalogists (Grant-In-Aid of Research to BJK) and Natural Sciences and Engineering Research Council of Canada (Discovery Grant to RMB and Canadian Graduate Scholarship to BJK) for funding.
Author contribution statement
BJK conceived and designed the experiments, conducted fieldwork, and analyzed the data. BJK and RMB secured funding and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Christian Voigt.
Rights and permissions
About this article
Cite this article
Klüg-Baerwald, B.J., Brigham, R.M. Hung out to dry? Intraspecific variation in water loss in a hibernating bat. Oecologia 183, 977–985 (2017). https://doi.org/10.1007/s00442-017-3837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3837-0