Abstract
A large number of herbaceous and woody plants from tropical woodland, savanna, and monsoon forest were analysed to determine the impact of environmental factors (nutrient and water availability, fire) and biological factors (microbial associations, systematics) on plant δ15N values. Foliar δ15N values of herbaceous and woody species were not related to growth form or phenology, but a strong relationship existed between mycorrhizal status and plant δ15N. In woodland and savanna, woody species with ectomycorrhizal (ECM) associations and putative N2-fixing species with ECM/arbuscular (AM) associations had lowest foliar δ15N values (1.0–0.6‰), AM species had mostly intermediate δ15N values (average +0.6‰), while non-mycorrhizal Proteaceae had highest δ15N values (+2.9 to +4.1‰). Similar differences in foliar δ15N were observed between AM (average 0.1 and 0.2‰) and non-mycorrhizal (average +0.8 and +0.3‰) herbaceous species in woodland and savanna. Leguminous savanna species had significantly higher leaf N contents (1.8–2.5% N) than non-fixing species (0.9–1.2% N) indicating substantial N acquisition via N2 fixation. Monsoon forest species had similar leaf N contents (average 2.4% N) and positive δ15N values (+0.9 to +2.4‰). Soil nitrification and plant NO3 − use was substantially higher in monsoon forest than in woodland or savanna. In the studied communities, higher soil N content and nitrification rates were associated with more positive soil δ15N and plant δ15N. In support of this notion, Ficus, a high NO3 − using taxa associated with NO3 − rich sites in the savanna, had the highest δ15N values of all AM species in the savanna. δ15N of xylem sap was examined as a tool for studying plant δ15N relations. δ15N of xylem sap varied seasonally and between differently aged Acacia and other savanna species. Plants from annually burnt savanna had significantly higher δ15N values compared to plants from less frequently burnt savanna, suggesting that foliar 15N natural abundance could be used as marker for assessing historic fire regimes. Australian woodland and savanna species had low leaf δ15N and N content compared to species from equivalent African communities indicating that Australian biota are the more N depauperate. The largest differences in leaf δ15N occurred between the dominant ECM Australian and African savanna (miombo) species, which were depleted and enriched in 15N, respectively. While the depleted δ15N of Australian ECM species are similar to those of previous reports on ECM species in natural plant communities, the 15N-enriched δ15N of African ECM species represent an anomaly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite growing knowledge of 15N natural abundance signatures in plant communities, we are yet to fully understand the causal determinants of 15N natural abundance in natural systems. One difficulty is that 15N fractionation occurs as a consequence of not only biological but also physico-chemical processes (Handley and Raven 1992). Among the factors influencing soil δ15N values are soil age, disturbance, frequency of fire, soil moisture and rainfall, net mineralisation/nitrification potential, and point sources of isotopically distinct N (Stewart and Schmidt 1999). In previous studies of northern Australian plant communities, plant δ15N values have been interpreted as being causally linked to rainfall as an inverse relationship exists between rainfall and plant δ15N (Schulze et al. 1998; Austin and Sala 1999; Handley et al. 1999). Similarly, fire (Handley et al. 1999; Cook 2001) and grazing (Schulze et al. 1999) have been suggested as potential factors influencing plant δ15N values. In addition to observed trends of plant δ15N across different ecosystems, strong variation in δ15N may occur between species within an ecosystem (Stewart and Schmidt 1999).
Early studies of plant δ15N relations suggested that δ15N provides a tracer that allows tracking of plant N sources, but it has become evident that δ15N of total soil N is mostly not reflected in plant δ15N, and due to analytical difficulties very limited information is available on the δ15N of plant available soil N sources. The lack of a direct relationship between soil δ15N and plant δ15N values may be due to (1) plants using N forms that differ in their isotopic composition from the δ15N of total soil N (Handley and Scrimgeour 1997; Högberg 1997), (2) mycorrhizal associations affecting plant δ15N (Högberg et al. 1996; Schmidt and Stewart 1997; Michelsen et al. 1998; Hobbie et al. 2000), and (3) isotopic fractionation occurring during N uptake (Evans 2001; Robinson 2001; Yoneyama et al. 1991, 2001). However, while understanding of plant δ15N relations in natural ecosystems is still somewhat limited, ecosystem research has demonstrated that robust patterns exist with respect to plant and soil δ15N within and across ecosystems allowing the use of 15N natural abundance as an integrator of N cycle processes (Robinson 2001).
Here we consider the extent to which plant δ15N values reflect the influence of environmental and biological factors through an investigation of tropical woodland, savanna and monsoon forest species. These vegetation types differ in exposure and susceptibility to fire, water and nutrient status. The main taxa of Australian woody savanna species include the dominant Eucalyptus (Myrtaceae) with ecto- (ECM) or ecto/va-mycorrhizal (ECM/AM) associations, while most other species are va-mycorrhizal (AM). Acacia and other potentially N2-fixing woody leguminous species have ECM/AM or AM associations, while Proteaceae are non-mycorrhizal but form cluster roots. Interspersed with Eucalyptus-dominated savannas in north Australia are pockets of fire-susceptible monsoon forests in areas characterised by higher nutrient and water availability and lower fire frequencies compared to savannas that preclude invasion from fire-tolerant savanna species (Bowman and Fensham 1991). In contrast to savannas, monsoon forests are dominated by AM species. In both communities, species differ in their N status and use of N sources (Schmidt et al. 1998; Schmidt and Stewart 1998). Seasonal differences in plant N acquisition occur because soil N appears to be taken up mostly during the wet season and internally recycled during the dry season. A strong relationship between soil and plant N status exists in these communities (Schmidt et al. 1998; Schmidt and Stewart 1998). Fire is a major factor in Australian savannas and the influence of fire on δ15N relations was investigated to determine whether 15N could help to reconstruct historic fire regimes. The vegetation types studied here afford a useful model system with which to investigate factors influencing plant δ15N values. These plant communities were compared with corresponding African communities.
Materials and methods
Study sites and sample collections
The study was carried out in the Alligator Rivers Region of Kakadu National Park within a 30 km radius of Jabiru (12.39°S, 132.55°E) and near Darwin (12.30°S, 131.6°E, Howard River East catchment) in northern Australia. The climate is distinctly monsoonal with a long dry season (May–October) followed by a wet season (December–March) during which 90% of the average annual precipitation of 1,475 mm is received. Maximum and minimum monthly temperatures range from 18 to 25°C and 31 to 37°C in the dry and wet seasons, respectively. Sampling occurred in mid dry season (July 1994), late dry season (September 1996, 1997), early wet season (December 1995), and mid wet season (February 1995, January 1998). An annually burnt subsection (segment L) of the CSIRO Kapalga fire research site was compared with savanna that had not been burnt for 4 years at Kapalga (segment M) and one that had been burnt 2 years prior to sampling near Jabiru.
Plant species
Eucalyptus tetrodonta and E. miniata dominate the most distinctive savanna community in northern Australia. Acacia species are prominent understorey species in this open forest community. Other genera of tree and shrub species sampled in this study include Alstonia, Buchanania, Calytrix, Clerodendrum, Cochlospermum, Dolichandrone, Erythrophleum, Ficus, Gardenia, Grevillea, Lophostemon, Melaleuca, Owenia, Parinari, Persoonia, Petalostigma, Planchonia, Terminalia, and Xanthostemon. Soils are mostly well drained sandy loams or loams which remain temporarily water saturated during the wet season. The sandstone escarpment in Kakadu National Park supports open Eucalyptus woodland with sparse canopy and ground cover (Brock 1988). Escarpment species were studied at Koongarra Saddle and included many species in the same genera as listed for the savanna woodland. In addition, Allosyncarpia, Bossiaea, Calycopeplus, Dampiera, Gompholobium, Maytenus, Myrtella, Pityrodia, Plagiocarpus, Solanum, and Vitex were sampled. A deciduous monsoon forest near "Holiday Village" was studied and genera sampled included Acacia, Adenanthera, Canarium, Cathormion, Cupaniopsis, Elaeocarpus, Eucalyptus, Exocarpus, Ficus, Ganophyllum, Glycosmis, Litsea, Planchonia, Strychnos, Terminalia, Trema, Vitex, and Wrightia. The monsoon forest has moderately drained clayey red soil close to the flood plains. Herbaceous species in each community were sampled and included members of the following families: Araceae, Asteraceae, Commelinacae, Convolvulaceae, Curcurbitaceae, Cyperaceae, Dilleniaceae, Euphorbiaceae, Fabaceae, Goodeniaceae, Haemodoraceae, Poaceae, Liliaceae, Polygalaceae, Portulacaceae, Taccaceae, Tiliaceae, Vitaceae, and Xanthorrhoeaceae.
δ15N analysis
Healthy mature leaves, twigs and roots were oven-dried at 60°C on the day of collection. Woody plant samples were treated as described by Schmidt and Stewart (1997). Plant samples were ground to a fine powder using a ball mill (Retsch MM-2, Haan, Germany) and analysed by continuous flow isotope ratio mass spectrometry (CF-IRMS, Tracer Mass, Europa Scientific, Crewe, UK). Samples with a low N content (0.3–0.5% N) were analysed by continuous flow isotope ratio mass spectrometry (20–20 CF-IRMS Europa Scientific, Crewe, UK). Leaves, woody plant parts and twig xylem sap were sampled in the late dry season from the same tree. Xylem sap was analysed for nitrogenous compounds (Schmidt and Stewart 1998) to calculate the amount required to obtain 20–100 g N for 15N analysis. Xylem sap was freeze-dried in portions of 0.1 cm3 to yield sufficient N, which generally required 0.6–1.5 cm3 of sap. Bulked soil samples were taken from the upper horizon at 0–5 cm, air-dried and analysed by continuous flow mass spectrometry (see above).
Soil analysis
Mixed bed ion exchange resin (Dowex-MR3, Sigma) was used to determine the rate at which NO3 − and NH4+ were generated in the soil (Schmidt et al. 1998). Five grams of resin was placed in 5×5 cm stapled polyethylene bags (Swiss Screens PE 48GG, 365 μm mesh) and inserted horizontally in the top 5 cm of soil with minimum soil disturbance. Soil samples were collected from the top 5 cm of the soil profile and five subsamples were bulked for each soil sample. Total soil N was determined by mass spectrometry (CF-IRMS, Tracer Mass, Europa Scientific, Crewe, UK).
Statistical analysis
Data were analysed using STATISTICA (Statsoft, Tulsa, Oklahoma). Significant differences at the P<0.05 level were determined by t-test (Mann Whitney) or ANOVA (followed by Duncan's multiple range test).
Results
Intra-plant variation in δ15N
Previous studies have shown considerable intra-plant variation in N isotope values and in order to assess the extent of this variation in the present study, we examined representative species from three communities (Fig. 1). All parts of putative N2-fixing species (Acacia, Erythrophleum) had negative δ15N values, except leaves of a monsoon forest Acacia species. Leaves and twigs of ECM myrtaceous species (Eucalyptus, Allosyncarpia) had negative δ15N values, while roots had positive values. AM species had variable δ15N values. Non-mycorrhizal savanna species (Grevillea, Banksia) and most species in the monsoon forest had positive δ15N values.
δ15N (‰) of different plant parts of species from escarpment woodland, savanna, and monsoon forest. The three bars per taxa represent (1) leaves, (2) twigs, (3) roots. Species in same genus with the same root specialisations were pooled. Samples for each species/genus were bulked from several leaves and branches from one tree in the dry season. Data represent averages and standard deviations. For species' details see Materials and methods
No significant seasonal variations in leaf δ15N values of 23 savanna and 11 monsoon forest species were observed (data not shown), while δ15N of xylem sap differed between seasons (Fig. 2). Xylem sap extracted from Acacia, Eucalyptus and Erythrophleum in the wet season had lower δ15N values than sap collected in the dry season. Xylem sap from mature Acacia trees collected in the wet season had negative δ15N while that of saplings and an old tree had positive δ15N values. Xylem sap of AM species Ficus and Planchonia and non-mycorrhizal Grevillea had positive δ15N values.
Xylem sap δ15N of savanna woodland species collected in wet and dry season. Unless otherwise stated, samples were taken from mature individuals. The old Acacia tree had no new growth and sparse foliage, saplings were approximately 2 m tall. Each value represents bulked xylem sap from several twigs of one tree. Standard deviations are shown where more than two trees were sampled
Foliar N content and δ15N of species with different root specialisations
Herbaceous N2-fixing species had significantly (P<0.05) higher leaf N contents than non-fixing AM or non-mycorrhizal species from escarpment woodland and savanna (Table 1). In the monsoon forest, foliar N content was similar in all herbaceous species. δ15N values of N2-fixing species were mostly (although not statistically significantly) lower than those of AM species, which in turn were lower than those of non-mycorrhizal species.
Acacia species had significantly (P<0.05) higher leaf N contents than Eucalyptus, AM, and proteaceous species in escarpment woodland and savanna, while similar leaf N contents were observed in all species in the monsoon forest (Table 2). While differences in leaf δ15N were observed between species with different root specialisations, inspection of the data showed no relationship between δ15N and phenology or growth habit in herbaceous and woody species (data not shown). In escarpment woodland and savanna, Acacia and Eucalyptus had significantly (P<0.001, P<0.05) lower δ15N values than AM species and species in the Proteaceae (Table 2). Species from monsoon forest had positive δ15N values.
In all communities δ15N of total soil N was higher than those of woody species with mycorrhizal associations and putative N2-fixing species with mycorrhizal associations (Table 2). Only non-mycorrhizal Proteaceae had more positive average δ15N values than δ15N of total soil N of escarpment woodland and savanna.
Effects of fire regime and soil N relations on plant δ15N values
Comparison of pooled foliar δ15N values of fifteen herbaceous and woody species from annually burnt and less frequently burnt (≥2 year burning intervals) savanna sites indicated that annual burning was associated with increased foliar δ15N values (P<0.05) (Fig. 3). Similarly, total soil δ15N was higher (3.2‰) in annually burnt savanna compared with infrequently burnt savanna (2.5‰) (Fig. 3).
Comparison of δ15N (‰) of species growing at infrequently and annually burnt savanna sites (n=15), and of species growing at savanna and monsoon forest (n=6). Averages±SEM are shown. Statistical differences (P<0.05) between species from two sites are indicated by different letters. Asterisk indicates total soil δ15N in each community
Five species from savanna and monsoon forest had significantly (P<0.05) higher δ15N values when growing in monsoon forest compared to savanna (Fig. 3) whereas Exocarpos latifolius, a root hemi-parasite, had similar positive δ15N values in both communities.
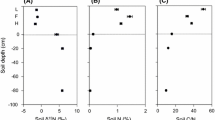
Plant available NH4 + and NO3 − levels determined using in situ ion exchange resin bags showed that the proportion of NO3 − relative to NH4 + was higher in soil from monsoon forest than soils from woodland and savanna (51–53% NO3 − of total available inorganic N) (Fig. 4). Similar levels of NH4 + and NO3 − were measured in savanna soil at annually and less frequently burnt savanna, although total soil N content was reduced in annually burnt savanna (0.06±0.01% N) compared with less frequently burnt savanna (0.12±0.01% N). Soil associated with Ficus in savanna had a similar NO3 − availability as soil from monsoon forest (Fig. 4).
Plant available soil N as determined with in situ ion exchange resin bags in the upper 5 cm of soil. Data represent averages from dry and wet season for each of the studied communities and from soil associated with Ficus in savanna (adapted from Schmidt et al. 1998)
Comparison of foliar N content and δ15N of Australian and African species
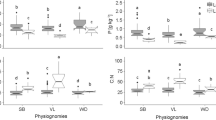
ECM, AM and N2-fixing species from Australian savanna had lower average foliar N contents than equivalent species from African miombo communities (Fig. 5). Similarly, ECM and N2-fixing species from Australian monsoon forest had a lower average leaf N content than their African counterparts. Only AM species in Australian monsoon forest had somewhat higher leaf N content than African rainforest species. Leaf δ15N values of ECM savanna species were low compared to ECM miombo species, while N2-fixing savanna and miombo species had similar δ15N values. Average δ15N of AM species differed between Zambian and Tanzanian miombo communities (+2.8 and 0.1‰) and Australian AM savanna species had intermediate δ15N values. In African rainforest, N2-fixing, AM, and ECM species had more positive δ15N values than species in Australian monsoon forest.
δ15N (‰, averages±SD) and foliar N content (% N of dry weight) of woody Australian woodland, savanna and monsoon forest species, and equivalent African Miombo woodland and rainforest species grouped according to their root specialisations. Putative N2-fixing species in the African communities are AM and ECM/AM in the Australian communities. Data for the African communities are from Högberg (1990), Högberg and Alexander (1995) and Högberg (personal communication). ECM status of Eucalyptus alba in monsoon forest was not assessed. Data represent averages and standard deviations
Discussion
Plant δ 15N values, mycorrhizal associations and N sources
Analysis of plants in tropical woodland, savanna and monsoon forest communities revealed that plants with different root specialisations have distinct δ15N values. In woodland and savanna, leaves of ECM species and to a lesser extent AM species had lower δ15N than those of non-mycorrhizal species confirming the notion that presence of ECM and AM associations is associated with isotopic depletion of the host (Stock et al. 1995; Högberg et al. 1996; Schmidt and Stewart 1997; Michelsen et al. 1998; Hobbie et al. 2000). Positive δ15N values of roots compared to negative δ15N values of stems and leaves in ECM savanna species support the view that discrimination against 15N occurs during fungal N assimilation, which results in 15N enrichment in fungal tissue associated with fine roots, while host tissue such as leaves or stems becomes depleted in 15N relative to fungal tissue (Högberg et al. 1996; Schmidt and Stewart 1997).
Woody AM species in woodland and savanna had mostly higher δ15N values than ECM(AM) and N2-fixing species, similar to previous studies where AM species consistently had higher δ15N values than species with ecto or ericoid mycorrhizal associations (Pate et al. 1993; Stock et al. 1995; Nadelhoffer et al. 1996; Schmidt and Stewart 1997; Michelsen et al. 1998). In our study the spread of δ15N values was greater in AM species than in any other group, ranging from negative δ15N values similar to those of ECM species to the isotopically enriched values that characterised non-mycorrhizal species. Woody AM species contained the most diverse taxa of the groups studied here, and the range of δ15N in AM species could reflect this heterogeneity. In addition to the possible effect of mycorrhizal fungal associations on plant δ15N values, soil N sources, soil N concentration and plant N assimilation characteristics influence plant δ15N (reviewed by Evans 2001; Robinson 2001). Low plant δ15N values may indicate that soil N sources are used which require access and assimilation via mycorrhizal associations, while N sources that are taken up and assimilated directly by the plant may result in higher plant δ15N values (Evans 2001). Some AM species had a higher NO3 − use than the majority of savanna species and the most conspicuous among these high NO3 −-assimilating physiotypes are Ficus species (Schmidt et al. 1998). Interestingly, Ficus also had the highest δ15N values of all AM species. Similarly, the herb Ptilotus had the highest NO3 − use and highest δ15N values of species in Banksia woodland (Pate et al. 1993). Nitrate reductase activity was high in leaves of Ficus and Ptilotus (Pate et al. 1993; Schmidt et al. 1998). It has been suggested that NO3 − reduction in leaves results in little discrimination against 15N while NO3 − reduction in the roots causes efflux of a 15N-enriched N fraction and plant 15N depletion relative to the source (Evans 2001). While intra-plant isotopic fractionation may affect annual species (Evans 2001), Ficus leaves, stems and roots were similarly enriched in 15N indicating little intra-plant isotopic fractionation. A possible explanation for high δ15N values of high NO3 − using species is the observation that plants discriminate more strongly against 15NH4 + than against 15NO3 − in hydroponic culture (Mariotti et al. 1980; Yoneyama et al. 1991, 2001). If stronger isotopic discrimination occurs with NH4 + as a N source compared to NO3 − as a N source, preferential use of NO3 − may contribute to the observed differences in δ15N values between species with high NO3 − use such as Ficus and AM species with a low NO3 − use. Lastly, differences in δ15N between plants with high or low NO3 − use could be caused if soil NO3 − was 15N enriched compared to soil NH4 +, for example if 15N depleted NO3 − is lost from the soil through denitrification (Högberg 1997).
The high δ15N of monsoon forest soil, the greater N content and availability of NO3 − compared to savanna and woodland soil, supports the notion that stronger mineralisation and nitrification result in more positive δ15N of soil N fractions (Handley and Raven 1992). Monsoon forest plants had higher δ15N values and a greater NO3 − use compared to the majority of plants in woodland and savanna (Schmidt et al. 1998). Tree species growing at both savanna and monsoon forest had significantly (P<0.05) higher δ15N values when growing in the monsoon forest (average 2.8±0.3‰) compared to savanna (average 0.7±0.8‰). The high δ15N values of plants growing at monsoon forest compared to savanna may be caused by (1) high δ15N of soil N sources as a result of high N turnover, nitrification and/or N loss, (2) high use of NO3 − and associated lower fractionation against 15N compared to NH4 + use, (3) low contribution of mycorrhizal fungi to plant N acquisition and associated lower discrimination against 15N during fungal N transfer.
Plant δ15N and N2 fixation
Leaf δ15N values of putative N2-fixing species were in the range often associated with N2 fixation, but were indistinguishable from δ15N of myrtaceous ECM species in woodland and savanna. Since putative N2-fixing Acacia species have ECM and AM associations, it is not possible to separate effects of N2 fixation and mycorrhizal associations on plant δ15N. However, the high leaf N content of Acacia and other putative N2-fixing species in comparison to non-fixing species in savanna and woodland suggests that a substantial proportion of plant N is derived from N2 fixation. Leguminous species (Bossiaea, Cajanus, Galactia, Gompholobium, Plagiocarpus) with AM associations from woodland and savanna had low δ15N and high leaf N contents indicating that the combination of AM and putative N2 fixation results in δ15N values similar to those of ECM/AM Acacia species. A previous study found large variations between δ15N of N2-fixing and non-fixing savanna species along an aridity gradient (Schulze et al. 1991) on which calculations of the contribution of N2 fixation to leaf N were based. However, the recent insights that an inverse relationship exists between rainfall and plant δ15N, and that mycorrhizal associations, among other factors, affect plant δ15N values prevents straightforward calculations of N2 fixation rates in savannas and other plant communities.
A strong difference existed in xylem sap δ15N of mature Acacia trees and adjacent young saplings and old tree in the wet season, since xylem sap δ15N of saplings and old tree was 5–6.3‰ higher than xylem sap of mature Acacia. The observed differences in xylem sap δ15N between differently aged Acacia may point to differences in N2 fixation rates and/or N assimilation characteristics associated with maturing and ageing of Acacia, and this finding needs to be explored further in combination with measurements of actinorhizal and mycorrhizal associations. Similar seasonal differences in xylem sap δ15N as those observed in mature Acacia were also observed in the putative N2-fixing species Erythrophleum chlorostachys and in non-fixing Eucalyptus. Xylem sap δ15N may reflect seasonal differences in plant N assimilation and transport, and it has been suggested that savanna trees rely soil N sources during the wet season when soil N availability is high, whereas trees remobilise and transport internally stored N sources during the dry season (Schmidt et al. 1998; Schmidt and Stewart 1998).
Plant δ15N and burning regimes
Annual burning of savanna resulted in a trend of soil and leaf 15N enrichment compared to less frequently burnt savanna, which contrasts with Cook's (2001) study at nearby sites. Total soil N content was lower in annually burnt savanna than in less regularly burnt savanna in our study (Schmidt et al. 1998) and in a previous study (Mordelet et al. 1996), while Cook (2001) found no difference in total soil N content of sites with different fire histories. Although is has previously been suggested that annual burning of savannas does not result in net N loss from the ecosystem (Holt and Coventry 1990), Cook (1994) estimated that annual burning causes net N losses of 1.5–2 g N m−2 year−1. It is currently of considerable interest to devise burning regimes for sustainable management of Australia's tropical savannas. Nitrogen loss from temperate plant communities resulted in 15N enrichment of the remaining soil N fractions (Högberg 1997). δ15N values from savanna with different fire frequencies and analysis of historic samples could be used to address the question of how naturally occurring fires and traditional Aboriginal burning practices have influenced savanna N relations in the past.
Comparing δ15N of Australian and African plant communities
15N natural abundance studies in African plant communities first indicated that mycorrhizal associations could affect plant δ15N values (Högberg 1990; Högberg and Alexander 1995). In order to understand plant δ15N values in relation to African plant communities, δ15N and leaf N content of Australian savanna and monsoon forest species were compared with equivalent species from African miombo and rainforest. Similar to Australian savanna, African miombo woodland is dominated by non-nodulated species with ECM associations (Högberg 1990). Similarities exist in vegetation structure and nutrient relations between African and Australian communities (Mott et al. 1985). However, African miombo species had consistently higher leaf N contents than equivalent species with similar root specialisations in Australian savanna. The overall lower leaf N content of Australian savanna species compared to African species supports the notion that Australian savanna and woodland biota are the more N depauperate, which can be linked to the low soil N status of these ecosystems.
There were consistent differences between δ15N values of African and Australian plants communities with African species having the more positive δ15N values; the only exception being AM species in Tanzanian woodland which had similar δ15N values as Australian savanna species. The suggested "δ15N lowering effect" of EC mycorrhizal associations on plant δ15N in Australian woodland and savanna contradicts the isotopic enrichment of African ECM species. Indeed Högberg's studies (Högberg 1990; Högberg and Alexander 1995) appear to be the only examples of 15N enrichment of ECM species relative to species with other root specialisations.
Differences in plant δ15N values across ecosystems in relation to soil N status and root specialisations indicate that 15N natural abundance is a marker for ecosystem N relations, however, but further knowledge is required to understand the interactions of soil-plant 15N natural abundance at the ecosystem scale. It would be particularly interesting to investigate how δ15N of soil NH4 + and NO3 − relate to δ15N of xylem sap in species with different mycorrhizal associations and N assimilation characteristics.
References
Austin AT, Sala OE (1999) Foliar δ15N is negatively correlated with rainfall along with the IGBP transect in Australia. Aust J Plant Physiol 26:293–295
Bowman DMJS, Fensham RJ (1991) Response of a monsoon forest-savanna boundary to fire protection in Weipa, northern Australia. Aust J Ecol 16:111–118
Brock J (1988) Top end native plants. John Brook, Darwin, Australia
Cook GD (1994) The fate of nutrients during fires in a tropical savanna. Aust J Ecol 19:359–365
Cook GD (2001) Effects of frequent fires and grazing on stable nitrogen isotope ratios in northern Australia. Aust Ecol 26:630–636
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6:121–126
Handley LL, Raven JA (1992) The use of natural abundance of N isotopes in plant physiology and ecology. Plant Cell Environ 15:965–985
Handley LL, Scrimgeour C (1997) Terrestrial plant ecology and 15N natural abundance: the present limits to interpretation for uncultivated systems with original data from a Scottish old field. Adv Ecol Res 27:133–212
Handley LL, Austin AT, Robinson D, Scrimgeour CM, Raven JA, Heaton THE, Schmidt S, Stewart GR (1999) The 15N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Aust J Plant Physiol 26:185–199
Hobbie EA, Macko SA, Williams W (2000) Correlations between foliar δ15N and nitrogen concentrations may indicate plant-mycorrhizal interactions. Oecologia 122:273–283
Högberg P (1990) 15N natural abundance as a possible marker of the ectomycorrhizal habit of trees in mixed African woodlands. New Phytol 115:483–486
Högberg P (1997) Tansley review no. 95. 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Högberg P, Alexander IJ (1995) Roles of root symbioses in African woodland and forest: evidence from 15N abundance and foliar analysis. J Ecol 83:217–224
Högberg P, Högbom L, Schinkel H, Högberg M, Johannisson C, Wallmark H (1996) 15N natural abundance of surface soils, roots and mycorrhizas in profiles of European forest soils. Oecologia 108:207–214
Holt JA, Coventry RJ (1990) Nutrient cycling in Australian savanna. J Biogeogr 17:427–432
Mariotti A, Mariotti F, Amarger N, Pizelle G, N'Gambi JM, Champigny ML, Moyse A (1980) Fractionnement isotopique de l'azote lors des processsus d'absorption des nitrates et de la fixation de l'azote atmosphérique par les plantes. Physiol Veg 18:163–181
Michelsen A, Quarmby C, Sleep D, Jonasson S (1998) Vascular plan 15N natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia 115:406–418
Mordelet P, Cook G, Abbadie L, Grably M, Mariotti A (1996) Natural 15N abundance of vegetation and soil in the Kapalga savanna, Australia. Aust J Ecol 21:336–340
Mott JJ, Williams J, Andrew MH, Gillison AN (1985) Australian savanna ecosystems. In: Tothill JC, Mott JJ (eds) Ecology and management of the world's savannas. Australian Academy of Science, Canberra, pp 56–82
Nadelhoffer KJ, Shaver G, Fry B, Giblin A, Johnson L, McKane R (1996) 15N natural abundance and N use by tundra plants. Oecologia 107:386–394
Pate JS, Stewart GR, Unkovich M (1993) 15N natural abundance of plant and soil components of a Banksia woodland ecosystem in relation to nitrate utilisation, life form, mycorrhizal status and N2-fixing abilities of component species. Plant Cell Environ 16:365–373
Reddell P, Milnes AR (1992) Mycorrhizas and rehabilitation of waste rock dumps. Aust J Bot 40:233–242
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Schmidt S, Stewart GR (1997) Waterlogging and fire impacts on N availability in a subtropical wet heathland (wallum). Plant Cell Environ 20:1231–1241
Schmidt S, Stewart GR (1998) Transport, storage and mobilization of N by trees and shrubs in the wet/dry tropics of northern Australia. Tree Physiol 18:403–410
Schmidt S, Stewart GR, Turnbull MH, Erskine PD, Ashwath N (1998) N relations of natural and disturbed plant communities in tropical Australia. Oecologia 117:95–104
Schulze E-D, Gebauer G, Ziegler H, Lange OL (1991) Estimates of nitrogen fixation by trees on an aridity gradient in Namibia. Oecologia 88:451–455
Schulze E-D, Williams RJ, Farquhar GD, Schulze W, Langridge J, Miller JM, Walker BH (1998) Carbon and nitrogen isotope discrimination and nitrogen nutrition of trees along a rainfall gradient in northern Australia. Aust J Plant Physiol 25:413–425
Schulze E-D, Farquhar GD, Miller JM, Schulze W, Walker BH, Williams RJ (1999) Interpretation of increased foliar δ15N in woody species along a rainfall gradient in northern Australia. Aust J Plant Physiol 26:296–298
Stewart GR, Schmidt S (1999) The evolution and ecology of plant mineral nutrition. In: Press MC, Scholes JD, Barker MG (eds) Physiological plant ecology. Blackwell, Oxford, pp 91–114
Stock WD, Wienand KT, Baker AC (1995) Impacts of invading N2-fixing Acacia species on patterns of nutrient cycling in two Cape ecosystems: evidence from soil incubation studies and 15N natural abundance values. Oecologia 101:375–382
Yoneyama T, Omata T, Nakata S, Yazaki J (1991) Fractionation of N isotopes during the uptake and assimilation of ammonia by plants. Plant Cell Physiol 32:1211–1217
Yoneyama T, Matsumaru T, Usui K, Engelaar WMHG (2001) Discrimination of nitrogen isotopes during absorption of ammonium and nitrate at different nitrogen concentration by rice (Oryza sativa L.) plants. Plant Cell Environ 24:133–139
Acknowledgements
We thank EPA of the Supervising Scientist, Jabiru, for logistic support and CSIRO Tropical Ecosystems Research Centre for access to the Kapalga fire trial. We are indebted to Drs. N. Ashwath, L. Hutley, M. Turnbull, P. Erskine, G. Woodall, I. Biggs and S. Richards for helping with field and laboratory work. G. Moss and J. Stewart provided excellent expert technical support with mass spectrometry. Many thanks to Prof. Peter Högberg for supplying additional data for the African communities. The Australian Research Council supported this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, S., Stewart, G.R. δ15N values of tropical savanna and monsoon forest species reflect root specialisations and soil nitrogen status. Oecologia 134, 569–577 (2003). https://doi.org/10.1007/s00442-002-1150-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-002-1150-y