Abstract
The global degradation of coral reefs is having profound effects on the structure and species richness of associated reef fish assemblages. Historically, variation in the composition of fish communities has largely been attributed to factors affecting settlement of reef fish larvae. However, the mechanisms that determine how fish settlers respond to different stages of coral stress and the extent of coral loss on fish settlement are poorly understood. Here, we examined the effects of habitat degradation on fish settlement using a two-stage experimental approach. First, we employed laboratory choice experiments to test how settlers responded to early and terminal stages of coral degradation. We then quantified the settlement response of the whole reef fish assemblage in a field perturbation experiment. The laboratory choice experiments tested how juveniles from nine common Indo-Pacific fishes chose among live colonies, partially degraded colonies, and dead colonies with recent algal growth. Many species did not distinguish between live and partially degraded colonies, suggesting settlement patterns are resilient to the early stages of declining coral health. Several species preferred live or degraded corals, and none preferred to associate with dead, algal-covered colonies. In the field experiment, fish recruitment to coral colonies was monitored before and after the introduction of a coral predator (the crown-of-thorns starfish) and compared with undisturbed control colonies. Starfish reduced live coral cover by 95–100%, causing persistent negative effects on the recruitment of coral-associated fishes. Rapid reductions in new recruit abundance, greater numbers of unoccupied colonies and a shift in the recruit community structure from one dominated by coral-associated fishes before degradation to one predominantly composed of algal-associated fish species were observed. Our results suggest that while resistant to coral stress, coral death alters the process of replenishment of coral reef fish communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and degradation have been major factors responsible for declining populations (Vitousek et al. 1997), loss of biodiversity (Brooks et al. 2002) and the disruption of ecosystem services (McCarty 2001; Malcolm et al. 2006) in terrestrial environments. It has been estimated that almost one-half of the land surface has been modified by human activities (Vitousek et al. 1997) and more extinctions have been attributed to habitat loss than any other factor (Dirzo and Raven 2003). Terrestrial habitat loss or alteration has been caused by a number of mechanisms, including human predation and extinction of important predators and herbivores (Lyons et al. 2004), habitat transformation for farming or development (Vitousek et al. 1997), introduction of exotic species (Seabloom et al. 2006) and most recently climate change (Sala et al. 2000; Walther et al. 2002).
One of the central issues in conservation biology has been to identify the characteristics that render species prone to habitat change (Lampila et al. 2005; Cushman 2006). A key factor appears to be their level of specialisation, particularly a strong association with features of a habitat that are susceptible to anthropogenic disturbance (McKinney 1997; Hughes et al. 2000; Kotze and O’Hara 2003). Habitat specialists may be highly dependent on particular habitats throughout their lives or they may pass through critical stages in their life cycle that render them highly responsive to changes in the quantity or quality of their habitat (Halpern et al. 2005; Moore and Elmendorf 2006). For open populations, the decline and extirpation of suitable recruitment habitat can potentially be a major determinant of population decline.
There is increasing evidence of the widespread loss or modification of a range of habitats in shallow marine environments (e.g. Farnsworth and Ellison 1997; Alongi 2002; Duarte 2002; McClanahan 2002; Steneck et al. 2002). The risk to biodiversity from loss of marine habitats is increasing as the scale of habitat loss expands (Dulvy et al. 2003; Munday 2004b; Kappel 2005). Coral reefs appear to be particularly susceptible to a range of natural and anthropogenic disturbances that have reduced coral cover on a global scale (Hoegh-Guldberg 1999; McClanahan 2002; Gardner et al. 2003; Hughes et al. 2003). While clearly significant for corals, recent work also indicates that coral reef fish assemblages often exhibit dramatic changes in structure and loss of biodiversity in relation to declining coral cover (Jones and Syms 1998; Halford et al. 2004; Jones et al. 2004; Graham et al. 2006; Wilson et al. 2006). While coral reef fish communities comprise the full spectrum of coral dependency, from specialists in obligate association with a single coral species (Munday et al. 1997; Munday 2004a), to those found on almost any substratum (Green 1996), the magnitude of the changes in fish communities in response to habitat change suggests a widespread reliance on the underlying coral reef habitat. However, the demographic mechanisms responsible for changes in fish community structure in response to habitat disturbance are poorly understood.
The life history transition during which larvae undergo metamorphosis into juveniles and take up residence on coral reefs is a critical period for reef fishes. Juveniles can exhibit strong habitat selection at settlement, including selection for particular coral substrata (Tolimieri 1995; Öhman et al. 1998; Holbrook et al. 2002) or particular depths or reef zones (Srinivasan 2003) and can also use the presence or absence of conspecifics or other fishes as settlement cues (Jones 1987; Sweatman 1988; Booth 1992, 1995). The dynamics and distribution of adult fish populations, and how they ultimately respond to disturbance, may be strongly influenced by habitat-limited recruitment (Schmitt and Holbrook 2000; Syms and Jones 2000; Booth and Beretta 2002). Recent work suggests that a large proportion of reef fishes may preferentially recruit into live branching corals, even many of those not necessarily associated with corals as adults (Jones et al. 2004). Species-specific differences in coral preferences and levels of specialisation are likely to influence how fish assemblages respond to live coral loss (Syms and Jones 2000; Munday 2004b; Gardiner and Jones 2005; Feary et al. 2007). However, our understanding of the extent of habitat selection at the time of recruitment, and the effects of coral death on fish recruitment require further investigation.
Although phase shifts in the structure of coral reef fish communities may begin with the effects of habitat change on recruitment, support for this hypothesis has been limited to a few monitoring studies (Lewis 1998; Booth and Beretta 2002; Jones et al. 2004) and a laboratory-based study (Öhman et al. 1998). Further experimental studies are needed to understand the extent of the relationship between fish recruitment and coral degradation. For example, while we know that reef fishes are choosy at settlement, we do not know at what stage of declining coral health they begin discriminating among corals. That is, do they avoid settling into bleached corals (which may recover) or do they only distinguish among living and dead (algal covered) substrata? Also, Wilson et al. (2006) showed that the nature of fish community changes depends upon the type of disturbance, distinguishing among disturbances that kill corals while leaving the structure intact [e.g. bleaching, crown-of-thorns starfish (COTS)] from those that destroy the structure as well (e.g. storms, mechanical damage). While several studies have experimentally established the response of fishes to the mechanical disturbance of reefs (Lewis 1998; Syms and Jones 2000), there have been no experimental demonstrations of the effects of the loss of living coral tissue only.
The aims of this study were two fold. Firstly, we set out to test whether the selection of benthic microhabitats by larvae of several common Indo-Pacific reef fishes was influenced by the health of coral colonies. To do this, habitat choice experiments were conducted in laboratory aquaria to examine the degree to which juveniles distinguished between live colonies, partially degraded colonies, and dead colonies with recent algal growth. We hypothesised that habitat specialists were more likely to distinguish between habitats within the aquaria than habitat generalists. We also hypothesised that the nature and strength of association between newly settled fishes and healthy coral would determine how they responded to habitat degradation. We tested this second hypothesis in an experiment conducted in the field. Coral colonies were experimentally degraded by introducing a coral predator, the COTS, and we monitored and compared natural settlement to degraded and control colonies.
Materials and methods
Laboratory preference experiment
Settlement-stage larvae of several common Indo-Pacific damselfishes (Pomacentridae) were collected using light traps (Stobutzki and Bellwood 1998) from the lagoon at Lizard Island on the northern Great Barrier Reef, Australia (14°40′S; 145°28′E). The species collected were assigned to three habitat use categories based on patterns of adult habitat use (Randall et al. 1997; Allen et al. 2003): live coral-associated species (Chromis viridis, Pomacentrus moluccensis), degraded coral associates (Chrysiptera flavipinnis, Chrysiptera rollandi, Pomacentrus amboinensis, Pomacentrus nagasakiensis), and species associated with dead, algal-covered corals (Dischistodus prosopotaenia, Pomacentrus chrysurus and Pomacentrus wardi). Larvae were held prior to trials in featureless glass aquaria supplied with filtered seawater and aeration.
In each trial, three coral colonies (Acropora cerealis), each in a different category of health, were placed in a large, circular 300-l tank filled with filtered seawater. All colonies were collected from the field in their natural state of health; there was no experimental manipulation of live coral cover. The three coral colonies were randomly arranged in a triangular configuration along the sides of the tank with equal distance between colonies. The condition of the three colonies was as follows: live (100% live coral cover), degraded (>75% reduction in live coral) and algal-covered (dead coral with 1- to 2-week-old algal growth) (personal observation). All colonies were 20–30 cm2 in diameter. Three larvae of the same species were utilised within each trial. Monthly peaks in pomacentrid settlement occur over a few nights each month (Milicich et al. 1992). During these temporal periods extremely large numbers of larvae will settle onto the reef and it is unlikely that for the majority of species settlement will occur in isolation from other individuals. Using three larvae within each trial ensured that species’ natural behaviour at settlement was shown. Larvae were introduced into the centre of the tank between 2000 and 2100 hours and their colony choice was recorded the following morning at 0500 hours, and subsequently every 30 min until 1300 hours. Twenty-one individuals of each species were tested over seven trials (n = 7). Individuals <10 cm from a colony were deemed to be associated with that colony. Coral colonies were only used in a single trial and tanks were cleaned between trials.
Since individuals within each trial could not be separately identified, the average abundance of all individuals (within each fish species) associating with each of the three colony categories was quantified over all seven trials. As the data did not satisfy the assumptions of normality and homogeneity of variance required by ANOVA, Kruskal–Wallis ANOVA on ranks was used to examine whether significant differences in habitat association were apparent within each fish species. Any significant differences found in habitat associations within species were then examined using post hoc Mann–Whitney U-tests, to determine the importance of each habitat in influencing species habitat associations. The Mann–Whitney U-test is a nonparametric alternative to the t-test for comparing differences in population means and has the advantage of not requiring normal probability distribution for the data. We identified potential metrics when these tests showed significant differences (P ≤ 0.05).
Field disturbance experiment
The field experiment was conducted in Kimbe Bay, West New Britain, Papua New Guinea (5°30′S, 150°05′E). All invertebrate predators were removed from ten colonies of A. cerealis (20–30 cm2 diameter) on each of three reef complexes (A, B, C). Over a 2- to 3-month period, naturally settling fishes were collected every 3–4 days from each colony using hand nets and clove oil as an anaesthetic (Munday and Wilson 1997). Surveys and collections were conducted at reef C from April to June 2005 and at reefs A and B from August to September 2005. After this 2- to 3-month period, all colonies on each reef were completely caged with small wire-mesh cages. On each reef a single COTS was introduced into each of five randomly selected cages (experimental), whereas the remaining five colonies were left unmanipulated (control). Cages remained in place for 2 days and were then removed. Experimental colonies lost from 95 to 100% of live coral cover, whereas there was no reduction in live coral on control colonies. Natural settlement of fishes was then quantified every 3–4 days from June to November 2005 on reef C, and September to November 2005 on reefs A and B. Recent work has shown that despite seasonal fluctuations in the abundance of fish larvae settling into reefs within Kimbe Bay coinciding with wet seasons (February–November) (Srinivasan and Jones 2006), the majority of common coral reef fishes show little fluctuation in successful settlement throughout the year. Thus, natural settlement rate was expected to vary little throughout the year, allowing us to compare new settler composition between collections throughout the experimental time period.
The average abundance and species richness of new settlers associated with replicate coral colonies (between experimental and control) was quantified within each reef, based on the entire sampling time before and then after introduction of COTS. Both the average abundance and average species richness of new settlers associated with coral colonies within each reef, over each sampling period, were then analysed using repeated measures multivariate ANOVA, with the average abundance and average species richness of new settlers as dependent variables. Factors in each repeated measures model were reefs (A, B, C), treatment (control, experimental) and colony surveys through time as the repeated measure.
Results
Laboratory preference experiment
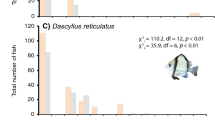
Significant differences in habitat association were apparent within the majority of study species, when comparing the average abundance of all individuals (within each fish species) associating with each of the three colony categories throughout trials (Table 1). Mann–Whitney U-tests showed that within the species showing significant differences in habitat association, several groups were apparent; species preferentially using the live habitat, species associating with both live and degraded habitats and species preferentially found in the degraded habitat (Table 2). The habitat use of P. moluccensis and C. viridis both conformed to adult categories, with both using live habitats. In comparison, both C. flavipinnis and P. amboinensis were more dependent on the live coral than adult categories would predict. Adults of both species consistently associate with degraded habitats, though within laboratory aquaria both species preferentially used either live or degraded habitats (Table 2). Pomacentrus chrysurus were predominantly found using the live habitat, while P. wardi associated with the degraded habitat (Table 2)
Temporal changes in habitat association between 0500 and 1300 hours were apparent between species (Fig. 1). High levels of movement between habitats were apparent during the first temporal period, with the majority of species associated with all three habitats in the first temporal period (Fig. 1). By the second or third temporal periods, however, the majority of species had associated with a particular habitat and invariably remained in that habitat for the duration of the trial (Fig. 1). Nevertheless, differences in movement between habitats were found between the study species (Fig. 1). Species not closely associating with the live habitat were less likely to remain in distinct habitats within the aquaria, moving more frequently throughout the habitats during trials, while those associating with the live habitat would move into this habitat in the first or second temporal period and remain in this habitat for the duration of the trial (Fig. 1).
Field experiment
Significant differences in both the abundance and species richness of new settlers associating with control and experimental treatments on all three reefs were apparent (Table 3), due to large reductions in new settlers associating with experimental colonies after live coral degradation (Fig. 2). Reductions in the abundance of new settlers on experimental colonies were most apparent on both A and B, with a 95 and 97% decrease in abundance after coral degradation, respectively, while a 75% decrease in new settler abundance was also apparent on C.
Reductions in the species richness of assemblages in experimental coral colonies were also apparent throughout reefs, with at least 40% decrease in the average species richness in experimental coral colonies on all three reefs, after caging (Fig. 2). Such large reductions in the abundance and species richness of new settlers associating with experimental colonies were largely due to reductions in the settlement of two species strongly associated with live coral, the coral goby Gobiodon quinquestrigatus (Gobiidae), which reduced in proportional abundance by 12% after coral loss and the damselfish, P. moluccensis, which decreased in proportion by 100% on degraded colonies (Fig. 3). In comparison, although fluctuations in both the abundance and species richness of newly settled fishes were observed on control colonies, the magnitude of these changes was significantly less than that observed on experimental reefs (Fig. 2).
Changes in the composition of new settlers associating with experimental, opposed to control, colonies were apparent after caging (Fig. 3). Within experimental colonies, increased abundances of combtooth blennies (Blenniidae) and triplefins (Tripterygiidae) (e.g. Ecsenius prooculis, Helcogramma spp.) were observed settling into the habitats, while decreased abundances of species more closely associated with live coral were found within the habitats (Fig. 3). On experimental colonies fishes closely associated with algal resources (e.g. blennies and triplefins) comprised only 1.8% of the assemblage 2 weeks before live caging, which increased to 18% two weeks after cages were taken off and over 90% after 8 weeks. In comparison, little change in the composition of new settling species associating with control colonies was apparent before and after caging (Fig. 3).
Significant temporal changes in both the abundance and species richness of new settlers were apparent between treatments (Table 3). Degradation of experimental colonies led to temporal reductions in both the abundance and species richness of new settlers associating with experimental colonies 2 weeks after coral loss (Fig. 4). In comparison, little change or an increase in the abundance and a decrease in species richness of new settlers were apparent in control colonies (Fig. 4).
Although no significant difference in percent occupancy was apparent between control and experimental colonies before caging (ANOVA, F = 0.344, df = 1, P = 0.565), reductions of live coral cover (following caging) on experimental colonies resulted in a significantly higher frequency of empty colonies during subsequent sampling periods than control colonies (ANOVA, F = 60.54, df = 1, P < 0.001). Across all three reef complexes, 4 weeks before live coral loss an average of 52% (± 7.4 SE) of experimental colonies contained at least one individual, whereas 4 weeks after caging and live coral degradation only 9% (± 4.2 SE) of experimental colonies were occupied. In contrast, the percent occupancy on control reefs increased during the experiment: 4 weeks before caging an average of 45% (± 10 SE) of control colonies were occupied by at least one individual, whereas 4 weeks after manipulation an average of 74% (± 6.4 SE) of control colonies were occupied.
Discussion
Our results support an emerging view that settlement is likely to be a crucial bottleneck that determines the impact of coral degradation on reef fish biodiversity and community structure (Booth and Wellington 1998; Jones et al. 2004). For a number of coral-associated reef fish species, reductions in the availability of live coral at settlement may decrease their numerical abundance within degraded reef systems. Such changes in settlement patterns with reef degradation may then lead to a shift in the community structure of reef-associated fish communities, away from live coral specialists to degraded reef associates. This mechanism may account for the dramatic changes to coral-associated reef fish populations in response to declining live coral cover (see also Halford et al. 2004; Jones et al. 2004; Garpe et al. 2006; Graham et al. 2006; Wilson et al. 2006).
The majority of study species preferentially associated with either live or partially degraded coral colonies within laboratory aquaria, and none of the species examined preferentially associated with dead, algal-covered colonies. This suggests that several coral-associated reef fish species may be resilient to the early stages of degradation. For these species, the extent of coral loss may be an important factor in structuring settlement patterns. Within this study degraded treatments held up to 25% live coral cover. At this spatial scale, the degraded coral colony may have held sufficient live coral cover for species to utilise the habitat. However, once coral death and algal overgrowth had occurred there was little to no settlement of species into the habitat. Such preferences for live and degraded habitats at settlement were apparent in fishes that both associate with live coral throughout their lives (e.g. P. moluccensis) (Booth 2002; Booth and Beretta 2002) and for many with little to no use of live coral colonies throughout the juvenile or adult stage (e.g. P. chrysurus) (Allen et al. 2003). Hence, adult responses to declining coral cover may be more a result of settlement preferences than adult habitat requirements (Jones et al. 2004).
Variations in the availability of appropriate settlement habitat may influence settlement patterns in a variety of reef-associated fishes (Holbrook et al. 2000), and can affect both local species richness and population abundance (Schmitt and Holbrook 2000). However, few studies have experimentally examined the role of habitat alteration in structuring reef fish settlement patterns, with most studies strictly observational, and therefore unable to distinguish habitat changes from other potential causative factors (Booth and Beretta 2002). In this study, reductions in live coral on experimentally degraded in situ colonies led to reductions in both the abundance and species richness of newly settling fishes, a pattern observed in other studies (Booth and Beretta 2002). Although reductions in the settlement of a range of fish species were apparent on experimentally degraded colonies, such community decline was primarily due to reduced settlement of the coral-associated goby, G. quinquestrigatus and the coral-associated damselfish, P. moluccensis. Both fishes dominated live coral colonies, settling in large numbers in this habitat. Degradation of colonies by COTS predation and subsequent growth of algae reduced the number of new settlers of both fishes, with virtually no individuals of either species found on degraded colonies.
A physical reduction in available shelter occurs when degraded coral colonies are colonized by algae (i.e. algae reduces the amount of available space between coral branches) (Munday 2001) and this may decrease the suitability of colonies for new settlers, independent of live coral loss (Öhman et al. 1998). However, relatively immediate reductions in the abundance and species richness of new settlers on dead in situ colonies that had accumulated only light algal growth (2 weeks) were apparent in this study. This observation suggests that the loss of live coral itself was responsible for decreased settlement (Garpe et al. 2006). An increasing array of work is now showing that a variety of cues (i.e. chemical, auditory and visual) can form vital indicators for reef naïve fish larvae to initiate benthic settlement behaviour (Lecchini et al. 2005a; Lecchini et al. 2005b). In the present study, degradation of experimental coral colonies may have reduced or even negated such settlement cues, resulting in significant declines in new settlers associating with the degraded habitat.
A range of factors may have increased the loss of new settlers on algal-covered corals between surveys (every 3–4 days), such as predation or interspecific competition for resources (Steele and Forrester 2002; Webster 2002). However, post-settlement exploration and movement of new settlers into preferred habitats may have occurred, independent of ontogenetic changes in habitat use (Webster and Hixon 2000; Munday 2001). Exploration of habitats at settlement is a well-known behaviour in both terrestrial and aquatic animals (Stamps and Krishnan 1995; Leis and Carson-Ewart 2002; Hawkins et al. 2003). Habitat exploration is thought to allow animals to examine potential habitats (Leis and Carson-Ewart 2002), targeting habitats where reproduction and survival (i.e. fitness) will be highest (Haughland and Larsen 2004). Within the present study, exploration of aquaria habitats at dawn was found, with species moving between habitats before associating with a preferred habitat. As individuals utilising preferred habitats can show increased juvenile growth (Jones 1997; Munday 2001) or survival (Wellington 1992; Munday 2001), post-settlement movement into preferred habitats may have positive effects on the demographic structure of reef fish species (Munday 2004a). It is possible that in situ settlement occurs in two phases, beginning with a coarse-scale selection of appropriate habitat, followed by early post-settlement movement into preferred substrata (sensu Finn and Kingsford 1996; McCormick and Makey 1997) and may be an important behavioural choice for successful settlement and recruitment in a range of reef-associated fishes in degraded habitats.
Associations with algal biomass (including seagrass habitats and mangroves) at settlement have been shown in a small number of taxonomic groups (e.g. Green 1998; Shima 2001; Dorenbosch et al. 2005a; Dorenbosch et al. 2005b); however, few studies have examined the response of reef fish settlement to increased algal resources following coral degradation. The majority of work has focused on the role of algal resources in structuring the abundance and species richness of adults within degraded coral reef habitats (McClanahan et al. 1999, 2001). Such work has shown that the algal biomass can provide a considerable array of resources for a range of coral reef fishes, including sites for foraging (Ceccarelli et al. 2001; Townsend and Tibbetts 2004), shelter (Wilson 2001; Clarke and Tyler 2003) and reproduction (Neat 2001). However, this study provides some of the first evidence that shifts in reef fish community structure from the numerical dominance of live coral associates to one where algal associates numerically dominate can occur through changes in fish settlement with coral degradation and algal biomass increase (but see Booth and Beretta 2002). Such alterations in reef fish settlement patterns following reef degradation may then have strong and persistent influences on the abundance and diversity of reef fish species closely associated with live coral habitats at settlement (Schmitt and Holbrook 2000).
This work has fundamental implications in understanding the response of coral-associated reef fishes to increasing levels of live coral degradation. As the availability of suitable live coral settlement habitat decline on degraded reefs, the abundance of several species may rapidly decline and be replaced by species that preferentially associate with degraded, algal-dominated habitats (Jones et al. 2004). We can predict that such changes in recruitment for these species may then interact with their longevity to determine the time scale of effects of live coral loss on community structure. Within fishes with short life spans and high population turnover rates, substantial reductions in new settler abundance and richness with live coral loss may have relatively immediate, negative effects on community replenishment (Munday and Jones 1998; Booth and Beretta 2002). In comparison, for longer lived species, we can predict that reductions in recruit abundance with live coral loss may have little effect on their population abundance in the short term, with significant effects on the community apparent at much longer time scales (Pratchett et al. 2006). Recent research has argued that phase delays in the response of the associated reef fish community to coral loss may be due to a coupling of the adult fish with the coral reefs’ physical complexity (Graham et al. 2006). Broad reductions in the structure of reef fish communities may then be linked to the breakdown of the coral reefs’ physical complexity (Lewis 1998; Syms and Jones 2000; Graham et al. 2006). Although the loss of the underlying coral reef structure will have detrimental effects on the community abundance of a range of reef-associated fishes (Sano et al. 1984; Gratwicke and Speight 2005; Wilson et al. 2006), we predict that reductions in the abundance and richness of newly settling coral-associated fishes with loss of the living coral tissue only may play a substantial role in altering the replenishment and ultimately the structure of their populations within degraded reef systems.
References
Allen GR, Steene RC, Humann P, DeLoach N (2003) Reef fish identification: Tropical Pacific. New World Publications, Fla.
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29:331–349
Booth DJ (1992) Larval settlement patterns and preferences by domino damselfish Dascyllus albisella gill. J Exp Mar Biol Ecol 155:85–104
Booth DJ (1995) Juvenile groups in a coral-reef damselfish: density-dependent effects on individual fitness and population demography. Ecology 76:91–106
Booth DJ (2002) Distribution changes after settlement in six species of damselfish (Pomacentridae) in One Tree Island lagoon, Great Barrier Reef. Mar Ecol Prog Ser 226:157–164
Booth DJ, Wellington G (1998) Settlement preferences in coral-reef fishes: effects on patterns of adult and juvenile distributions, individual fitness and population structure. Aust J Ecol 23:274–279
Booth DJ, Beretta GA (2002) Changes in a fish assemblage after a coral bleaching event. Mar Ecol Prog Ser 245:205–212
Brooks TM, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Rylands AB, Konstant WR, Flick P, Pilgrim J, Oldfield S, Magin G, Hilton-Taylor C (2002) Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol 16:909–923
Ceccarelli DM, Jones GP, McCook LJ (2001) Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr Mar Biol Annu Rev 39:355–389
Clarke RD, Tyler JC (2003) Differential space utilization by male and female spinyhead blennies, Acanthemblemaria spinosa (Teleostei: Chaenopsidae). Copeia 2003:241–247
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
Dirzo R, Raven PH (2003) Global state of biodiversity and loss. Annu Rev Environ Resour 28:137–167
Dorenbosch M, Grol MGG, Nagelkerken I, van der Velde G (2005a) Distribution of coral reef fishes along a coral reef-seagrass gradient: edge effects and habitat segregation. Mar Ecol Prog Ser 299:277–288
Dorenbosch M, Grol MGG, Christianen MJA, Nagelkerken I, van der Velde G (2005b) Indo-Pacific seagrass beds and mangroves contribute to fish density coral and diversity on adjacent reefs. Mar Ecol Prog Ser 302:63–76
Duarte CM (2002) The future of seagrass meadows. Environ Conserv 29:192–206
Dulvy NK, Sadovy Y, Reynolds JD (2003) Extinction vulnerability in marine populations. Fish Fish 4:25–64
Farnsworth EJ, Ellison AM (1997) The global conservation status of mangroves. Ambio 26:328–334
Feary DA, Almany GR, Jones GP, McCormick MI (2007) Coral degradation and the structure of tropical reef fish communities. Mar Ecol Prog Ser 333:243–248
Finn MD, Kingsford MJ (1996) Two-phase recruitment of Apogonids (Pisces) on the Great Barrier Reef. Mar Freshwater Res 47:423–432
Gardiner NM, Jones GP (2005) Habitat specialisation and overlap in a guild of coral reef cardinalfishes (Apogonidae). Mar Ecol Prog Ser 305:163–175
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
Garpe KC, Yahya SAS, Lindahl U, Öhman MC (2006) Long-term effects of the 1998 bleaching event on reef fish assemblages. Mar Ecol Prog Ser 315:237–247
Graham NAJ, Wilson SK, Jennings S, Polunin NVC, Bijoux JP, Robinson J (2006) Dynamic fragility of oceanic coral reef ecosystems. Proc Natl Acad Sci USA 103:8425–8429
Gratwicke B, Speight MR (2005) The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667
Green AL (1996) Spatial, temporal and ontogenetic patterns of habitat use by coral reef fishes (family Labridae). Mar Ecol Prog Ser 133:1–11
Green AL (1998) Spatio-temporal patterns of recruitment of labroid fishes (Pisces: Labridae and Scaridae) to damselfish territories. Environ Biol Fishes 51:235–244
Halford A, Cheal AJ, Ryan DAJ, Williams DM (2004) Resilience to large-scale disturbance in coral and fish assemblages on the Great Barrier Reef. Ecology 85:1892–1905
Halpern BS, Gaines SD, Warner RR (2005) Habitat size, recruitment, and longevity as factors limiting population size in stage-structured species. Am Nat 165:82–94
Haughland DL, Larsen KW (2004) Exploration correlates with settlement: red squirrel dispersal in contrasting habitats. J Anim Ecol 73:1024–1034
Hawkins LA, Armstrong JD, Magurran AE (2003) Settlement and habitat use by juvenile pike in early winter. J Fish Biol 63:174–186
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshwater Res 8:839–866
Holbrook SJ, Forrester GE, Schmitt RJ (2000) Spatial patterns in abundance of a damselfish reflect availability of suitable habitat. Oecologia 122:109–120
Holbrook SJ, Brooks AJ, Schmitt RJ (2002) Variation in structural attributes of patch-forming corals and in patterns of abundance of associated fishes. Mar Freshwater Res 53:1045–1053
Hughes JB, Daily GC, Ehrlich PR (2000) Conservation of insect diversity: a habitat approach. Conserv Biol 14:1788–1797
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystroem M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Jones GP (1987) Some interactions between residents and recruits in two coral reef fishes. J Exp Mar Biol Ecol 114:169–182
Jones GP (1997) Relationships between recruitment and postrecruitment processes in lagoonal populations of two coral reef fishes. J Exp Mar Biol Ecol 213:231–246
Jones GP, Syms C (1998) Disturbance, habitat structure and the ecology of fishes on coral reefs. Aust J Ecol 23:287–297
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci USA 101:8251–8253
Kappel CV (2005) Losing pieces of the puzzle: threats to marine, estuarine, and diadromous species. Front Ecol Environ 3:275–282
Kotze DJ, O’Hara RB (2003) Species decline-but why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 135:138–148
Lampila P, Mönkkönen M, Desrochers A (2005) Demographic responses by birds to forest fragmentation. Conserv Biol 19:1537–1546
Lecchini D, Planes S, Galzin R (2005a) Experimental assessment of sensory modalities of coral-reef fish larvae in the recognition of their settlement habitat. Behav Ecol Sociobiol 58:18–26
Lecchini D, Shima J, Banaigs B, Galzin R (2005b) Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia 143:326–334
Leis JM, Carson-Ewart BM (2002) In situ settlement behaviour of damselfish (Pomacentridae) larvae. J Fish Biol 61:325–346
Lewis AR (1998) Effects of experimental coral disturbance on the population dynamics of fishes on large patch reefs. J Exp Mar Biol Ecol 230:91–110
Lyons SK, Smith FA, Brown JH (2004) Of mice, mastodons and men: human-mediated extinctions on four continents. Evo Ecol Res 6:339–358
Malcolm JR, Liu C, Neilson RP, Hansen L, Hannah L (2006) Global warming and extinctions of endemic species from biodiversity hotspots. Conserv Biol 20:538–548
McCarty JP (2001) Ecological consequences of recent climate change. Conserv Biol 15:320–331
McClanahan TR (2002) The near future of coral reefs. Environ Conserv 29:460–483
McClanahan TR, Hendrick V, Rodrigues MJ, Polunin NVC (1999) Varying responses of herbivorous and invertebrate-feeding fishes to macroalgal reduction on a coral reef. Coral Reefs 18:195–203
McClanahan TR, McField M, Huitric M, Bergman K, Sala E, Nystrom M, Nordemar I, Elfwing T, Muthiga NA (2001) Responses of algae, corals and fish to the reduction of macroalgae in fished and unfished patch reefs of Glovers Reef Atoll, Belize. Coral Reefs 19:367–379
McCormick MI, Makey LJ (1997) Post-settlement transition in coral reef fishes: overlooked complexity in niche shifts. Mar Ecol Prog Ser 153:247–257
McKinney ML (1997) Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu Rev Ecol Syst 28:495–516
Milicich MJ, Meekan MG, Doherty PJ (1992) Larval supply: a good predictor of recruitment of three species of reef fish (Pomacentridae). Mar Ecol Prog Ser 86:153–166
Moore KA, Elmendorf SC (2006) Propagule vs. niche limitation: untangling the mechanisms behind plant species’ distributions. Ecol Lett 9:797–804
Munday PL (2001) Fitness consequences of habitat use and competition among coral-dwelling fishes. Oecologia 128:585–593
Munday PL (2004a) Competitive coexistence of coral-dwelling fishes: the lottery hypothesis revisited. Ecology 85:623–628
Munday PL (2004b) Habitat loss, resource specialization, and extinction on coral reefs. Global Change Biol 10:1642–1647
Munday PL, Wilson SK (1997) Comparative efficacy of clove oil and other chemicals in anaesthetization of Pomacentrus amboinensis, a coral reef fish. J Fish Biol 51:931–938
Munday PL, Jones GP (1998) The ecological implications of small body size among coral-reef fishes. Oceanogr Mar Biol Annu Rev 36:373–411
Munday PL, Jones GP, Caley MJ (1997) Habitat specialisation and the distribution and abundance of coral-dwelling gobies. Mar Ecol Prog Ser 152:227–239
Neat FC (2001) Male parasitic spawning in two species of triplefin blenny (Tripterygiidae): contrasts in demography, behaviour and gonadal characteristics. Environ Biol Fishes 61:57–64
Öhman MC, Munday PL, Jones GP, Caley MJ (1998) Settlement strategies and distribution patterns of coral-reef fishes. J Exp Mar Biol Ecol 225:219–238
Pratchett MS, Wilson SK, Baird AH (2006) Declines in the abundance of Chaetodon butterflyfishes following extensive coral depletion. J Fish Biol 69:1269–1280
Randall JE, Allen GR, Steene RC (1997) Fishes of the Great Barrier Reef and Coral Sea. University of Hawaii, Hawaii
Sala OE, Chapin FS III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Biodiversity global biodiversity scenarios for the year 2100. Science 287:1770–1774
Sano M, Shimizu M, Nose Y (1984) Changes in structure of coral reef fish communities by destruction of hermatypic corals: observational and experimental views. Pac Sci 38:51–80
Schmitt RJ, Holbrook SJ (2000) Habitat limited recruitment of coral reef damselfish. Ecology 81:3479–3494
Seabloom EW, Williams JW, Slayback D, Stoms DM, Viers JH, Dobson AP (2006) Human impacts, plant invasion, and imperiled, plant species in California. Ecol Appl 16:1338–1350
Shima JS (2001) Recruitment of a coral reef fish: roles of settlement habitat and postsettlement losses. Ecology 82:2190–2199
Srinivasan M (2003) Depth distributions of coral reef fishes: the influence of microhabitat structure, settlement, and post-settlement processes. Oecologia 137:76–84
Srinivasan M, Jones G (2006) Extended breeding and recruitment periods of fishes on a low latitude coral reef. Coral Reefs 25:673–682
Stamps JA, Krishnan VV (1995) Territory acquisition in lizards. III. Competing for space. Anim Behav 49:679–693
Steele MA, Forrester GE (2002) Early postsettlement predation on three reef fishes: effects on spatial patterns of recruitment. Ecology 83:1076–1091
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Stobutzki IC, Bellwood DR (1998) Nocturnal orientation to reefs by late pelagic stage coral reef fishes. Coral Reefs 17:103–110
Sweatman H (1988) Field evidence that settling coral reef fish larvae detect resident fishes using dissolved chemical cues. J Exp Mar Biol Ecol 124:163–174
Syms C, Jones GP (2000) Disturbance, habitat structure, and the dynamics of a coral-reef fish community. Ecology 81:2714–2729
Tolimieri N (1995) Effects of microhabitat characteristics on the settlement and recruitment of a coral reef fish at two spatial scales. Oecologia 102:52–63
Townsend KA, Tibbetts IR (2004) The ecological significance of the combtoothed blenny in a coral reef ecosystem. J Fish Biol 65:77–90
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of earth’s ecosystems. Science 277:494–499
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Webster MI (2002) Role of predators in the early post-settlement demography of coral-reef fishes. Oecologia 131:52–60
Webster MS, Hixon MA (2000) Mechanisms and individual consequences of intraspecific competition in a coral-reef fish. Mar Ecol Prog Ser 196:187–194
Wellington GM (1992) Habitat selection and juvenile persistence control the distribution of two closely related Caribbean damselfishes. Oecologia 90:500–508
Wilson S (2001) Multiscale habitat associations of detrivorous blennies (Blenniidae: Salariini). Coral Reefs 20:245–251
Wilson SK, Graham NAJ, Pratchett M, Jones GP, Polunin NVC (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Global Change Biol 12:2220–2234
Acknowledgements
This project was supported by grants from the Australian Coral Reef Society, Great Barrier Reef Marine Park Authority, Mahonia Na Dari Research and Conservation Centre, Walindi Plantation Resort and The Nature Conservancy to D. A. F., a National Science Foundation fellowship (IRFP no. 0202086) to G. R. A., a James Cook University Merit Research Grant to M. I. M. and an Australian Research Council Discovery grant to G. P. J. Many thanks to C. Denny, D. Godoy, T. Knight, L. Peacock, J. Pickering, B. Ponde, L. Romaso and S. van Dijken for invaluable field assistance. All experiments conducted in this study comply with Australian and Papua New Guinean laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Rights and permissions
About this article
Cite this article
Feary, D.A., Almany, G.R., McCormick, M.I. et al. Habitat choice, recruitment and the response of coral reef fishes to coral degradation. Oecologia 153, 727–737 (2007). https://doi.org/10.1007/s00442-007-0773-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0773-4