Abstract
Agricultural workers are exposed to many contaminants and suffer from respiratory and other symptoms. Dusts, gases, microbial products and pesticide residues from farms have been linked to effects on the health of agricultural workers. Growing sets of data from in vitro and in vivo models demonstrate the role of the innate immune system, especially Toll-like receptor 4 (TLR4) and TLR9, in lung inflammation induced following exposure to contaminants in agricultural environments. Interestingly, inflammation and lung function changes appear to be discordant indicating the complexity of inflammatory responses to exposures. Whereas the recent development of rodent models and exposure systems have yielded valuable data, we need new systems to examine the combined effects of multiple contaminants in order to increase our understanding of farm-exposure-induced negative health effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Historical perspectives on occupational hazards of farming
Some of the oldest known occupational hazards are associated with farming. As early as 1555, Olaus Magnus wrote that grain dust could damage vital organs of thrashers in his book titled, “Historia de gentibus septintrionalibus” (Schenker et al. 1998). Subsequently, Bernardino Ramazzini documented the risks of inhaling grain dust in his work “De Morbis Artificum Diatriba” (Schenker et al. 1998). Despite the recognition of the respiratory hazards of farm work as early as 1555, only during the last few decades systematic investigations into the respiratory health of agriculture workers have been started. This has led to the development of field of agricultural medicine within the broader domain of occupational medicine. The biomedical data collected so far counter the myth that agricultural environments in rural settings are inherently safer than urban environments.
Development of concentrated animal feeding operations and negative impact on barn worker’s health
The model of animal production operations started to change in the 20th century mainly to alter and enhance food production systems. These changes were largely attributable to a need to increase food production to keep up with the rapid increase in the world population. The increase in the world population was the result of reduced child mortality and increased life expectancy because of advances in medical care. The availability of methods to produce fertilizers and new genomics methods led to significant increases in agriculture productivity. To improve the utilization of the feed and the land used for animal-based food production, intensive animal production systems such as concentrated animal feeding operations (CAFOs) were developed (Casey et al. 2015). These CAFOs achieve efficiency in production but generate a variety of occupational contaminants that negatively impact barn workers, nearby communities and the environment (for a review, see Charavaryamath and Singh 2006). The majority of the contaminants are of animal origin (manure, urine and gas) with contributions from other sources such as feed, pesticides and biological/chemical feed additives. Among the animal origin contaminants, microbes or microbial products are at the center because of their abundance and health impact. The barn environment contains many bacteria, bacterial DNA, lipopolysaccharide (LPS), peptidoglycan (PGN), fungal cell wall components and viral particles (Corzo et al. 2013). Among the 150 or more gaseous contaminants recorded, methane (CH4), ammonia (NH3), hydrogen sulfide (H2S) and carbon dioxide (CO2) are monitored and assessed for their impact on human and animal health (for a review, see Schenker et al. 1998).
Barn workers, veterinarians and people in the vicinity of CAFOs are exposed to many microbial and chemical molecules and experience symptoms ranging from irritation of the skin, mucus membranes and eyes, runny nose, stuffy nose, cough, asthma, exacerbation of pre-existing asthma, asthma-like syndrome, bronchitis, chest tightness and excessive mucus production, changes in mood and depression (Charavaryamath and Singh 2006; Viegas et al. 2013). Studies have been carried out in which healthy human volunteers have been exposed to the swine barn environment followed by the measurement of lung function and markers of inflammation. The recent development of rat and mouse models to mimic the exposure experienced by human workers is facilitating advances in the elucidation of the molecular and cellular mechanisms of lung inflammation following exposure to barn air (Charavaryamath and Singh 2006; Senthilselvan et al. 2009; Sethi et al. 2013). Notwithstanding the advances made so far, a significant amount of work still needs to be done on the pulmonary effects of exposure to the agriculture environment.
To mitigate rising food demands, agricultural farming has witnessed a high use of pesticides. “Pesticide” is a generic name that includes insecticide, herbicide and fungicide (Mamane et al. 2015). Pesticides significantly enhance agriculture productivity (Rekha et al. 2006) and are used on about one-third of agricultural products, making their use indispensable in agricultural production (Liu et al. 2002). However, heavy use of these chemicals results in direct or indirect toxicity and a variety of effects on non-target organisms (Ansari and Kumar 1988). Occupational exposure to various pesticides occurs mainly via dermal routes followed by inhalation and ingestion, whereas the dietary route mainly accounts for non-occupational exposure (Baldi et al. 2012). Although most pesticides are neurotoxic, respiratory dysfunctions have also been observed following direct (inhalation) or indirect (oral) exposure to pesticides in workers engaged with pesticides. The deleterious effects of pesticide exposure on target organs such as liver and kidney are well documented; however, similar data are lacking with respect to non-target organs such as lungs.
Occupational exposure and lung inflammation
Organic dusts
Multiple research groups have identified organic dusts as a major problem in lung inflammation on farms, particularly in CAFOs. Exposure studies in animals (Charavaryamath et al. 2005; Schneberger et al. 2016) and human subjects, evaluation of pulmonary health of workers (Senthilselvan et al. 1997a) and testing of dust mitigation strategies (Senthilselvan et al. 1997b) have shown that organic dust and its components are a primary concern in these environments. The data have also shown that all dusts are not equal. Therefore, total dust in the air may not always be directly comparable with the symptoms seen, meaning that other factors such as particle size, concentration of bacterial products (e.g., LPS and PGN), weight and composition are likely to be of great importance to the generated symptoms (Kirychuk et al. 2010; Hawley et al. 2015). The fraction of dust within the respirable range (1–10 μm) is a likely factor in deciding the inflammatory potential of barn dust and can vary depending on the type of facility (Dosman et al. 2006); however, clear effects of inhaled particles are observed outside of this range in cell cultures that differ in responses to respirable-sized particles (Hawley et al. 2015). These factors may also affect one another, such that LPS are suggested to be enriched in smaller particles (Jones et al. 1984).
Work on these particulates has focused largely on the bacterial components from these environments. Much of the research has been directed primarily towards three components of these dusts: LPS, lipoproteins and proteoglycans. Whereas these components are clearly vital to the responses seen in workers, these components are accepted not to be sufficient to explain all observations. Therefore, we will probably find more contributors to these dust-induced innate immune responses (Schneberger et al. 2016). Similarly, we might find components that are inhibitory or modulatory as opposed to stimulatory (Poole et al. 2011). As an example, organic barn dusts have recently been discovered to contain proteolytic properties (Romberger et al. 2015) that can stimulate protease-activated receptors (PARs) 1 and 2 in the bronchial epithelium. These receptors require the cleavage of the N-terminal domain by proteases in order to induce signaling when the new terminal domain binds the receptor (Dery et al. 1998). Cooperation of PARs with other innate receptors such as Toll-like receptor 4 (TLR4) has also been suggested (Rallabhandi et al. 2008). In the PAR study, a reduction in pro-inflammatory cytokines and reduced bronchoalveolar lavage (BAL) cellular infiltrates were seen when protease activity was blocked. These proteolytic properties of organic dusts require further work, as some chemokines are produced in forms that become more potent upon such cleavage (Berahovich et al. 2005), although the study suggests that the main effect is probably through these PAR receptors.
Gases
Barns for animals such as pigs and poultry are kept closed for biosecurity reasons and also in harsh winter climates. The closed environment leads to the build up of a variety of gases and microbial molecules. Three gases are commonly recognized as being elevated in most CAFO operations, namely ammonia (NH3), hydrogen sulfide (H2S) and carbon dioxide (CO2), although they are far from being the only ones (mentioned earlier).
NH3 is highly soluble and should not penetrate far beyond the upper airway (Landahl and Herrmann 1950). It is a respiratory irritant, however and may be adsorbed to dust particulates in the air. Donham and colleagues (2002) reported synergy between ammonia and organic-dust exposure in poultry operations; this synergy explains the decline in the forced expiratory volume (FEV) and forced expiratory flow (FEF) of the lungs of workers (Donham et al. 2002). Others have reported interactions with endotoxin exposure in pigs (Gustin et al. 1994). A later study of humans with grain dust and ammonia (Sigurdarson et al. 2004) and pigs in a controlled exposure study (Done et al. 2005) failed to yield similar inflammation, whereas another suggested some effect of ammonia in response to Mycoplasma hypopneumonia and a clear effect of particulates on the same condition (Michiels et al. 2015). Thus, the impact of ammonia on or with dust exposures on the immune response remains uncertain.
H2S is commonly recognized as a serious potential health hazard in CAFO operations, although it is mostly associated with issues of waste management and removal. This gas can easily penetrate to the alveolar level and is often cited as a potential health hazard in these facilities, although the hazardous effects often relate to the neurological depression of respiration rather than to inflammation (Truong et al. 2006). This gas has also been shown to potentially cause cellular damage via the generation of reactive oxygen species (Eghbal et al. 2004). H2S further appears to be anti-inflammatory in several exposure systems, although some debate remains with regards to the importance of kinetics (Whiteman et al. 2010), because most of these systems do not involve the inhalation delivery of H2S. One study did indeed suggest potential advantages to H2S in mitigating damage attributable to LPS exposure (Faller et al. 2012). Unfortunately, no work appears to exist on the co-exposure effects of this gas on organic-dust immune responses.
CO2 may be elevated as high as 5000 ppm or greater in some facilities (Clark and McQuitty 1988; Ouellette et al. 1999) but is often discounted as having any effect as responses to CO2 are typically mild and do not include lung irritation at levels that can be detected in such barn facilities (Langford 2005). Studies with cell cultures and animal models have yielded mixed results as to the pro- or anti-inflammatory nature of hypercapnia (Abolhassani et al. 2009; Wang et al. 2010) but have involved levels far in excess of workplace levels. A pilot project that we recently undertook to address this by using a barn-dust exposure system showed that, even at the 8-h 5000-ppm acceptable safe limit, significant changes occurred to the levels of interleukin-6 (IL-6), KC, CCL9 and MMP9 in mice (J. DeVasure, K. Bailey, D. Romberger, T. Wyatt, and D. Schneberger, unpublished data). Some of these, such as CCL9 showed clear CO2 dose-specific increases at the mRNA and protein level. In almost all cases, these changes were only present upon co-exposure with barn-dust extracts, suggesting that gas exposures can modify responses to such dusts while being relatively innocuous on their own.
Pesticides
Farm workers are more prone to pesticide exposure via dermal and inhalation routes and commonly exhibit high prevalence of cough, breathlessness, phlegm, sinusitis, throat discomfort, asthma, chronic bronchitis and allergic rhinitis (Mamane et al. 2015). Pesticide applicators using paraquat, parathion, malathion and thiocarbamate frequently suffer from reduced lung function (Mekonnen and Agonafir 2004) and evoked respiratory symptoms of wheezing (Hoppin et al. 2002). Workers processing pesticides show a high prevalence of acute respiratory symptoms during the work shift (Zuskin et al. 2008). Chronic exposure to pesticides such as chlorpyrifos, carbamate and diazinon are associated with higher incidences of lung cancer (Beane Freeman et al. 2005) and sarcoidosis (Newman et al. 2004). These epidemiological studies indicate that direct or indirect exposures to various types of pesticides adversely affect the pulmonary health of exposed workers.
The prevalence of pesticides in the food chain is responsible for short- and long-term exposures to these pesticides. Oral exposures to various classes of pesticides have been linked with pulmonary damage in various laboratory animals (Abdelsalam 1987; Karaoz et al. 2002; Yavuz et al. 2008; Merkowsky et al. 2016; Pandit et al. 2016). Organophosphate pesticides (OPs) commonly induce pulmonary edema characterized by bronchoconstriction, congestion and intra-alveolar hemorrhage (Abdelsalam 1987). Acute OP poisoning (150 mg/kg) results in intra-parenchymal vascular congestion, thrombosis and hemorrhage, together with an increase in the proliferation of the respiratory epithelium, in the number of alveolar macrophages, in emphysematous changes and in bronchoalveolar hemorrhage in rabbits (Yavuz et al. 2008).
Similarly, chlorpyriphos, an OP, induced mononuclear cell infiltration in peribronchial and perivascular areas, hyperplasia of type II pneumocyte and increased connective tissue in the lungs of rats (Karaoz et al. 2002). In our previous study, we also demonstrated that chlorpyriphos at 3 mg/kg per day orally for 60 days causes perivascular and peribronchial mononuclear cell infiltration and septal congestion in the lungs of mice (unpublished data). Inhalation of chlorpyrifos and cypermithrin mixture leads to fibrosis with collagen proliferation, aggregation of the lymphoid cells in peribronchiolar tissue, severe dilation and congestion of the blood vessels and hyperplasia in the bronchiolar and lining epithelium of rat (Noaishi et al. 2013).
Diazinon, another OP, has predominately household usages and its exposure induces hemorrhage, infiltration of macrophages and mononuclear cells, edematous tissue, pyknotic nuclei and necrotic cells in the lungs of guinea pig (Rady 2009) and rat (Najafi et al. 2014). Fenitrothion (20 mg/kg) by oral gavage for 28 consecutive days causes the disruption of the alveolar walls and lining of the terminal bronchiole, swollen alveolar cells, infiltration of inflammatory cells and cell necrosis in rats (Budin et al. 2012). Aspiration of OP results in pulmonary neutrophil sequestration, alveolar hemorrhage and interstitial edema together with the disruption of the alveolar-capillary membrane (Hulse et al. 2014). Further, early-life exposures to OPs such as dialkylphosphate adversely affect lung function (Raanan et al. 2015). Paraquat, a herbicide, induces severe lung injury characterized by interstitial edema, leukocyte infiltration, alveolar hemorrhage, fibroblast proliferation and increased collagen deposition leading to pulmonary fibrosis (Satomi et al. 2004; Tomita et al. 2007).
We recently reported the first data concerning pulmonary inflammation in mice following oral exposure to fipronil (Merkowsky et al. 2016) and imidacloprid (Pandit et al. 2016). Imidacloprid orally at 1/20th of LD50 for 30 days resulted in lung inflammation characterized by peribronchial and perivascular infiltration and an expanded perivascular space in mice (Pandit et al. 2016). An accumulation of inflammatory cells around the terminal bronchioles, dilation of the perivascular spaces, enlargement of the airway epithelial cells and an increase in the accumulation of inflammatory cells in the alveolar septa and the alveoli were noted following both oral and intranasal exposure to fipronil (Merkowsky et al. 2016). Dopamine and amiloride-sensitive cation channels aid in lung liquid clearance and disorders of their regulation can elicit pulmonary hemorrhage and edema (Adir and Sznajder 2003). The differential expression of dopamine receptors D2 and D4 together with amiloride-sensitive cation channel 2 resulted in pulmonary hemorrhage and edema in rats following exposure to paraquat (Satomi et al. 2004). The data suggest that oral exposures to various classes of pesticides elicit lung inflammation frequently characterized by pulmonary edema, hemorrhages and fibrosis.
Pulmonary fibrosis is another major lesion observed following exposure to various pesticides. Paraquat selectively accumulates (6 to 10 times) in the epithelial cells of the lung (Dinis-Oliveira et al. 2008) in an energy-dependent mechanism (Rose et al. 1976) suggesting that some transporter and/or ion channels are cystic fibrosis transmembrane conductance regulators (CFTR; Satomi et al. 2004) and may be involved in the onset of cystic fibrosis (Naren et al. 1999). Further, pesticide induces the altered expression of various tissue-specific genes, may be responsible for the development of pulmonary fibrosis and will be discussed later while dealing with pesticide-induced immunomodulations in the next section.
Use of animal models to understand mechanisms of lung inflammation
The use of animal models to understand the cell and molecular mechanisms of lung inflammation is becoming an invaluable tool. Donham and Leininger housed rabbits and guinea pigs in the barn for 12 months and reported diffuse interstitial histiocytic pneumonia, epithelial hyperplasia and metaplasia with submucosal plasma cell and heterophil infiltration in tracheal and turbinate tissues and the presence of precipitins to barn-dust extract in the plasma (Donham and Leininger 1984). The authors concluded that 12 months of continuous housing in the swine barn environment induces chronic low-grade inflammation. These early studies in which laboratory animals were housed continuously in the barn failed however to model the occupational exposure of full-time barn workers.
Our group designed a rat model of human occupational exposure to swine barn air, wherein rats were exposed to the swine barn air for 8 h per day, for 1, 5 (Monday to Friday), or 20 days (for 4 working weeks, Monday to Friday). During the weekends, rats spent the full 48 h in the standard laboratory animal housing facility. During exposure, rat cages were hung at 2 m above the ground. We showed that single and multiple exposures induced lung inflammation and airway reactivity with an increase in mucus-producing cells with long-term exposure. Further, a 20-day exposure dampened airway reactivity indicating an adaptive response. Our study also recorded high amounts of LPS and viable bacteria in the barn air during the exposure periods (Charavaryamath et al. 2005). To our knowledge, this was the first known rat model of human occupational exposure to swine barn air. Our model was unique in that we mimicked human exposure of full-time barn workers (8 h/day, Monday to Friday) and recapitulated some of the features of human occupational lung diseases with in vivo cell and molecular details (Charavaryamath et al. 2005). Using a similar rat model of single-day barn exposure, we demonstrated the recruitment of pulmonary intravascular monocytes/macrophages (PIMMs) at 48 h post barn exposure (Gamage et al. 2007). PIMMs are resident cells in species such as horses, cattle and pigs, whereas recruitment of PIMMs is seen in infectious and inflammatory conditions in other species (Charavaryamath et al. 2006; Schneberger et al. 2012). These cells are important since they have been shown to produce both pro- and anti-inflammatory cytokines to regulate lung inflammation (Charavaryamath et al. 2006). Depletion of PIMMs has been shown to curtail lung inflammation in various models of lung inflammation thereby underscoring their importance (Singh et al. 2004; Parbhakar et al. 2005; Gill et al. 2008). Our group has also published extensively on the biology of recruited and resident PIMMs in various species of domestic animal (for a review, see Schneberger et al. 2012). We have also shown that barn-exposed rats retain the ability to mount an effective inflammatory response to a secondary microbial challenge such as Escherichia coli LPS (Fig. 1; Charavaryamath et al. 2008b). Following barn exposure, we revealed the recruitment of neutrophils and monocyte/macrophages into lung tissue and lavage fluid following 1-, 5- and 20-day exposure to barn air (Charavaryamath et al. 2005, 2008a). Further, following 5- and 20-day exposure, we demonstrated the peribronchiolar and perivascular recruitment of eosinophils (Charavaryamath et al. 2005). Interestingly, eosinophil recruitment is not observed after a 1-day exposure to barn air. Following the barn exposure of rats, a secondary Escherichia coli LPS challenge results in a robust inflammatory response indicating the priming of lung innate immunity (Charavaryamath et al. 2008b).
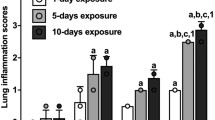
Immunohistochemical expression of interleukin-1β (IL-1β) protein (arrows) in rat lung sections. Lung sections from controls (a, b) and 1-day barn-exposed rats (c, d) without and with Escherichia coli lipopolysaccharide (LPS) challenge, respectively and from 5-day barn-exposed rats (e) without Escherichia coli lipopolysaccharide (LPS) challenge (data not shown for 5-day barn-exposed rats with E. coli LPS challenge). f Quantitative representation of IL-1 β protein concetrations from control, 1-day and 5-day barn-exposed rats without and with E. coli LPS challenge (*P < 0.001). Reproduced with permission from Biomed Central (Charavaryamath et al. 2008b)
These data showed that rodent models can mimic the lung inflammation observed in workers in the pig barns.
The swine barn environment is rich in bacterial endotoxin/LPS and LPS is sensed by the host pattern recognition receptor (PRR) known as TLR4. In order to delineate the role of LPS signaling via TLR4, we exposed C3HeB/FeJ mice (wild-type for tlr4) and C3HeB/HeJ mice (mutant for tlr4) to swine barn air in a similar experimental design at the same study setting. We demonstrated that swine-barn-air-induced lung inflammation but not airway reactivity, is dependent on an intact tlr4 gene (Figs. 2, 3). We also observed tlr4-independent airway epithelial damage in both the stains of mice (Charavaryamath et al. 2008a). These experiments were crucial, since 10 % of the human population shows polymorphism in the tlr4 gene and exhibits selective resistance to the effects of LPS exposure (Lorenz et al. 2001). This might explain why some newly employed workers experience severe symptoms and leave the swine production industry, whereas those who continue to work seem to experience the “healthy worker survivor effect” (Arrighi and Hertz-Picciotto 1994).
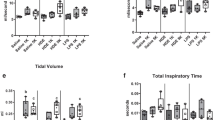
Airway hyper-responsiveness (AHR) to increasing doses of methacholine was similar in Toll-like receptor 4 (TLR4) wild-type (WT; a) and muntant (b) mice. After 5- and 20-day exposure, significantly increased and dampened AHR were seen, respectively. Reproduced from Charavaryamath et al. (2008a) with permission from Taylor and Francis
Total and differential leukocytes in bronchoalveolar lavage fluid (BALF) were counted after Wright’s staining. One-day barn-exposed WT mice showed higher total leukocyte numbers (a), absolute neutrophils (b), absolute macrophages (c) and absoloute lymphocytes (d) than 1-day barn-exposed mutants and all other WT groups. Five-day barn-exposed mutants showed higher absolute lymphocyte numbers comapred with 20-day exposed mutants (d). Reproduced from Charavaryamath et al. (2008a) with permission from Taylor and Francis
Occupational exposure and pulmonary immune modulations
Barn-air exposure
The importance of endotoxin and peptidoglycan in barn-dust inflammation rests in large part on the responses of the innate immune system. The innate immune system is organized to act rapidly to potential pathogens, responding to conserved microbial motifs that it recognizes as foreign and initiating immune responses. Since the discovery of Toll receptors in insects (Hashimoto et al. 1988) and their homologs in mammals and other species (Medzhitov et al. 1997), a large and diverse family of innate immune receptors have and probably will continue to be found.
Possibly the most studied innate immune receptors are in the TLR family. The number of TLRs vary between species, with ten TLRs being recognized to date in humans. These receptors can detect a broad range of lipids, proteoglycans, proteins, RNAs and DNAs (Akira and Takeda 2004). In all cases, TLRs signal through the same pathway (except for TLR3, with TLR4 signaling through the shared and alternate pathway), recruiting the MyD88 protein, which initiates a signaling cascade leading to induction of a number of immune-related pathways such as P38 and NF-κB (nuclear factor kappa-B; Akira and Takeda 2004).
Subsequently, additional families of innate immune receptors have been added to the TLRs. The NOD-like receptors are cytoplasmic receptors that mimic some of the binding range of the TLRs, signaling to the NF-κB and mitogen-activated protein (MAP) kinase pathways (Chen et al. 2009). NALP receptors constitute another 14 receptors that are part of the NOD family. Additional receptors include carbohydrate-binding C-type lectins such as collectins (discussed later) and RIG-1-like receptors (Kawai and Akira 2008), pentraxins (Du Clos 2013) and complement (Dunkelberger and Song 2010), to name just a few. Whereas many of these have yet to be explored with regard to barn dusts, some work has shown changes to complement (Von Essen et al. 1988) and NOD signaling (Poole et al. 2011). For the purposes of this review, we will focus primarily on TLRs, especially TLR2 and TLR4, in relation to organic-dust exposures.
TLR4 is perhaps the best studied of the TLRs. While it is most noted for binding to LPS (with CD14, MD-2 and LBP), the range of ligands has expanded over time to encompass other microbial products and markers of cellular or tissue stress/damage (Vaure and Liu 2014). Evidence exists for the wide expression of the receptor in the lung on the bronchial epithelium, pulmonary epithelial cells, vascular endothelium, and macrophages (Janardhan et al. 2006) but activation can also be restricted by required cofactors such as MD-2, e.g., in the stimulation of airway epithelial cells (Jia et al. 2004). In humans, endotoxin exposure in swine barns has long been known to be related to respiratory health status (Vogelzang et al. 1998) and studies on human subjects have shown that the status of the TLR4 gene is associated with increased lung dysfunction after barn exposure (Senthilselvan et al. 2009), clearly establishing TLR4 and its ligand LPS as an important factor in organic-dust-induced lung inflammation. These results have been extended beyond swine barns to chicken barn dusts (Kirychuk et al. 2010) showing that these dusts enhance TLR4 expression, at least in the short term but not with long-term chronic exposure (Pallvi et al. 2016).
TLR2 is typically associated with the detection of peptidoglycans, lipoteichoic acid, lipopeptides, lipoproteins and zymosan (Zahringer et al. 2008). This TLR can dimerize with TLR1, TLR6, or TLR10, greatly increasing the range of possible ligands that it can detect (Oliveira-Nascimento et al. 2012; Kang et al. 2009; Oosting et al. 2014). In the lungs, TLR2 is expressed on type-2 epithelial cells, bronchial epithelium and alveolar macrophages (Bailey et al. 2008). Studies have shown that Gram-positive bacteria tend to predominate in the barn environment (Martin et al. 1996) suggesting that TLR2 may be even more important that TLR4 in determining the response to these organic dusts.
Some work has also been done with TLR9 and organic dusts (Batzer et al. 2007; Bauer et al. 2013). TLR9 detects microbial DNA and thus would be expected to respond to any bacterial DNA in dusts. Similar to the other TLRs, widespread expression of the receptor occurs in the lung (Schneberger et al. 2013). Like TLR4 and TLR2, the expression of the receptor can also be found on dendritic cells (Hoene et al. 2006) and neutrophils (Hayashi et al. 2003). Although nowhere near as strong a response as TLR2 and TLR4, changes have been seen in BAL cellular composition in TLR9 knockout mice exposed to the barn environment (Schneberger et al. 2016) and changes to levels of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) have been detected in the lung. Another study of these same animals revealed what appeared to be reduced numbers of type-II cells in exposed knockout animals after chronic barn-air exposure (Sethi et al. 2013). These results help to illustrate that multiple active components make up such organic dusts and that the response generated to them spans multiple receptors. Whereas TLR9 may not express the magnitude of response seen with TLR2 and TLR4, it also appears to contribute to organic-dust immune responses.
Although intracellular signaling often occurs through shared pathways, the regulation and diversity of responses can also be achieved through a variety of cofactors that are required for function or that enhance or inhibit function. For example, CD14, MD-2 and LBP are required as co-factors for functional binding of TLR4 (Heumann et al. 2003). In cases of tissue or cell injury, released factors such as HMGB1 are known to signal or enhance response of TLRs 2, 4 and 9 (Park et al. 2004; Ivanov et al. 2007) in various cell types. Many TLRs also have a function in the detection of stress or injury. For example, TLR9 can detect and respond to mitochondrial DNA and thus can also sense necrosis (Zhang et al. 2010), while TLR4 can distinguish between long- and short-chain hyaluronan (Jiang et al. 2005), the latter of which is a signal of tissue damage.
With most TLRs sharing a common pathway leading to NF-ĸB activation, interest has been shown in knocking out the adapter protein MyD88, which initiates signaling along most all TLR pathways (Kawai et al. 1999). Knockout of MyD88 is highly effective in greatly reducing lavage cell numbers in response to organic dust, in addition to a number of key cytokines and chemokines both in acute and chronic exposures. Oddly, parenchymal inflammation is greater in the dust-exposed MyD88 knockout animals. Many of these aggregates are composed mainly of B and T cells (Bauer et al. 2013). Similarly, whereas MyD88-knockout bronchial epithelial cells are less responsive to the organic-dust-induced slowing of ciliary beat, wound closure is also inhibited, suggesting a need for TLR signaling in efficient wound repair (Poole et al. 2015). The use of bone marrow mouse chimera has revealed an interesting observation that airway hyper-responsiveness to barn dust is mediated by MyD88 in structural cells and not leukocytes (Poole et al. 2015). This occurs in spite of the secretion of pro-inflammatory cytokines in the same system being highly reliant on MyD88 expression in hematopoetic/lymphocytic cells. Further, neutrophilic influx into the lung is reliant on MyD88 expression in both cell populations showing the importance of both immune and non-immune cells in the overall lung response to organic dusts (Poole et al. 2015).
More recently, the discovery of the induction of A20 by endotoxin and organic dusts has garnered attention for being necessary for protection from the development of asthma (Schuijs et al. 2015). This ubiquitin-modifying enzyme has roles in modifying responses from a broad range of receptor pathways such as TLR, NOD, TNF, IL-17 and the T-cell complex that all lead to NF-ĸB activation, showing that the effects of exposure to organic dusts can alter a broad array of immune response mechanisms (Catrysse et al. 2014). RIG-1 signaling is also modified by A20, suggesting more than just NF-ĸB signaling is altered (Lin et al. 2006).
Although NF-ĸB activation is of great importance to responses to barn dusts, evidence has been presented of other pathways of significance in these responses. Data from the Romberger, Wyatt and Poole laboratories show the role of protein kinase C (PKC) in swine-barn-dust-induced swift responses in a bronchial epithelial cell system; the response peaks at 1–2 h and is not induced by LPS alone (Romberger et al. 2002). Two isoforms of PKC, in particular PKC-α and PKC-ε, have further been shown to be essential to TNF-α, IL-6 and IL-8 secretions in response to swine dusts (Romberger et al. 2002; Wyatt et al. 2010), although some variation has been observed between the different types of dusts (Wyatt et al. 2007). More recently, these groups looked at means to inhibit this PKC cytokine cascade. Using a cAMP-dependent protein kinase (PKA)-activating agent, they were able to inhibit TNF-α production and, as a result, the downstream PKC and cytokine/chemokine activation and production (Wyatt et al. 2010). Another such attempt to enhance PKA was performed by using agonists to the β-adrenergic receptor, such as salbutamol and salmeterol, which show similar results (Romberger et al. 2016). In another attempt at limiting PKC activity, dimethylarginine dimethylaminohydrolase (DDAH) was found to be able to inhibit PKC and cytokine response to swine dusts, suggesting further levels of control to intracellular signaling to these dusts (Bailey et al. 2014). These data were the first to show the role of PKC in barn-dust-induced cellular responses in bronchial epithelial cells.
Pesticide exposure
Pesticide exposures have been associated with marked immune dysregulation and adverse cytokine profiles (Duramad and Holland 2011) suggesting that the immune system is a target of the toxic effect of several pesticides. Cytokines are the important markers revealing the nature of the immune disturbances induced by pesticide immunotoxicity (Rowsey and Gordon 1999). Increased levels of circulating cytokines and chemokines and neutrophil sequestration in the lungs are characteristics of systemic inflammation (Abraham 2003).
OPs have been involved in the manipulation of cytokines expression (Alluwaimi and Hussein 2007) and OP-induced asthma is characterized by elevated Th2 cytokines such as IL-4, IL-5 and IL-13 (Duramad et al. 2006). Parathion, chlorpyrifos and diazinon increase the mRNA expression of TNF-α, IL-1β, platelet derived growth factor (PDGF) and transforming growth factor-β (TGF-β) in a concentration-dependent manner without affecting viability in a human monocyte cell line differentiated into macrophage-like cells (THP1; Proskocil et al. 2014). IL-6 and IL-13 play an important role in oxidative stress (Rangasamy et al. 2005) and their mRNA expression is up-regulated together with antioxidant defense system enzymes (Mt1, Mt2, Hmox1, Gcl, GR, Txn1) after 6 h of paraquat exposure in lungs of mice (Tomita et al. 2007).
Paraquat upregulates various cytokine, chemokines and growth factors in lungs of mice (Ishida et al. 2006; Qian et al. 2015) and rats (Tomita et al. 2002). A significant increase occurs in the gene expression of TNF-α and monocyte chemo-attractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α and MIP-2, fibrogenic growth factors such as TGF-β, platelet-derived growth factor (PDGF)-A, acidic fibroblast growth factor and hepatoctyte growth factor in mice (Ishida et al. 2006). Further, paraquat-induced lung injury is characterized by significantly increased (P < 0.01) serum TGF-β1, IL-10 and TNF-α in rats (Jiang et al. 2015) and IL-1, IL-6, TNF-α and total protein levels in BAL fluid in mice (Qian et al. 2015). Paraquat exposure induces the differential pulmonary expression of neurotransmitter receptor genes, transporter genes, voltage-gated ion channel genes, lipid metabolism enzyme genes, G-protein involved in endocytosis and exocytosis genes and apoptosis-related genes in rat (Satomi et al. 2004). The synergistic effects of these molecules are presumed to be the cause of pulmonary fibrosis following paraquat exposure (Ishida et al. 2006).
Pesticides may cause toxic effects by stimulating various pro-inflammatory cells such as alveolar macrophages (Banks and Lein 2012). The alveolar macrophages during asthma secrete various pro-inflammatory mediators (Barnes 1994) that cause airway hyper-responsiveness (Zhang et al. 2007). Parathion activates alveolar macrophages to release TNF-α, which is linked to dysfunction of neuronal M2 receptor and airway hyper-reactivity in guinea pig (Proskocil et al. 2013). The role of alveolar macrophages is further underscored by the finding that their depletion by clodronate abrogates parathion-induced airway hyper-reactivity in guinea pig (Proskocil et al. 2013). The exposure to paraquat increases Fas and FasL expression together with CASP3 to suggest the involvement of apoptosis in lung injury (Tomita et al. 2007).
We recently reported that TLRs such as TLR4 and TLR9 together with pro-inflammatory mediators may play a role in the pulmonary effects in mice exposed to fipronil (Merkowsky et al. 2016), ethion (unpublished data) and imidacloprid (Pandit et al. 2016). TLR9, a membrane-bound receptor, is expressed on a variety of cells including macrophages and neutrophils and its capacity to bind non-methylated CpG DNA sequences (Schneberger et al. 2016) makes it central to the recognition and phagocytosis of bacteria (Murad and Clay 2009). TLR9 activates the innate immune system in response to microbial/endogenous DNA such as mitochondrial DNA (mtDNA; Zhang et al. 2010) and its expression correlates positively with the severity of lung damage (Qian et al. 2015). During pesticide-induced cellular damage and necrosis in the lung, the damaged mitochondria may leak into the extracellular space thereby triggering the release of mitochondrial-damage-associated molecular patterns (DAMPs) such as mtDNA (West et al. 2011). Hence, paraquat-induced acute lung injury with elevated pulmonary TLR9 mRNA expression might be attributable to the activation of TLR9 by mtDNA (Qian et al. 2015).
Chlorpyrifos can exhibit airway hyper-reactivity without altering acetyl cholinesterase (AChE) and M3 muscarinic receptor function on airway smooth muscle (Fryer et al. 2004) suggesting a uniqueness in the onset of airway hyper-reactivity following exposure to OPs. However, a lack of similar data exists for other classes of pesticides, especially newly evolved pesticides. Thus, the exploration of this field will be of interest in order to unfold the cellular mechanism of pulmonary immune-modulations following exposure to a specific pesticide.
Role of surfactants
Surfactant proteins (SP) are important constituents of pulmonary defense and are a lipoprotein complex of phospholipids and apoproteins (Pastva et al. 2007). Immune surfactants A (SP-A) and D (SP-D) are now seen as critical components of innate immunity not only for the direct neutralization of pathogens and allergens but also for the inhibition or enhancement of inflammation depending on the circumstance (Kishore et al. 2006). Generated in the lung and often in other locations, immune surfactants serve to bind and inhibit pathogens, whereas non-immune surfactants B and C have roles in the reduction of lung surface tension (Weaver and Conkright 2001). The immune surfactants or collectins, on the other hand, also carry out a diverse array of innate defense roles. They can bind a number of carbohydrate motifs, affecting direct killing and the neutralization of a broad array of pathogens (Kishore et al. 2006). Apart from this, these surfactants are known to bind the SIRP-α cell receptor and inhibit P38 activation under normal conditions. However, changes in SP-A or D structure as a result of binding pathogens or nitric oxide s-nitrosylation of SP-D (Guo et al. 2008) alter this binding and so these surfactants instead bind CD91/calreticulin and activate the very same P38 pathway (Gardai et al. 2003). This last action initiates a number of cellular changes that may influence innate immunity, including the alteration of TLR2, TLR4 and TLR9 expression (An et al. 2002). SP-A has further been shown to block LPS binding to CD14 (Sano et al. 1999) and LPS binding to protein (Stamme et al. 2002) and subsequently inhibit TLR4 responses, revealing yet another way in which these molecules can modify innate immunity. Conversely, non-immune surfactant protein C can enhance LPS binding to CD14 (Augusto et al. 2003) suggesting that even the “non-immune” surfactants play roles in lung innate immunity.
Pulmonary SPs are also involved in the pathophysiology of lung injury following exposure to pesticides (Gil et al. 2007). Phospholipid transfer protein is frequently expressed in lungs (Jiang and Bruce 1995) and is an important regulator of surfactant biology (Pownall et al. 1991). Pulmonary SP-A and SP-D mediate host-defense functions (Wright 2005) and are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury (Bridges et al. 2000). SP-D reduces apoptosis in alveolar macrophages to prevent abnormal alveolar remodeling during lung injury (Clark et al. 2002). Lung damage following paraquat poisoning is secondary to derangement of the pulmonary surfactant system (Fisher et al. 1975). An increase occurs in the SP-D concentration in the BAL fluid of rat following exposure to paraquat (Gil et al. 2007). However, paraquat exposure decreases the mRNA expression of SP-D in the lung of mice (Tomita et al. 2007). The data reflect the species-specific role of SPs during pesticide-induced lung damage; this needs to be explored in depth.
Although surfactants appear not to have been studied in terms of organic dust, preliminary work in our laboratory has shown SP-A and SP-D reductions in A549 lung cell lines exposed to hog barn dusts. Further studies of SP-D have suggested that excess SP-D can inhibit the production of cytokines such as IL-8 in response to these dusts (unpublished observations). This further suggests that some of the inflammatory response generated in lungs to these dusts might be attributable to reductions in these immune surfactants. However, given their potential multiple roles in inflammation, much more work needs to be done with regard to such exposures.
Pesticide and endotoxin interaction
Endotoxin/LPS is a major pathogenic element that can induce systemic inflammation during Gram-negative bacterial infection, as it is an integral cell wall component of these bacteria (Sugiyama et al. 2008). LPS/endotoxin has been reported to induce lung damage in mice (Takeda and Akira 2004; Pandit et al. 2016) and rats (Shang et al. 2009). LPS challenge increases the production of IL-1β/IL-18 (Eltom et al. 2014) resulting in pulmonary inflammation (Sugiyama et al. 2008). LPS-induced TLR4 activation increases the levels of IL-1β/IL-18 in the BAL fluid via an ICE protease-activating factor (IPAF)-dependent and caspase-independent pathway (Eltom et al. 2014) and of IL-1β and TNFα in the airways and epithelial cells via the NF-κB pathway (Takeda and Akira 2004). Further, monocytes have integral activated caspase-1 that releases active IL-1β after a single stimulation with LPS (Netea et al. 2009).
Pesticides and bacterial interactions mimic the over-expression of certain genes/cytokines resulting in host tissues injury (Duramad et al. 2006; Gagnaire et al. 2007). Workers, especially those employed in agriculture, animal-confinement buildings and grain-handling facilities, are exposed to higher levels of endotoxins (Burch et al. 2010). Endotoxin is typically associated with the negative respiratory symptoms in poultry workers (Just et al. 2009) suggesting that endotoxin inhalation is responsible for pulmonary dysfunction such as acute airway obstruction, hypersensitivity pneumonitis, chronic bronchitis and decreased lung function in humans (Thorn 2001). LPS exposure together with fipronil alters the expression of cytokines in human monocytic cells (Sidiropoulou et al. 2011). Similarly, chlorpyrifos and its metabolites do not induce cytokine expression in vitro, whereas in combination with LPS, they stimulate the expression of higher levels of IFN-γ than LPS alone (Duramad et al. 2006).
In another study, the treatment of pregnant mothers with methamidophos and the intranasal challenge with respiratory syncytial virus (RSV) to the offspring mice suppressed the levels of IL-6 and IFN-γ in the BAL fluid of mice (Watanabe et al. 2013). Further, methamidophos treatment did not alter the pulmonary viral titers but moderately suppressed lung inflammation and the gene expression of the cytokines in the lungs of the offspring mice. The study led to the conclusion that the exposure of the mothers to methamidophos during pregnancy and nursing resulted in an irregular pulmonary immune response in the offspring mice.
We have previously demonstrated that LPS co-exposure with imidacloprid (Pandit et al. 2016), fipronil (Merkowsky et al. 2016) and chlorpyriphos (unpublished data) increases vulnerability to pulmonary damage. Treatment with imidacloprid orally at 1/20th of LD50 for 90 days in combination with LPS leads to marked lung damage together with a significant increase in total cell and neutrophil counts in BAL and peripheral blood without altering the mRNA expression of TLR-4 and TNF-α (Pandit et al. 2016). The limited data available on pesticide-endotoxin interaction suggest that this aspect is a potential area of pulmonary research in the future.
Resolution of inflammation
An exciting new area of research is the elucidation of the mechanisms involved in the resolution of inflammation. These anti-inflammatory and pro-inflammation-resolving lipid mediators such as lipoxins, resolvins and protectins promote the reduction of pro-inflammatory processes (Bannenberg 2010). This does not necessarily mean a reduction in lung immunity, as several factors are shown to increase resistance to pathogens (Chiang et al. 2012). Whereas little has been done regarding organic dusts, recent work with maresin has shown that it can inhibit the organic-dust-induced expression of IL-6, IL-8 and TNF-α (Nordgren et al. 2013). Animal studies have confirmed a reduction in BAL neutrophil infiltration, IL-6 and TNF-α, although characteristic dust-induced lymphoid aggregates remain (Nordgren et al. 2015). With such work exposures often being chronic, this suggests a new area of potential interest.
Concluding remarks and future directions
Whereas research over the years has elucidated multiple components within dust binding to a wide variety of innate immune receptors, we still have no clear definition of all the components responsible for responses seen to organic barn dusts. Size fractionation of dusts has revealed immune activity in multiple fractions. Although some components such as LPS and proteoglycans have emerged as major components, they do not define all of the effects seen. Work by several groups has also suggested the contribution of gases in these barn workplace environments modifying responses to dusts. To date, very little work has been carried out in this area, despite several gases having long been determined as irritants. Initially, some of the more common gases will need to be tested in isolation, moving to combinations that may approximate poor ventilation scenarios. Although additional single stimulatory factors may yet still emerge, future study models will need to look at the combined effects of multiple potential workplace stimulants such as dust, pesticides and gases. Whether this is addressed through more worksite exposure models or laboratory models, the complicated nature of the problem will eventually require more complex exposure systems to be addressed. The farming workplace environment is an intricate one and whereas we have learned a great deal since the first reports of hazards in 1555, much remains to be done if we are to preserve the health of those that provide food for us all.
References
Abdelsalam EB (1987) Organophosphorus compounds. I. Toxicity in domestic animals. Vet Res Commun 11:211–219
Abolhassani M, Guais A, Chaumet-Riffaud P, Sasco AJ, Schwartz L (2009) Carbon dioxide inhalation causes pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol 296:L657–L665
Abraham E (2003) Neutrophils and acute lung injury. Crit Care Med 31:S195–S199
Adir Y, Sznajder JI (2003) Regulation of lung edema clearance by dopamine. Isr Med Assoc J 5:47–50
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Alluwaimi AM, Hussein Y (2007) Diazinon immunotoxicity in mice: modulation of cytokines level and their gene expression. Toxicology 236:123–131
An H, Xu H, Yu Y, Zhang M, Qi R, Yan X, Liu S, Wang W, Guo Z, Qin Z, Cao X (2002) Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunol Lett 81:165–169
Ansari BA, Kumar K (1988) Cypermethrin toxicity: effect on the carbohydrate metabolism of the Indian catfish, Heteropneustes fossilis. Sci Total Environ 72:161–166
Arrighi HM, Hertz-Picciotto I (1994) The evolving concept of the healthy worker survivor effect. Epidemiology 5:189–196
Augusto LA, Synguelakis M, Johansson J, Pedron T, Girard R, Chaby R (2003) Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide. Infect Immun 71:61–67
Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ (2008) Toll-like receptor 2 is upregulated by hog confinement dust in an IL-6-dependent manner in the airway epithelium. Am J Physiol Lung Cell Mol Physiol 294:L1049–L1054
Bailey K, Wyatt T, Wells S, Klein E, Robinson J, Romberger D, Poole J (2014) Dimethylarginine dimethylaminohydrolase (DDAH) overexpression attenuates agricultural organic dust extract-induced inflammation. J Environ Immunol Toxicol 2:72–78
Baldi I, Lebailly P, Rondeau V, Bouchart V, Blanc-Lapierre A, Bouvier G, Canal-Raffin M, Garrigou A (2012) Levels and determinants of pesticide exposure in operators involved in treatment of vineyards: results of the PESTEXPO Study. J Expo Sci Environ Epidemiol 22:593–600
Banks CN, Lein PJ (2012) A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 33:575–584
Bannenberg GL (2010) Therapeutic applicability of anti-inflammatory and proresolving polyunsaturated fatty acid-derived lipid mediators. Sci World J 10:676–712
Barnes PJ (1994) Cytokines as mediators of chronic asthma. Am J Respir Crit Care Med 150:S42–S49
Batzer G, Lam DP, Paulus P, Boasen J, Ng N, Horner AA (2007) Using house dust extracts to understand the immunostimulatory activities of living environments. Immunobiology 212:491–498
Bauer C, Kielian T, Wyatt TA, Romberger DJ, West WW, Gleason AM, Poole JA (2013) Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am J Respir Cell Mol Biol 48:781–789
Beane Freeman LE, Bonner MR, Blair A, Hoppin JA, Sandler DP, Lubin JH, Dosemeci M, Lynch CF, Knott C, Alavanja MC (2005) Cancer incidence among male pesticide applicators in the Agricultural Health Study cohort exposed to diazinon. Am J Epidemiol 162:1070–1079
Berahovich RD, Miao Z, Wang Y, Premack B, Howard MC, Schall TJ (2005) Proteolytic activation of alternative CCR1 ligands in inflammation. J Immunol 174:7341–7351
Bridges JP, Davis HW, Damodarasamy M, Kuroki Y, Howles G, Hui DY, McCormack FX (2000) Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J Biol Chem 275:38848–38855
Budin SB, Saimin H, Taib IS, Jayusman PA, Mohamed J (2012) A histological studies of rats’ lung subacutely treated with Fenitrothion. Int J Collab Res Intern Med Public Health 4:744–752
Burch JB, Svendsen E, Siegel PD, Wagner SE, von Essen S, Keefe T, Mehaffy J, Martinez AS, Bradford M, Baker L, Cranmer B, Saito R, Tessari J, Linda P, Andersen C, Christensen O, Koehncke N, Reynolds SJ (2010) Endotoxin exposure and inflammation markers among agricultural workers in Colorado and Nebraska. J Toxicol Environ Health A 73:5–22
Casey JA, Kim BF, Larsen J, Price LB, Nachman KE (2015) Industrial food animal production and community health. Curr Environ Health Rep 2:259–271
Catrysse L, Vereecke L, Beyaert R, van Loo G (2014) A20 in inflammation and autoimmunity. Trends Immunol 35:22–31
Charavaryamath C, Singh B (2006) Pulmonary effects of exposure to pig barn air. J Occup Med Toxicol 1:10
Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B (2005) Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res 6:50
Charavaryamath C, Janardhan KS, Caldwell S, Singh B (2006) Pulmonary intravascular monocytes/macrophages in a rat model of sepsis. Anat Rec A Discov Mol Cell Evol Biol 288:1259–1271
Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B (2008a) Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 34:19–35
Charavaryamath C, Keet T, Aulakh GK, Townsend HG, Singh B (2008b) Lung responses to secondary endotoxin challenge in rats exposed to pig barn air. J Occup Med Toxicol 3:24
Chen G, Shaw MH, Kim YG, Nunez G (2009) NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol 4:365–398
Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484:524–528
Clark PC, McQuitty JB (1988) Air quality in farrowing barns. Can Agric Eng 30:173–178
Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KB (2002) Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol 169:2892–2899
Corzo CA, Culhane M, Dee S, Morrison RB, Torremorell M (2013) Airborne detection and quantification of swine influenza A virus in air samples collected inside, outside and downwind from swine barns. PLoS ONE 8:e71444
Dery O, Corvera CU, Steinhoff M, Bunnett NW (1998) Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol 274:C1429–C1452
Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F (2008) Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 38:13–71
Done SH, Chennells DJ, Gresham AC, Williamson S, Hunt B, Taylor LL, Bland V, Jones P, Armstrong D, White RP, Demmers TG, Teer N, Wathes CM (2005) Clinical and pathological responses of weaned pigs to atmospheric ammonia and dust. Vet Rec 157:71–80
Donham KJ, Leininger JR (1984) Animal studies of potential chronic lung disease of workers in swine confinement buildings. Am J Vet Res 45:926–931
Donham KJ, Cumro D, Reynolds S (2002) Synergistic effects of dust and ammonia on the occupational health effects of poultry production workers. J Agromed 8:57–76
Dosman JA, Fukushima Y, Senthilselvan A, Kirychuk SP, Lawson JA, Pahwa P, Cormier Y, Hurst T, Barber EM, Rhodes CS (2006) Respiratory response to endotoxin and dust predicts evidence of inflammatory response in volunteers in a swine barn. Am J Ind Med 49:761–766
Du Clos TW (2013) Pentraxins: structure, function, and role in inflammation. ISRN Inflamm 2013:379040
Dunkelberger JR, Song WC (2010) Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50
Duramad P, Holland NT (2011) Biomarkers of immunotoxicity for environmental and public health research. Int J Environ Res Public Health 8:1388–1401
Duramad P, Tager IB, Leikauf J, Eskenazi B, Holland NT (2006) Expression of Th1/Th2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and allergen Der p1. J Appl Toxicol 26:458–465
Eghbal MA, Pennefather PS, O’Brien PJ (2004) H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 203:69–76
Eltom S, Belvisi MG, Yew-Booth L, Dekkak B, Maher SA, Dubuis ED, Jones V, Fitzgerald KA, Birrell MA (2014) TLR4 activation induces IL-1beta release via an IPAF dependent but caspase 1/11/8 independent pathway in the lung. Respir Res 15:87
Faller S, Zimmermann KK, Strosing KM, Engelstaedter H, Buerkle H, Schmidt R, Spassov SG, Hoetzel A (2012) Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Med Gas Res 2:26
Fisher HK, Clements JA, Tierney DF, Wright RR (1975) Pulmonary effects of paraquat in the first day after injection. Am J Physiol 228:1217–1223
Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA (2004) Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol 286:L963–L969
Gagnaire B, Gay M, Huvet A, Daniel JY, Saulnier D, Renault T (2007) Combination of a pesticide exposure and a bacterial challenge: in vivo effects on immune response of Pacific oyster, Crassostrea gigas (Thunberg). Aquat Toxicol 84:92–102
Gamage LN, Charavaryamath C, Swift TL, Singh B (2007) Lung inflammation following a single exposure to swine barn air. J Occup Med Toxicol 2:18
Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM (2003) By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115:13–23
Gil HW, Oh MH, Woo KM, Lee EY, Oh MH, Hong SY (2007) Relationship between pulmonary surfactant protein and lipid peroxidation in lung injury due to paraquat intoxication in rats. Korean J Intern Med 22:67–72
Gill SS, Suri SS, Janardhan KS, Caldwell S, Duke T, Singh B (2008) Role of pulmonary intravascular macrophages in endotoxin-induced lung inflammation and mortality in a rat model. Respir Res 9:69
Guo CJ, Atochina-Vasserman EN, Abramova E, Foley JP, Zaman A, Crouch E, Beers MF, Savani RC, Gow AJ (2008) S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol 6:e266
Gustin P, Urbain B, Prouvost JF, Ansay M (1994) Effects of atmospheric ammonia on pulmonary hemodynamics and vascular permeability in pigs: interaction with endotoxins. Toxicol Appl Pharmacol 125:17–26
Hashimoto C, Hudson KL, Anderson KV (1988) The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269–279
Hawley B, Schaeffer J, Poole JA, Dooley GP, Reynolds S, Volckens J (2015) Differential response of human nasal and bronchial epithelial cells upon exposure to size-fractionated dairy dust. J Toxicol Environ Health A 78:583–594
Hayashi F, Means TK, Luster AD (2003) Toll-like receptors stimulate human neutrophil function. Blood 102:2660–2669
Heumann D, Lauener R, Ryffel B (2003) The dual role of LBP and CD14 in response to Gram-negative bacteria or Gram-negative compounds. J Endotoxin Res 9:381–384
Hoene V, Peiser M, Wanner R (2006) Human monocyte-derived dendritic cells express TLR9 and react directly to the CpG-A oligonucleotide D19. J Leukoc Biol 80:1328–1336
Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP (2002) Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am J Respir Crit Care Med 165:683–689
Hulse EJ, Clutton RE, Drummond G, Eddleston M (2014) Translational toxicological research: investigating and preventing acute lung injury in organophosphorus insecticide poisoning. J R Army Med Corps 160:191–192
Ishida Y, Takayasu T, Kimura A, Hayashi T, Kakimoto N, Miyashita T, Kondo T (2006) Gene expression of cytokines and growth factors in the lungs after paraquat administration in mice. Leg Med (Tokyo) 8:102–109
Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Birson CA, Bianchi ME, Wang H, Chu WM (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CPG-DNA. Blood 110:1970–1981
Janardhan KS, McIsaac M, Fowlie J, Shrivastav A, Caldwell S, Sharma RK, Singh B (2006) Toll like receptor-4 expression in lipopolysaccharide induced lung inflammation. Histol Histopathol 21:687–696
Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB Jr (2004) Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol 287:L428–L437
Jiang XC, Bruce C (1995) Regulation of murine plasma phospholipid transfer protein activity and mRNA levels by lipopolysaccharide and high cholesterol diet. J Biol Chem 270:17133–17138
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11:1173–1179
Jiang L, Zhang Y, Sun Y, Hu L, Gao D (2015) Artesunate attenuates lung injury in paraquat-intoxicated rats via downregulation of inflammatory cytokines. Clin Lab 61:1601–1607
Jones W, Morring K, Olenchock SA, Williams T, Hickey J (1984) Environmental study of poultry confinement buildings. Am Ind Hyg Assoc J 45:760–766
Just N, Duchaine C, Singh B (2009) An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J Occup Med Toxicol 4:13
Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, Lee JO (2009) Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31:873–884
Karaoz E, Gultekin F, Akdogan M, Oncu M, Gokcimen A (2002) Protective role of melatonin and a combination of vitamin C and vitamin E on lung toxicity induced by chlorpyrifos-ethyl in rats. Exp Toxicol Pathol 54:97–108
Kawai T, Akira S (2008) Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci 1143:1–20
Kawai T, Adachi O, Ogawa T, Takeda K, Akira S (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115–122
Kirychuk SP, Reynolds SJ, Koehncke NK, Lawson J, Willson P, Senthilselvan A, Marciniuk D, Classen HL, Crowe T, Just N, Schneberger D, Dosman JA (2010) Endotoxin and dust at respirable and nonrespirable particle sizes are not consistent between cage- and floor-housed poultry operations. Ann Occup Hyg 54:824–832
Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T (2006) Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol 43:1293–1315
Landahl HD, Herrmann RG (1950) Retention of vapors and gases in the human nose and lung. Arch Ind Hyg Occup Med 1:36–45
Langford NJ (2005) Carbon dioxide poisoning. Toxicol Rev 24:229–235
Lin R, Yang L, Nakhaei P, Sun Q, Sharif-Askari E, Julkunen I, Hiscott J (2006) Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J Biol Chem 281:2095–2103
Liu CJ, Men WJ, Liu YJ (2002) The pollution of pesticides in soils and its bioremediation. Syst Sci Compr Stud Agric 18:295–297
Lorenz E, Frees KL, Schwartz DA (2001) Determination of the TLR4 genotype using allele-specific PCR. Biotechniques 31:22–24
Mamane A, Baldi I, Tessier JF, Raherison C, Bouvier G (2015) Occupational exposure to pesticides and respiratory health. Eur Respir Rev 24:306–319
Martin WT, Zhang Y, Willson P, Archer TP, Kinahan C, Barber EM (1996) Bacterial and fungal flora of dust deposits in a pig building. Occup Environ Med 53:484–487
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394–397
Mekonnen Y, Agonafir T (2004) Lung function and respiratory symptoms of pesticide sprayers in state farms of Ethiopia. Ethiop Med J 42:261–266
Merkowsky K, Sethi RS, Gill JP, Singh B (2016) Fipronil induces lung inflammation in vivo and cell death in vitro. J Occup Med Toxicol 11:10
Michiels A, Piepers S, Ulens T, Van Ransbeeck N, Del Pozo Sacristan R, Sierens A, Haesebrouck F, Demeyer P, Maes D (2015) Impact of particulate matter and ammonia on average daily weight gain, mortality and lung lesions in pigs. Prev Vet Med 121:99–107
Murad YM, Clay TM (2009) CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs 23:361–375
Najafi G, Tehrani AA, Jalali AS, Babaei M, Najafi A (2014) Sublethal dose of diazinon induces pulmonary toxicity in rat: histopathological evidence. J Interdiscip Histopathol 2:26–31
Naren AP, Cormet-Boyaka E, Fu J, Villain M, Blalock JE, Quick MW, Kirk KL (1999) CFTR chloride channel regulation by an interdomain interaction. Science 286:544–548
Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA (2009) Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113:2324–2335
Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, Terrin ML, Weinberger SE, Moller DR, McLennan G, Hunninghake G, DePalo L, Baughman RP, Iannuzzi MC, Judson MA, Knatterud GL, Thompson BW, Teirstein AS, Yeager H Jr, Johns CJ, Rabin DL, Rybicki BA, Cherniack R, ACCESS Research Group (2004) A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med 170:1324–1330
Noaishi MA, Afify MMM, Allah AAA (2013) Study the inhalation exposure effect of pesticides mixture in the white rat. Nat Sci 11:45–54
Nordgren TM, Heires AJ, Wyatt TA, Poole JA, LeVan TD, Cerutis DR, Romberger DJ (2013) Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir Res 14:51
Nordgren TM, Bauer CD, Heires AJ, Poole JA, Wyatt TA, West WW, Romberger DJ (2015) Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl Res 166:57–69
Oliveira-Nascimento L, Massari P, Wetzler LM (2012) The role of TLR2 in infection and immunity. Front Immunol 3:79
Oosting M, Cheng SC, Bolscher JM, Vestering-Stenger R, Plantinga TS, Verschueren IC, Arts P, Garritsen A, van Eenennaam H, Sturm P, Kullberg BJ, Hoischen A, Adema GJ, van der Meer JW, Netea MG, Joosten LA (2014) Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc Natl Acad Sci U S A 111:E4478–E4484
Ouellette CA, Feddes JJR, Wegner II, Barber EM (1999) A portable environmental monitoring system to assess barn worker indoor air quality. J Agric Saf Health 5:383–394
Pallvi SRS, Mukhopadhyay CS, Verma R (2016) Expression of TLR4 mRNA in lungs of mice after single and multiple exposures to poultry barn air. Indian J Anim Sci 86:128–130
Pandit AA, Choudhary S, Verma R, Singh B, Sethi RS (2016) Imidacloprid induced histomorphological changes and expression of TLR-4 and TNFα in lung. Pestic Biochem Physiol 131:9–17
Parbhakar OP, Duke T, Townsend HG, Singh B (2005) Depletion of pulmonary intravascular macrophages partially inhibits lipopolysaccharide-induced lung inflammation in horses. Vet Res 36:557–569
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377
Pastva AM, Wright JR, Williams KL (2007) Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc 4:252–257
Poole JA, Kielian T, Wyatt TA, Gleason AM, Stone J, Palm K, West WW, Romberger DJ (2011) Organic dust augments nucleotide-binding oligomerization domain expression via an NF-{kappa}B pathway to negatively regulate inflammatory responses. Am J Physiol Lung Cell Mol Physiol 301:L296–L306
Poole JA, Wyatt TA, Romberger DJ, Staab E, Simet S, Reynolds SJ, Sisson JH, Kielian T (2015) MyD88 in lung resident cells governs airway inflammatory and pulmonary function responses to organic dust treatment. Respir Res 16:111
Pownall HJ, Hickson-Bick D, Massey JB (1991) Effects of hydrophobicity on turnover of plasma high density lipoproteins labeled with phosphatidylcholine ethers in the rat. J Lipid Res 32:793–800
Proskocil BJ, Bruun DA, Jacoby DB, van Rooijen N, Lein PJ, Fryer AD (2013) Macrophage TNF-alpha mediates parathion-induced airway hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol 304:L519–L529
Proskocil B, Lein P, Jacoby D, Fyer A (2014) Organophosphorus pesticides directly simulate macrophages to increase expression of growth factors and cytokines. Eur Respir J 44 (Suppl 58):4779
Qian J, Liu L, Chen L, Lu X, Zhu C (2015) Increased toll-like receptor 9 expression is associated with the severity of paraquat-induced lung injury in mice. Hum Exp Toxicol 34:430–438
Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, Eskenazi B (2015) Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environ Health Perspect 123:179–185
Rady MI (2009) Effects of exposure to diazinon on the lung and small intestine of guinea pig, histological and some histochemical changes. Braz Arch Biol Technol 52:317–326
Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, Fasano A, Vogel SN (2008) Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem 283:24314–24325
Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S (2005) Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202:47–59
Rekha B, Naik SN, Prasad R (2006) Pesticide residue in organic and conventional food-risk analysis. J Chem Health Saf 13:12–19
Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA (2002) Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 93:289–296
Romberger DJ, Heires AJ, Nordgren TM, Souder CP, West W, Liu XD, Poole JA, Toews ML, Wyatt TA (2015) Proteases in agricultural dust induce lung inflammation through PAR-1 and PAR-2 activation. Am J Physiol Lung Cell Mol Physiol 309:L388–L399
Romberger DJ, Heires AJ, Nordgren TM, Poole JA, Toews ML, West WW, Wyatt TA (2016) Beta2-adrenergic agonists attenuate organic dust-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 311:L101–L110
Rose MS, Lock EA, Smith LL, Wyatt I (1976) Paraquat accumulation: tissue and species specificity. Biochem Pharmacol 25:419–423
Rowsey PJ, Gordon CJ (1999) Tumor necrosis factor is involved in chlorpyrifos—induced changes in core temperature in the female rat. Toxicol Lett 109:51–59
Sano H, Sohma H, Muta T, Nomura S, Voelker DR, Kuroki Y (1999) Pulmonary surfactant protein A modulates the cellular response to smooth and rough lipopolysaccharides by interaction with CD14. J Immunol 163:387–395
Satomi Y, Tsuchiya W, Mihara K, Ota M, Kasahara Y, Akahori F (2004) Gene expression analysis of the lung following paraquat administration in rats using DNA microarray. J Toxicol Sci 29:91–100
Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman J, Douwes J, Dowling K, Enarson D, Green F, Heederik D, Husman K, Kennedy S, Kullman G, Lacasse Y, Lawson B, Malmberg P, May J, McCurdy S, Merchant J, Myers J, Nieuwenhuijsen M, Olenchock S, Saiki C, Schwartz D, Seiber J, Thorne P, Wagner G, White N, Xu X, Chan-Yeung M (1998) Respiratory health hazards in agriculture. Am J Respir Crit Care Med 158:S1–S76
Schneberger D, Aharonson-Raz K, Singh B (2012) Pulmonary intravascular macrophages and lung health: what are we missing? Am J Physiol Lung Cell Mol Physiol 302:L498–L503
Schneberger D, Caldwell S, Kanthan R, Singh B (2013) Expression of Toll-like receptor 9 in mouse and human lungs. J Anat 222:495–503
Schneberger D, Aulakh G, Channabasappa S, Singh B (2016) Toll-like receptor 9 partially regulates lung inflammation induced following exposure to chicken barn air. J Occup Med Toxicol 11:31
Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, von Metius E, Chanez P, Lambrecht BN, Hammad H (2015) Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 349:1106–1110
Senthilselvan A, Dosman JA, Kirychuk SP, Barber EM, Rhodes CS, Zhang Y, Hurst TS (1997a) Accelerated lung function decline in swine confinement workers. Chest 111:1733–1741
Senthilselvan A, Zhang Y, Dosman JA, Barber EM, Holfeld LE, Kirychuk SP, Cormier Y, Hurst TS, Rhodes CS (1997b) Positive human health effects of dust suppression with canola oil in swine barns. Am J Respir Crit Care Med 156:410–417
Senthilselvan A, Dosman JA, Chenard L, Burch LH, Predicala BZ, Sorowski R, Schneberger D, Hurst T, Kirychuk S, Gerdts V, Cormier Y, Rennie DC, Schwartz DA (2009) Toll-like receptor 4 variants reduce airway response in human subjects at high endotoxin levels in a swine facility. J Allergy Clin Immunol 123:1034–1040
Sethi RS, Schneberger D, Singh B (2013) Characterization of the lung epithelium of wild-type and TLR9(-/-) mice after single and repeated exposures to chicken barn air. Exp Toxicol Pathol 65:357–364
Shang Y, Jiang Y, Xu S, Wu Y, Wu Z, Yuan S, Yao S (2009) Reduction of pulmonary inflammatory response by erythropoietin in a rat model of endotoxaemia. Chin Med J 122:834–838
Sidiropoulou E, Sachana M, Hargreaves AJ, Woldehiwet Z (2011) Effects of diazinon-oxon and fipronil on IL-1β and TNF-α cytokine production in human THP-1 promyelocytic cells. Toxicol Lett 205:S151
Sigurdarson ST, O’Shaughnessy PT, Watt JA, Kline JN (2004) Experimental human exposure to inhaled grain dust and ammonia: towards a model of concentrated animal feeding operations. Am J Ind Med 46:345–348
Singh B, Pearce JW, Gamage LN, Janardhan K, Caldwell S (2004) Depletion of pulmonary intravascular macrophages inhibits acute lung inflammation. Am J Physiol Lung Cell Mol Physiol 286:L363–L372
Stamme C, Muller M, Hamann L, Gutsmann T, Seydel U (2002) Surfactant protein a inhibits lipopolysaccharide-induced immune cell activation by preventing the interaction of lipopolysaccharide with lipopolysaccharide-binding protein. Am J Respir Cell Mol Biol 27:353–360
Sugiyama K, Muroi M, Tanamoto K (2008) A novel TLR4-binding peptide that inhibits LPS-induced activation of NF-kappaB and in vivo toxicity. Eur J Pharmacol 594:152–156
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16:3–9
Thorn J (2001) The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflamm Res 50:254–261
Tomita M, Nohno T, Okuyama T, Nishimatsu S, Adachi J (2002) Paraquat-induced gene expression in rat lung tissues using a differential display reverse transcription-polymerase chain reaction. Arch Toxicol 76:530–537
Tomita M, Okuyama T, Katsuyama H, Miura Y, Nishimura Y, Hidaka K, Otsuki T, Ishikawa T (2007) Mouse model of paraquat-poisoned lungs and its gene expression profile. Toxicology 231:200–209
Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O’Brien PJ (2006) Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 38:733–744
Vaure C, Liu Y (2014) A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 5:316
Viegas S, Mateus V, Almeida-Silva M, Carolino E, Viegas C (2013) Occupational exposure to particulate matter and respiratory symptoms in Portuguese swine barn workers. J Toxic Environ Health A 76:1007–1014
Vogelzang PF, van der Gulden JW, Folgering H, Kolk JJ, Heederik D, Preller L, Tielen MJ, van Schayck CP (1998) Endotoxin exposure as a major determinant of lung function decline in pig farmers. Am J Respir Crit Care Med 157:15–18
Von Essen SG, Robbins RA, Thompson AB, Ertl RF, Linder J, Rennard S (1988) Mechanisms of neutrophil recruitment to the lung by grain dust exposure. Am Rev Respir Dis 138:921–927
Wang N, Gates KL, Trejo H, Favoreto S Jr, Schleimer RP, Sznajder JI, Beitel GJ, Sporn PH (2010) Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J 24:2178–2190
Watanabe W, Yoshida H, Hirose A, Akashi T, Takeshita T, Kuroki N, Shibata A, Hongo S, Hashiguchi S, Konno K, Kurokawa M (2013) Perinatal exposure to insecticide methamidophos suppressed production of proinflammatory cytokines responding to virus infection in lung tissues in mice. Biomed Res Int 2013:151807
Weaver TE, Conkright JJ (2001) Function of surfactant proteins B and C. Annu Rev Physiol 63:555–578
West AP, Shadel GS, Ghosh S (2011) Mitochondria in innate immune responses. Nat Rev Immunol 11:389–402
Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK (2010) The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal 12:1147–1154
Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68
Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ (2007) Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase Cepsilon in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 293:L1163–L1170
Wyatt TA, Slager RE, Heires AJ, Devasure JM, Vonessen SG, Poole JA, Romberger DJ (2010) Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am J Respir Cell Mol Biol 42:706–715
Yavuz Y, Yurumez Y, Ciftci IH, Sahin O, Saglam H, Buyukokuroglu M (2008) Effect of diphenhydramine on myocardial injury caused by organophosphate poisoning. Clin Toxicol (Phila) 46:67–70
Zahringer U, Lindner B, Inamura S, Heine H, Alexander C (2008) TLR2—promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205–224
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107
Zhang Y, Cardell LO, Adner M (2007) IL-1beta induces murine airway 5-HT2A receptor hyperresponsiveness via a non-transcriptional MAPK-dependent mechanism. Respir Res 8:29
Zuskin E, Mustajbegovic J, Schachter EN, Kern J, Deckovic-Vukres V, Trosic I, Chiarelli A (2008) Respiratory function in pesticide workers. J Occup Environ Med 50:1299–1305
Acknowledgements
Dr. Baljit Singh’s research program, especially the work providing the data included in this review article, is supported by grants from the Natural Sciences and Engineering Research Council of Canada and Saskatchewan Lung Association. Dr. Sethi’s work is funded by Guru Angad Dev Veterinary and Animal Sciences University, India and the Indian Council of Medical Research (ICMR), New Delhi. The research work of Dr. Charavaryamath and Dr. Schneberger carried out during their PhD training program in Dr. Singh’s laboratory was supported by scholarships from the Canadian Institutes of Health Research and University of Saskatchewan. Current work in Dr. Charavaryamath’s laboratory is supported by funding from Iowa State University, Ames, Iowa, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sethi, R.S., Schneberger, D., Charavaryamath, C. et al. Pulmonary innate inflammatory responses to agricultural occupational contaminants. Cell Tissue Res 367, 627–642 (2017). https://doi.org/10.1007/s00441-017-2573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2573-4