Abstract
One of the great mysteries of coral-reef fish ecology is how larvae locate the relatively rare patches of coral-reef habitat on which they settle. The present study aimed to estimate, by experiments in aquaria, the sensory modalities of coral-reef fish larvae for senses used in searching for their species’ settlement habitat. Larval recognition of settlement habitat can be based on the detection of conspecifics and/or of characteristics of coral habitat using visual, chemical and mechanical cues. For this study, larvae were captured with crest nets and were then introduced into experimental tanks that allowed testing of each type of cue separately (visual, chemical or mechanical cues). Among the 18 species studied, 13 chose their settlement habitat due to the presence of conspecifics and not based on the characteristics of coral habitat, and 5 species did not move toward their settlement habitat (e.g. Scorpaenodes parvipinnis, Apogon novemfasciatus). Among the different sensory cues tested, two species used the three types of cues (Parupeneus barberinus and Ctenochaetus striatus: visual, chemical and mechanical cues), six used two types (e.g. Myripristis pralinia: visual and chemical cues; Naso unicornis: visual and mechanical cues), and five used one type (e.g. Chrysiptera leucopoma: visual cues; Pomacentrus pavo: chemical cues). These results demonstrate that many coral-reef fish larvae could in practice use sensory cues for effective habitat selection at settlement, and have the ability to discriminate species-specific sensory cues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To move in space and time, living systems should perceive, localise, see and identify the information emanating from the environment that surrounds them and from the other organisms belonging to the same or different species. To touch, see, feel, taste and hear provides information on the environment so that any movement of a few centimetres or thousands of kilometres enables an organism to make the best choice in the search of food, sexual partner or settlement habitat. Animal movements, then, have important consequences, at different ecological levels and scales, for a variety of ecological processes (see Westcott and Graham 2000). Movement influences an animal’s risk of predation, the resources it encounters, interactions with conspecifics, and the biotic and abiotic conditions it experiences (see Weins et al. 1995). Movement also provides insights into an organism’s interaction with the environment. Such influences combine to make movement an underlying determinant of population and metapopulation dynamics within species (Turchin 1996). However, without perceiving the information emanating from the environment that surrounds them, the animal’s movements are inefficient (see Cassier et al. 2000).
Some coral-reef fish larvae have swimming abilities sufficient to control their pattern of oceanic dispersion and their return to adult habitat (e.g. Leis and Carson-Ewart 1997; Stobutzki and Bellwood 1997; Dudley et al. 2000). However, these swimming abilities will be useful only if larvae can detect suitable habitat at settlement, because it is unlikely that successful settlement is solely a matter of chance (for review, see Doherty 2002). Perhaps one of the greatest challenges facing the majority of marine reef organisms with larval stages that potentially disperse and develop in offshore waters is how to relocate the relatively rare patches of coral-reef habitat on which they settle and ultimately reside as adults (for review, see Myrberg and Fuiman 2002). The answer must lie partly in the sensory modalities of fishes. Two research topics must be distinguished: (1) how do pelagic larvae recognise the island to colonise, and (2) how do reef larvae recognise their settlement habitat? At present, most studies address the sensory recognition of islands to colonise (e.g. Tolimieri et al. 2000; Kingsford et al. 2002; Leis et al. 2002). The present study examines the sensory modalities of coral-reef fish larvae to recognise their settlement habitat at Moorea Island (French Polynesia).
The life-cycle of most fish species on coral reefs includes a planktonic larval phase (in the open ocean), which usually lasts from 3 to 6 weeks, followed by a sedentary reef phase (in the lagoon) for the juveniles and adults (for review, see Victor 1991). During the oceanic phase, the larvae may move far from their native island due to currents (Milicich 1994) and/or their swimming abilities (Stobutzki and Bellwood 1997). Then larvae return to the reef (natal or not) to continue their development into juveniles, and then to adults. Generally, larvae enter the lagoon across the reef crest by night (colonisation phase; Dufour and Galzin 1993). In the hours following this colonisation, larvae undergo metamorphosis and choose suitable habitats (settlement phase), based mainly on the characteristics of coral habitat and the presence or absence of conspecifics (individuals of same species), as well as other species. This settlement phase has been widely studied among coral-reef fish (for review, see Doherty 2002), yet little information is available on the behavioural responses of larvae to the sensory cues from the environment (for review, see Myrberg and Fuiman 2002).
Cues allowing the larvae to detect the settlement habitat of their species can be emitted by the coral habitat itself (specific shape of coral colony or specific odour of anemone) and/or by the conspecifics already settled. Sweatman (1988) studied in situ the chemical modalities of two species of Pomacentridae (Dascyllus aruanus and D. reticulatus). He used a pump system to collect water from coral colonies containing conspecifics and inject it into coral colonies that did not have any fish. The coral colonies that were injected with the water had a higher settlement rate of juveniles of D. aruanus and D. reticulatus than the colonies without pumped-in water. Booth (1992) demonstrated in aquaria that for D. albisella (Pomacentridae) sight played a role in choosing the settlement habitat. Lastly, Elliott et al. (1995) demonstrated in situ that larvae of Amphiprion detected the presence of anemones by smell up to 8 m. Only these three studies have dealt with recognition of settlement habitats by coral-reef fishes.
The present study aimed to estimate, by experiments in aquaria, the sensory modalities of coral-reef fish larvae for senses used in searching for their species’ settlement habitat. Larval recognition of settlement habitat can be based on the detection of conspecifics and/or characteristics of coral habitat using visual (e.g. shape of a coral colony), chemical (e.g. an odour of anemone) and mechanical (e.g. vibratory or sound waves of fish) cues.
Methods
The present study was carried out from February to May 2002. The list of target species was not predetermined, but depended on daily catches by crest nets. The crest nets placed on the reef crest of Moorea Island allowed the capture of fish larvae just before they entered the lagoon to settle (Dufour and Galzin 1993). The conspecifics (individuals of the same species as that of the larvae tested), used as cue transmitters, were juveniles caught with crest nets and maintained in aquaria for 15–21 days. The coral habitat was a living or dead spherical coral colony of 6 cm radius, or coral rubble in a spherical mass of 6 cm radius. A preliminary study has determined the settlement coral habitat of each species tested (Lecchini 2003).
Description and principle of crest nets
Fish larvae were sampled with four crest nets, similar to those used by Dufour and Galzin (1993). Each net had a rectangular mouth (1.8 m wide, 1 m height) oriented across the water flow, and was made of 2-mm mesh, which was fine enough to retain all incoming larvae. Cod-ends were attached to the nets in the afternoon to minimise the catch of debris during earlier daylight hours when few fish larvae are normally captured (Dufour and Galzin 1993), and detached in the early morning to remove the captured larvae. Fish captured during the night were collected at dawn, transferred to and subsequently maintained in aquaria containing UV-sterilised seawater filtered through a 50-µm filter. All experiments described here were conducted during evenings immediately following capture of larvae (i.e. within 24 h of collection).
Description and principle of the experimental system
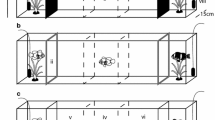
The objective of the experiment was to test the three types of sensory cues (visual, chemical and mechanical cues) separately in order to learn if the larvae could use them to recognise their settlement habitat. The special experimental system consisted of an aquarium with five compartments (A–E), three of them interconnected (A, B and C), to which were added two aquaria with one compartment (tank), on each side (Fig. 1). Larvae were introduced in the central compartment of the aquarium (noted A) and could remain in it or move toward the adjacent compartments (noted B and C) through funnels (anti-return system).
The aquarium system used to evaluate the sensory modalities of coral-reef fish larvae at settlement. The special experimental system consists of an aquarium with five compartments (A–E), with A, B and C interconnected via funnels and D and E isolated from central compartments via plastic panels affixed with removable opaque barriers. Additional tanks (labelled no. 1 and 2) are isolated from the five-compartment aquarium and mounted upon separate platforms to prevent transfer of vibratory signals. Larvae are introduced into the central compartment of the aquarium (A) and can remain in it or move toward the adjacent compartments (B and C) due to funnels (anti-return system).
To ensure that only the sensory cues tested were responsible for the movements of larvae, the experiment was done in a laboratory room isolated from outside light and outside noises (experiments were done at night). Indoor light came from neon lights that were regularly distributed in the room (to avoid different larval responses according to light levels and direction). The water in the aquaria was collected in the lagoon via a pump system. This water was then poured into tanks, filtered through a tray of sand (50-µm pores), and sterilised by ultraviolet light. This relatively pure water was renewed with each test.
To ensure the responses of larvae would be independent of one another (not group effect), each test involved the placement of 1 larva in an aquarium (20 aquaria and 40 tanks were set up for the study). Twenty larvae were individually tested in the recognition of each type of sensory cue. Thus, the visual, chemical and mechanical cues from conspecifics were tested during the same night with 60 larvae, caught the night before. The visual and chemical cues from coral habitats were tested during the same night (different from the conspecifics experiment night) with 40 larvae, caught the night before.
To test the visual cues, conspecifics or a coral habitat were placed in the tanks. As the tanks and the aquarium did not have physical contact and were mounted on polystyrene plates, neither vibrations nor chemicals could be transmitted to the larvae in the central compartment in the aquarium (Katzir 1981). In this treatment, only the visual cues could be responsible for the movements of larvae.
To test the chemical cues, compartment B or C was filled independently of the other two compartments (a sheet of plastic was placed in front of the corresponding hole to seal it) with water in which some conspecifics (five conspecifics in 3 l of water) or a coral habitat (spherical coral colony or coral rubble in a spherical mass with a 6 cm radius in 3 l of water) had been immersed for 6 h. The larvae were placed in the central compartment (filled with water in which no fish or coral habitat had been immersed), and the sheet of plastic was removed so that chemicals could diffuse into the central chamber (to avoid a potential artefact due to the presence of plastic sheet in the aquarium, a second and similar plastic sheet was placed and then removed between compartment A and compartment B or C filled with water in which no fish or coral habitat had been immersed). In this treatment, only the chemical cues could be responsible for the movements of larvae.
The mechanical cues were tested solely with conspecifics. The conspecifics were placed in either compartment D or E. The glass panes that separated compartment D from B and E from C were obscured by an opaque plastic sheet (attached tightly to the glass) to eliminate any visual cues. Furthermore, the chemical cues could not spread between these compartments. In this treatment, only the mechanical cues (i.e. vibratory and/or sound waves of conspecifics) could be responsible for the movements of larvae.
Experiment 1: detection of the visual, chemical and mechanical cues emitted by the conspecifics
If larvae are attracted to conspecifics, the larvae must recognise cues emitted by conspecifics and not cues from other species (heterospecifics). To show this conspecific attraction, the experimental protocol was as follows.
(i) Experiment to verify the absence of external effects on the aquarium system
One larva was placed in the central compartment of each of the aquaria and after a period of 2 min, the compartment in which each larva was present was noted. One chi-square test was carried out to determine if the observed distribution was identical to the theoretical distribution (ten larvae per compartment: B and C). As the aquaria were equipped with anti-return systems (one-way funnels between the central and the adjacent compartments), the theoretical distribution, after random diffusion and if sufficient sampling time has elapsed, will end up as (50%; 0%; 50%): no larvae in the central compartment, and half the larvae on one side, the other half on the other side (across the aquaria). A second possibility was that 20 larvae remained for the most part in the central compartments (no movements). If either condition was met, we assumed no external effects were present in the aquarium system and the study proceeded.
A preliminary study (carried out on 11 of the 18 species tested in the present study), where larvae were monitored closely, allowed us to determine the best time for sampling to achieve an equilibrium state (Lecchini 2003). Species that are slow movers may remain in the central compartment if the sampling time is too short. We observed during the pilot experiment that if the larva did not move for 2 min, it generally stayed in the central compartment for at least 5 min (maximal time tested). Yet, in situ, the response of larvae to the settlement cues should be to quickly reduce the high predation at settlement (Steele and Forrester 2002; Doherty et al. 2004). We have thus defined the best sampling time as 2 min.
(ii) Experiment to test for repulsion in response to the heterospecifics (example of the visual cues)
Five heterospecifics (juveniles of any fish species among the different species captured with crest nets but other than that of the larvae tested, see Table 1) were placed in tank no. 2. One larva was placed in the central compartment of each of the aquaria, and after a period of 2 min the compartment in which each larva was present was noted. The chi-square test was carried out to compare the observed distribution to a baseline distribution (number of larvae in compartments A, B and C in the absence of external effects on the aquarium system). The species of heterospecifics was used in the following experimental protocol only if the test larvae were not repulsed by the heterospecifics (larvae present significantly in compartment B).
(iii) Response to specific cues from conspecifics (example of the visual cues)
Five heterospecifics were placed in tank no. 2 and five conspecifics in tank no. 1. One larva was placed in the central compartment of each of the aquaria and after a period of 2 min the compartment in which each larva was present was noted. The chi-square test was carried out to determine if the larvae responded to their conspecifics. We compared the observed distribution to a baseline distribution (number of larvae in compartments A, B and C in the absence of external effects).
This experimental protocol was used to test all three types of cues. To test the mechanical cues, five conspecifics and five heterospecifics were placed in compartments D and E, respectively. To test the chemical cues, compartment B was filled with water that had contained conspecifics (five individuals immersed in 3 l of water for 6 h) and compartment C with water that had contained heterospecifics (five individuals immersed in 3 l of water for 6 h). The central compartment was filled with water that had contained no fish.
As we conducted three successive chi-square tests for each sensory signal (visual, chemical and mechanical) of each species, we performed a correction of Bonferroni test for multiple tests (Holm 1979). To obtain a significant difference, the P-value of the chi-square test must be lower than the Pk-value (Pk=0.05/3=0.016; 0.05 is the probability threshold at 5%; 3 is the number of multiple tests performed with the same data).
Experiment 2: detection of visual and chemical cues emitted by coral habitats
The objective of the experiment was the same as for experiment 1, except the cues were emitted by a coral habitat (living or dead spherical coral colony of 6 cm radius, or coral rubble in a spherical mass of 6 cm radius). These coral habitats were collected in a Moorea Island lagoon and corresponded to the typical settlement habitat of the larvae tested (Lecchini 2003). The experimental protocol was the same as for experiment 1, except the mechanical cues were not tested.
Experiment 3: preferential movement of larvae toward cues from conspecifics or coral habitats
The objective of the experiment was to learn if the larvae were preferentially attracted to the cues from conspecifics or coral habitats. The experimental protocol was the same as for experiment 1 (protocol steps i and iii), except that the heterospecifics were replaced by the coral habitat, and protocol step ii was not conducted.
Experiment 4: use of senses at different developmental stages (larval vs juvenile)
The objective of the experiment was to determine if juveniles of 21 days post-colonisation (measured after the end of the larval stage) recognised cues from conspecifics using the same senses as larvae of their species. The experimental protocol was the same as for experiment 1, except larvae were replaced by juveniles.
Results
Experiment 1 was carried out using 18 species of coral-reef fish from 10 families (Table 1). In the absence of a sensory signal (experiment to verify the absence of external effects on the aquarium system), the 20 larvae either stayed mainly in the central compartment of the aquarium (e.g. Apogon novemfasciatus, chemical cues, B: 3 larvae, A: 16 larvae, C: 1 larva) or had a homogeneous distribution between compartments B and C (e.g. Chrysiptera leucopoma, visual cues, chi2=0.65, chi20.05,1=3.84, P=0.54>Pk=0.016), thus validating the absence of external effects on the aquarium system. The test with heterospecifics (experiment to test for repulsion in response to the heterospecifics) did not provoke significant repulsion of larvae (e.g. Stegastes nigricans, visual cues, chi2=2.25, chi20.05,2=5.99, P=0.32>Pk). Thus, no variability was observed in the two first steps of the experimental protocol, whatever the species and the sensory cues tested (for the P-value of chi2 tests, see raw data of Lecchini 2003).
When the larvae were in the presence of cues from conspecifics and heterospecifics, ten species used visual cues, ten used chemical cues and three used mechanical cues to move significantly to the conspecifics (e.g. Abudefduf sordidus, visual cues, chi2=9.90, chi20.05,2=5.99, P=0.007<Pk; Myripristis pralinia, chemical cues, chi2=125.03, chi20.05,2=5.99, P=0.0001<Pk; Parupeneus barberinus, mechanical cues, chi2=8.11, chi20.05,2=5.99, P=0.015<Pk). Thus, two species used three types of sensorial cues (P. barberinus and Ctenochaetus striatus), six used two types (e.g. M. pralinia: visual and chemical cues; Naso unicornis: visual and mechanical cues), five used one type (e.g. Chrysiptera leucopoma: visual cues; Pomacentrus pavo: chemical cues) and five species did not respond to sensory cues (e.g. Scorpaenodes parvipinnis, Crenimugil crenilabis). Larvae among families and also among species of the same family (Pomacentridae, Acanthuridae) were highly variable in sensory responses.
In experiment 2, none of the four species studied were attracted to the visual or chemical cues from coral habitats (e.g. Pomacentrus pavo, visual cues, chi2=3.52, chi20.05,2=5.99, P=0.18>Pk; chemical cues, chi2=1.06, chi20.05,2=5.99, P=0.68>Pk). Experiment 3 confirmed that the attracting cues (visual and chemical) came from the conspecifics and not from the coral habitats (e.g. M. pralinia, visual cues, chi2=137.02, chi20.05,2=5.99, P=0.0001<Pk; chemical cues, chi2=46.33, chi20.05,2=5.99, P=0.0001<Pk). Experiments 1 and 3 demonstrated that the use of sensory cues by larvae of the same species was consistent in the two experiments.
Finally, for the five species tested in experiment 4, juveniles (21 days post-colonisation) and larvae (1 day post-colonisation) both recognised conspecifics using the same sensory cues. Only Chrysiptera leucopoma used different senses at different developmental stages, with larvae recognising conspecifics by visual cues and juveniles by chemical cues.
Discussion
Among the 18 species studied, 13 chose their settlement habitat due to the presence of conspecifics and not based on the characteristics of coral habitat, and 5 species did not move toward their settlement habitat. Among the different sensory cues tested, two species used the three types of cues (visual, chemical and mechanical cues), six used two types (visual and chemical cues or visual and mechanical cues), and five used one type (visual or chemical cues). Our results suggest that fishes may use a range of sensory modalities for effective habitat selection at settlement, and have the ability to discriminate species-specific sensory cues.
Relevance of settlement phase
The division of populations in spatially separated subpopulations or local populations may have profound effects on the dynamics and persistence of populations. Two fundamental processes underlying the demographic and genetic implications of fragmented populations are dispersal and settlement (e.g. Hansson 1991; Hanski 1999). Oceanic dispersion and reef settlement rates are expected to have great impact on the dynamics and sizes of local populations, and thus on the regional risk of extinction. For instance, although small subpopulations may be more likely to become extinct as a result of demographic and environmental stochasticity, their persistence could greatly increase with the “rescue” effect of settlement (Brown and Kodric-Brown 1977). Similarly, if a local population becomes extinct, dispersal is the only way by which it may be recolonised. Thus, in the classical concept of metapopulation, the long-term persistence and stability of the systems depend on the effects of dispersal rates and settlement success (Hanski and Gilpin 1997). The settlement success is characterised by the probability of oceanic larvae of perceiving, localising, seeing and identifying the settlement habitat and/or conspecifics. Thus, settlement is a key process in population biology, shaping the characteristic texture of populations, communities and ecosystems in space and time (Weins 2001).
Validity of the experimental system
Some experimental artefacts may result from enclosing fish in aquaria. The behaviour of larvae and conspecifics is likely to be affected by the stress of having a limited foraging space. This stress could inhibit the normal movements of larvae and/or the emission of cues from conspecifics. Brown and Godin (1997) demonstrated that sticklebacks under stress emitted molecules that repulsed, and not molecules that attracted. Moreover, with the experimental protocol used, a negative result (no movements toward conspecifics) does not allow us to conclude that larvae lack the sensory modalities to recognise their settlement habitat or other fishes. They could remain in the central compartment even after recognising cues from conspecifics or coral habitats. All these considerations prevent direct comparison of the sensory modalities of larvae observed in aquaria to their responses in a natural environment. However, we have opted for experiments in aquaria in order to work in a closed and controllable environment where only the sensory cues studied would be responsible for the movements of larvae. Moreover, the different steps of each experiment were conducted with similar larvae and similar conspecifics or heterospecifics that had all been subjected to identical protocols for capture and maintenance prior to being released in the aquaria. Overall, these experiments allowed us to individually test the importance of visual, chemical and mechanical modalities of fish larvae for 18 different species.
Sensory modalities of larvae in the detection of conspecifics
Among the 18 species studied in experiment 1, 13 moved toward conspecifics and 5 did not move. In experiment 4, the juveniles of species studied detected conspecifics with the same sensory modalities as at the larval stage, except for one species: Chrysiptera leucopoma. These results are in accordance with the studies done on the development of sensory organs of coral-reef fish, which lead to the conclusion that larval colonisation of the reef is accomplished using sensory modalities similar to those of juveniles (for review, see Myrberg and Fuiman 2002).
For the five species that did not migrate (Table 1), the reasons may not be due to a lack of sensory modalities but could be due to stress in aquaria and/or no attraction of larvae in situ. The latter hypothesis would mean that the larvae either settle indifferently on a coral patch (e.g. Sale 1978; Sale and Douglas 1984), select their settlement habitat according to characteristics of the coral habitat (Miyagawa 1989; Elliott et al. 1995), or use other environmental factors such as the food availability in the habitat, or the absence of predators or heterospecifics (e.g. Schmitt and Holbrook 1985; Booth 2002).
Among the 13 species that migrated, 10 used sight to recognise conspecifics. Sight is a well-developed sense in coral-reef fish larvae (Myrberg and Fuiman 2002). In addition, the aquarium experiments were done in the light and at short distance (<80 cm). Wootton (1991) estimated vision to be effective at 40 m for fish (in general) and Leis and Carson-Ewart (1999) estimated it to be effective up to 10 m for larvae of Plectopomus leopardus at settlement phase. Sight therefore functions at short distances to help the larvae detect the “right” settlement habitat.
The chemical cues from conspecifics were detected by the smell or taste of ten coral-reef fish species. The attractant effects of conspecifics’ odours were also demonstrated in competent megalopae of fiddler crabs, Uca pugnax (O’Connor and Judge 1997), and several hermit crab species (Harvey 1996). Likely, the chemical communication substance released by Decopoda conspecifics might have a polypeptide- or neurotransmitter-related structure (see Gebauer et al. 2002), as observed in several other marine invertebrates (Yamamoto et al. 1999; Browne and Zimmer 2001). In fish, some scientists suggested that the chemical communication substances would be some amino acids, biliary salts or the vitamins from the mucus of the skin, or the urine or excrement of fish (Fontaine et al. 1982; Sola and Tosi 1993; Baker and Montgomery 2001). Hellstrom and Doving (1986) suggested that depending on the nature of chemical cues, fish used smell or taste to recognise a predator or a habitat. Thus, a study is in progress in which High Performance Liquid Chromatography is used to identify the nature of these chemical cues (D. Lecchini, unpublished work).
The mechanical cues from conspecifics were detected by the inner ear or the lateral line of three species. We have, however, no evidence that the large amplitude vibrations detectable by the lateral line and produced by movements of small fishes penetrate a plate of glass. Moreover, larvae in the ocean use the sound generated by the waves on the reef crest and/or the “nocturnal chorus” of the lagoon to detect the island to colonise (Tolimieri et al. 2000; Leis et al. 2002). The nocturnal chorus is produced by the “teeth” of shrimp, fish and sea urchins grinding on corals (Cato 1992). The low number of species using the mechanical cues may then be explained by the intermittent or lack of sound generation from conspecifics in the aquaria. However, the pelagic larvae can detect islands by sound because the waves always generate noise on the reef crest and the nocturnal chorus is continuous at night.
Recognition of settlement habitat due to the presence of conspecifics, and not of the coral habitat itself
The settlement phase has been widely studied for coral-reef fish and invertebrates, and it has often been demonstrated that larvae chose a suitable habitat according to the presence of conspecifics and the characteristics of coral habitat (e.g. Giese et al. 1991; Ohman et al. 1998; Mercier et al. 2000; Holbrook et al. 2002). In our experiments 2 and 3, we demonstrated that fish larvae recognised their settlement habitat due to the presence of conspecifics and not by the characteristics of coral habitat. Elliott et al. (1995) demonstrated that larvae of Amphiprion were attracted to the odour of anemones and not to the odour of conspecifics already settled on the anemones. In crabs, Gebauer demonstrated that larvae of Sesarma curacaoense were attracted by chemical cues of conspecifics (Gebauer et al. 2002), whereas Chasmagnathus granulata larvae were attracted by conspecifics and habitat (Gebauer et al. 1998). Sweatman (1988) studied the chemical modalities of Dascyllus aruanus and D. reticulatus. He found that the coral colonies that were injected with the water had a higher settlement rate of juveniles of D. aruanus and D. reticulatus than the colonies without pumped-in water. However, some larvae settled on coral habitats without conspecifics.
Overall, it is difficult to conclude on the role of coral habitat in attracting larvae as our study is only of four species, and too few studies have explored the preferential movements of larvae toward cues from conspecifics or coral habitats. Moreover, the conclusions of our study are limited as environmental conditions may affect larvae after colonisation and those conditions are not present in the aquaria. In situ, larvae must not only detect the cues from conspecifics but also those from predators and heterospecifics. Thus, future studies in aquaria should be validated by in-situ experiments to test other environmental cues impacting larvae during the settlement phase.
References
Baker CF, Montgomery JC (2001) Species specific attraction of migratory banded kokopu juveniles to adult pheromones. J Fish Biol 58:1221–1229
Booth DJ (1992) Larval settlement patterns and preferences by domino damselfish Dascyllus albisella Gill. J Exp Mar Biol Ecol 155:85–104
Booth DJ (2002) Distribution changes after settlement in six species of damselfish (Pomacentridae) in One Tree Island lagoon, Great Barrier Reef. Mar Ecol Prog Ser 226:157–164
Brown GE, Godin JJ (1997) Antipredator responses to conspecific and heterospecific skin extracts by threespine sticklebacks: alarm pheromones revisited. Behaviour 134:1123–1134
Brown JH, Kodric-Brown A (1977) Turnover rates in island biogeography: effect of immigration on extinction. Ecology 58:445–449
Browne KA, Zimmer R (2001) Controlled field release of a waterborne chemical signal stimulates planktonic larvae to settle. Biol Bull 200:87–91
Cassier P, Bohatier J, Descoins C, Nagnan Le Meillour P (2000) Communication chimique et environnement. Belin, Paris
Cato DH (1992) The biological contribution to the ambient noise in waters near Australia. Acoust Aust 20:76–80
Doherty PJ (2002) Variable replenishment and the dynamics of reef fish populations. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, San Diego, pp 327–358
Doherty PJ, Dufour V, Galzin R, Hixon MA, Planes S (2004) High mortality at settlement is a population bottleneck for a tropical surgeonfish. Ecology (in press)
Dudley B, Tolimieri N, Montgomery J (2000) Swimming ability of the larvae of some reef fishes from New Zealand waters. Mar Freshwater Res 51:783–787
Dufour V, Galzin R (1993) Colonisation patterns of reef fish larvae to the lagoon at Moorea Island, French Polynesia. Mar Ecol Prog Ser 102:143–152
Elliott JK, Elliott JM, Mariscal RN (1995) Host selection, location, and association behaviors of anemone fishes in field settlement experiments. Mar Biol 122:377–389
Fontaine M, JeanJean S, Monzikoff A (1982) Intervention possible de la riboflavine (vitamine B2) comme télémédiateur chimique dans les écosystèmes aquatiques. C R Acad Sci Sér III:165–167
Gebauer P, Walter I, Anger K (1998) Effects of substratum and conspecific adults on the metamorphosis of Chasmagnathus granulata (Decapoda) megalopae. J Exp Mar Biol Ecol 223:185–198
Gebauer P, Paschke K, Anger K (2002) Metamorphosis in a semiterrestrial crab, Sesarma curacaoense: intra and interspecific settlement cues from adults odors. J Exp Mar Biol Ecol 268:1–12
Giese AC, Pearse JS, Pearse VB (1991) Reproduction of marine invertebrates. Boxwood, Calif
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I, Gilpin ME (1997) Metapopulation biology: ecology, genetics and evolution. Academic, San Diego
Hansson L (1991) Dispersal and connectivity in metapopulations. Biol J Linn Soc 42:89–103
Harvey AW (1996) Delayed metamorphosis in Florida hermit crabs: multiple cues and constraints (Crustacea: Decapoda: Paguridae and Diogenidae). Mar Ecol Prog Ser 141:27–36
Hellstrom T, Doving KB (1986) Chemoreception of taurocholate in anosmic and sham-operated cod, Gadus morhua. Behav Brain Res 21:155–162
Holbrook SL, Brooks AJ, Schmitt RJ (2002) Predictability of fish assemblages on coral patch reefs. Mar Freshwater Res 53:181–188
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Katzir G (1981) Aggression by the damselfish Dascyllus aruanus L. towards conspecifics and heterospecifics. Anim Behav 29:835–841
Kingsford MJ, Leis JM, Shanks A, Lindeman K, Morgan S, Pineda J (2002) Sensory environments, larval abilities and local self-recruitment. Bull Mar Sci 70:309–340
Lecchini D (2003) Identification of habitat use strategies between the colonisation and recruitment stages of coral reef fish in the lagoon of Moorea (French Polynesia): approach by behavioural ecology. PhD Thesis, Ecole Pratique des Hautes Etudes and University of Pierre and Marie Curie
Leis JM, Carson-Ewart BM (1997) In situ swimming speeds of the late pelagic larvae of some Indo-Pacific coral reef fishes. Mar Ecol Prog Ser 159:165–174
Leis JM, Carson-Ewart BM (1999) In situ swimming and settlement behaviour of larvae of an Indo-Pacific coral reef fish, the coral trout Plectropomus leopardus (Serranidae). Mar Biol 134:51–64
Leis JM, Carson-Ewart BM, Cato DH (2002) Sound detection in situ by the larvae of a coral reef damselfish (Pomacentridae). Mar Ecol Prog Ser 232:259–268
Mercier A, Battaglene SC, Hamel JF (2000) Settlement preferences and early migration of the tropical sea cucumber Holothuria scabra. J Exp Mar Biol Ecol 249:89–110
Milicich MJ (1994) Dynamic coupling of coral reef fish replenishment and oceanographic processes. Mar Ecol Prog Ser 110:135–144
Miyagawa K (1989) Experimental analysis of the symbiosis between anemonefishes and sea anemones. Ethology 80:19–46
Myrberg AA, Fuiman LA (2002) The sensory world of coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, San Diego, pp 123–148
O’Connor NJ, Judge ML (1997) Flexibility in timing of molting of fildler crab megalopae: evidence from in situ manipulation of cues. Mar Ecol Prog Ser 146:55–60
Ohman MC, Munday PL, Jones GP, Caley MJ (1998) Settlement strategies and distribution patterns of coral reef fishes. J Exp Mar Biol Ecol 225:219–238
Sale PF (1978) Coexistence of coral reef fishes a lottery for living space. Environ Biol Fish 3:85–102
Sale PF, Douglas WA (1984) Temporal variability in the community structure of fish on coral patch reefs and the relation of community structure to reef structure. Ecology 65:409–422
Schmitt RJ, Holbrook SJ (1985) Patch selection by juvenile black surfperch under variable risk: interactive influence of food quality and structural complexity. Mar Freshwater Res 11:12–21
Sola C, Tosi L (1993) Bile salts and taurine as chemical stimuli for glass eels, Anguilla anguilla: a behavioural study. Environ Biol Fish 37:197–204
Steele MA, Forrester GE (2002) Early postsettlement predation on three reef fishes: effects on spatial patterns of recruitment. Ecology 83:1076–1091
Stobutzki IC, Bellwood DR (1997) Sustained swimming abilities of the late pelagic stages of coral reef fishes. Mar Ecol Prog Ser 149:35–41
Sweatman HPA (1988) Field evidence that settling coral reef fish larvae detect resident fishes using dissolved chemical cues. J Exp Mar Biol Ecol 124:163–174
Tolimieri N, Jeffs A, Montgomery JC (2000) Ambient sound as a cue for navigation by the pelagic larvae of reef fishes. Mar Ecol Prog Ser 207:219–224
Turchin P (1996) Fractal analyses of animal movement: a critique. Ecology 77:2086–2090
Victor BC (1991) Settlement strategies and biogeography of reef fishes In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, pp 231–260
Weins JA (2001) The landscape context of dispersal. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal. Oxford University Press, New York, pp 96–109
Weins JA, Stenseth NC, Van Horne B, Ins RA (1995) Ecological mechanisms and landscape ecology. Oikos 66:369–380
Westcott DA, Graham DL (2000) Patterns of movement and seed dispersal of a tropical frugivore. Oecologia 122:249–257
Wootton RJ (1991) Ecology of teleost fishes. Chapman & Hall, Paris
Yamamoto H, Shimizu K, Tachibana A, Fusetani N (1999) Roles of dopamine and serotonin in larval attachment of the barnacle, Balanus amphitrite. J Exp Zool 284:746–758
Acknowledgements
The authors wish to thank Christiane and Douchka Rivière for their assistance in the field. We thank J.T. Williams (Smithsonian Institution, Washington) for improving the English readability of the manuscript. This research was supported by the Ecole Pratique des Hautes Etudes (UMR 8046 CNRS-EPHE), and a Lavoisier Fellowship (French Ministry of Foreign Affairs) awarded to D. Lecchini. Additional logistic and financial support was provided by the Centre de Recherches Insulaires et Observatoire de l’Environnement (CRIOBE). The authors have adhered to the Guidelines for the Use of Animals in Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Krause
Rights and permissions
About this article
Cite this article
Lecchini, D., Planes, S. & Galzin, R. Experimental assessment of sensory modalities of coral-reef fish larvae in the recognition of their settlement habitat. Behav Ecol Sociobiol 58, 18–26 (2005). https://doi.org/10.1007/s00265-004-0905-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0905-3