Abstract
Agricultural workplaces consist of multiple airborne contaminants and inhalation exposures induce respiratory effects in workers. Endotoxin (LPS) and glyphosate are two common airborne contaminants in agricultural environments. We have previously shown that exposure to a combination of LPS and glyphosate synergistically modulates immune reactions as compared to individual exposures. The immunopathogenesis of acute and chronic exposure to complex agricultural exposures including LPS and glyphosate is not known; therefore, we further investigated the lung cellular inflammatory differences in mice exposed to either a combination, or individual, LPS, and glyphosate for 1 day, 5 days, and 10 days. Exposure to a combination of LPS and glyphosate resulted in greater cellular inflammatory effects in lungs as compared to individual exposures to LPS or glyphosate. Repeated exposures to the combination of LPS and glyphosate resulted in robust infiltration of inflammatory cells in the perivascular, peribronchiolar, and alveolar regions, and increases of alveolar septal thicknesses and perivascular spaces in the lungs with intense intercellular adhesion molecule (ICAM) − 1 staining in the perivascular region, but minimal staining in the pulmonary artery endothelium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural workplace airborne exposures are a known risk for respiratory effects in workers. Long-term agricultural exposures have been associated with chronic respiratory symptoms and decline in lung function in workers (Senthilselvan et al. 1997, 2010; Kirychuk et al. 2003). Leukocyte accumulation has been shown in the bronchial epithelium and submucosal regions of lung biopsies (Schwartz et al. 1992) and in bronchoalveolar lavage (BAL) and nasal lavage fluid of agricultural workers (Zejda and Dosman 1993; Larsson et al. 1994, 1999; Cormier et al. 1997). The immunopathogenesis in relation to airborne agricultural exposures is not understood. Agricultural workplace airborne exposures are complex and exposure to multiple contaminants is common. Two common agricultural workplace airborne contaminants are lipopolysaccharides (LPS) and glyphosate. Lipopolysaccharides mainly derive from gram negative bacteria are ubiquitous in agricultural workplaces (Muzio et al. 2000). Glyphosate is a common active ingredient in herbicides (Duke and Powles 2008) and is the most widely used herbicide ingredient worldwide. Exposure to glyphosate has been associated with wheeze in agricultural workers (Hoppin et al. 2017).
Acute (Janardhan et al. 2006; Tschernig et al. 2008) and chronic exposure (Vernooy et al. 2002) to LPS has been shown to induce recruitment of inflammatory cells in the perivascular, peribronchiolar, and alveolar regions of lungs. LPS exposure has been shown to increase ICAM-1 expression on pulmonary endothelial cells and alveolar epithelial cells (Beck-Schimmer et al. 1997, 2002). Following mice treatment with irradiation ICAM-1 expression has been shown to increase in the pulmonary microvascular endothelium but not in the endothelium of larger vessels, and blocking of ICAM-1 expression by antibody treatment attenuated the migration of inflammatory cells in lungs (Hallahan and Virudachalam 1997), whereas glyphosate exposure in mice for seven days has been shown to induce infiltration of leukocytes and release of type 2 cytokines (IL-5 and IL-13) in lungs (Kumar et al. 2014). In our work, a synergistic inflammatory response was induced when mice were exposed to a combination of LPS and glyphosate. Exposure to a combination of LPS and glyphosate for 5 days induced synergistic release of proinflammatory cytokines TNF-α, IL-6, and KC as compared to individual exposures to LPS and glyphosate and the inflammatory response modulated after 10 days of exposure.
The pulmonary pathogenesis from exposure to LPS and glyphosate is not known. We hypothesized that exposure to a combination of LPS and glyphosate would increase recruitment of inflammatory cells in lung tissue and the effects on lung tissue would be enhanced with repeated exposures as compared to that of singular LPS or glyphosate exposures. Further, given that ICAM-1 expression has been shown to increase with agricultural dust exposures (Romberger et al. 2002), we evaluated differences in ICAM-1 expression between the exposure types. Given that chronic exposures in agricultural workers results in differential respiratory effects depending on type of exposure, we further hypothesized that with repeated exposures the cellular inflammation would reveal persistent tissue effects that differed by exposure type. We evaluated cellular inflammatory effects in lung tissue and ICAM-1 expression after singular and repeated intranasal exposure to a combination, and individual, LPS and glyphosate.
Materials and methods
Mice exposures
The study design has been previously described (Pandher et al. 2021). In short, the study involved four treatment groups: control, LPS alone, glyphosate alone, and combined LPS and glyphosate. Mice were intranasally given saline (control), LPS (0.5 µg), glyphosate (1 µg), or a combination of LPS (0.5 µg) and glyphosate (1 µg). The design involved three treatment lengths: once, or once daily for 5 days, or once daily for 10 days. N = 5 mice per group. At the end of exposure periods, mice were sacrificed by CO2 inhalation and lavaged before the collection of lung tissue. All experimental procedures were approved by the Animal Research Ethics Board of the University of Saskatchewan (AUP 20,160,106).
Lung collection and processing
The lungs of the mice were ligated with thread at the right bronchus, and the left lung was inflated with 0.3 ml of 4% paraformaldehyde (PFA) in situ through a cannula inserted into the trachea. The inflated left lung was removed and immersed in 4% PFA for 16 h at 4 °C. The fixed lung tissues were processed through a series of alcohols in an Intelsint RVG/1 Histology Vacuum tissue processor (Intelsint; Turin, Italy) and followed by embedding in paraffin blocks using a Tissue Tek II tissue embedding station (Sakura Finetek; Nagano, Japan). Lung sections of 5-µm thickness were cut from paraffin blocks on an American Optical Rotary Microtome (Model 820, American Optical, Buffalo, NY USA) and placed onto pre-charged slides (ThermoFisher Scientific, Waltham, MA USA).
Histopathology and scoring for cellular inflammation
Lung sections from all treatment groups were stained with hematoxylin and eosin stain and mounted using Surgipath MM24 Mounting Media (Leica Biosystems, Richmond, IL USA) before analysis. Photomicrographs were taken using a bright field microscope equipped with Infinity 5–5 Microscope camera (Teledyne Lumenera, ON Canada). The primary type of inflammatory cells (polymorphonuclear cells and/or monomorphonuculear cells) infiltrating the perivascular, peribronchiolar, and alveolar septal regions were scored for each of the treatment groups and treatment lengths, as well as levels of alveolar thickness and perivascular space differences. Scoring was performed under 40 × objective lens on five fields per section from each treated mouse (N = five mice per treatment group; five fields per section of each mouse). Lung sections were scored in an independent blinded manner by four investigators using the criteria in Table 1 (Singh et al. 2005). Each investigator provided a lung histology score for each of the parameters from Table 1 for each of the treatment groups. An average lung histology score was calculated for each parameter and each group.
Immunohistochemistry
Lung sections on slides were stained with an ICAM-1 antibody using immunohistochemistry as described previously (Pandher et al. 2021). Briefly, lung sections were deparaffinized in xylene and rehydrated in descending concentrations of ethanol. The tissue sections were incubated with hydrogen peroxide (0.5% H2O2 in methanol) for 20 min to quench the endogenous peroxidase activity. This was followed by incubation with pepsin (2 mg/ml of 0.01 N HCL) for 30 min to unmask the antigen and with 1% bovine serum albumin for 30 min to block non-specific binding. Lung sections were incubated overnight at 4 °C with a primary antibody against ICAM-1 (dilution: 1:100; clone: EPR16608, Rockland Immunochemicals, Gilbertsville, PA USA). Following overnight incubation, the secondary anti-rabbit antibody (dilution: 1:200; ThermoFisher Scientific, Waltham, MA USA) conjugated to horseradish peroxidase was added on sections for one hour at room temperature. The colored reaction was developed using a commercial kit (Vector laboratories, Burlington, ON Canada). Counterstaining of sections was performed with methyl green stain (Vector Laboratories, Burlington, ON Canada). Some sections were stained with positive control (von Willebrand factor antibody; dilution 1:200; ThermoFisher Scientific, Waltham, MA USA) and omitted with primary antibody or a secondary antibody to assess non-specific binding. The optimum primary antibody concentration was determined by varying concentration staining.
The images were captured with Infinity 5–5 Microscope camera (Teledyne Lumenera, ON Canada) mounted on a bright field microscope. Scoring was performed on randomly selected five fields per section of each treated mouse under 40 × (N = five mice per treatment group; five fields per section of each mouse in a group). An experienced investigator blinded to the treatment groups semi-quantified the cell specific expression of ICAM-1 in the perivascular region, alveolar region, and pulmonary arteries using scoring criteria outlined in Table 2 (Singh et al. 2005).
Statistical analysis
Data analysis and graph preparation was performed using GraphPad Prism software (Graph-Pad Software, San Diego, CA USA). Statistical differences were determined using one-way ANOVA followed by Tukey’s multiple comparisons tests. A p-value of < 0.05 was considered significant.
For graphing of data, “a” indicates a significant difference compared with the control group, “b” indicates a significant difference compared with the LPS group, “c” indicates a significant difference compared with the glyphosate group, “1” indicates a significant difference compared with 1-day exposure group, and “2” indicates a significant difference compared with 5-day exposure group.
Results
Histopathological examination
Cellular infiltration in the perivascular, peribronchiolar, and alveolar regions, alveolar septal thickness, and perivascular space increases were scored to differentiate cellular inflammation in the lungs, and to semi-quantify differences in response between exposures to the combination of LPS and glyphosate and individual LPS and glyphosate (Table 3). Exposure to the combination of LPS and glyphosate resulted in significantly greater pulmonary inflammatory scores in all the regions as compared to the LPS alone and glyphosate alone treatments (Table 3). Further, average scores increased with increasing length of exposure in the combined LPS and glyphosate exposure (Fig. 1). Exposure to the combination of LPS and glyphosate for 1-day resulted in average scores of 1 (Fig. 1) with one or two concentric rows of perivascular and peribronchial inflammation (Fig. 2j), few loosely arranged cells in the alveolar septa (Figs. 2j and 4g), sporadic areas of alveolar wall thickening (Fig. 4g), and small increases in the pervascular spaces (Fig. 2j). The LPS alone 1-day exposures also resulted in similar increases in inflammatory scores in the perivascular, peribroncial, and aleolar septal areas as the combined LPS and glyphosate exposures, but little change in the alveolar thickness and perivascular space (Table 3; Fig. 1). LPS 1-day exposures resulted in one or two concentric rows of perivascular and peribronchial inflammation, few loosely arranged cells in the alveolar septa; however, alveolar walls were not thickened and there were no increases in the pervascular spaces (Table 3; Figs. 2d and 4d). The 1-day exposures to glyphosate alone revealed little inflammation (Table 3; Figs. 1 and 2g). The control group average scores remained at 0 with little inflammation for all exposure periods (Table 3; Figs. 2a–c and 4a–c).
Scoring of inflammation parameters in stained lung sections of mice after exposure to control, LPS, glyphosate (Gly), or combined LPS and glyphosate (LPS + Gly) for 1 day, 5 days, and 10 days. A lung histology score was computed using the sum of the 4 individual scores for each of the measured parameters generated by four investigators (N = 5 mice per treatment group; five fields per section of each mouse in a group). The average lung histology score of each group was used for statistical analysis. Data presented as mean ± SD. Significance (p < 0.05) is denoted as such: “a” indicates a significant difference compared with the control group; “b” indicates a significant difference compared with the LPS group; “c” indicates a significant difference compared with the glyphosate group; “1” indicates a significant difference compared with 1-day exposure group
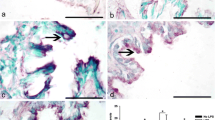
Hematoxylin and eosin-stained lung sections from mice after exposure for 1 day, 5 days, and 10 days to control (a–c), LPS (d–f), glyphosate (Gly; g–i), or combined LPS and glyphosate (LPS + Gly; j–l). Group representative images showing perivascular infiltration (square), peribronchiolar infiltration (circle), alveolar infiltration (diamond), perivascular space increase (double arrow), blood vessels congestion (triangle), alveolar septa thickness increase (bent up arrow), and sloughing of bronchial epithelial surface (lightning bolt). Magnification: × 200 (a–l). Scale bar: 200 µm (a–l). PA pulmonary artery, B bronchus
After 5 days of exposure, average scores for the combined LPS and glyphosate exposure group increased across all measurements from an average of 1 to an average of 2.5 (Table 3; Fig. 1) with three or more concentric rows of perivascular (Figs. 2k and 3a, d) and peribronchial inflammation (Figs. 2k and 3b, e), many cells in the peripheral parts of the alveolar septa (Figs. 2k; 3c, f; and 4h) frequent areas of alveolar wall thickening (Figs. 3c, f, and 4h), and medium increases in the pervascular spaces (Figs. 2k and 3a, d). These changes were greater than those of the LPS alone exposure in which the perivascular and peribronchial inflammation remained similar to that of the 1-day exposure (Table 3; Fig. 1), with slight increases in alveolar septal infiltration and thickness (Figs. 2e and 4e), and a small increase in perivascular spaces (Fig. 2e). Inflammation in the glyphosate alone treatment group had very little increase in inflammation across all measurements (Table 3; Figs. 1 and 2h) as compared to the 1-day exposure.
The perivascular (a, d), peribronchiolar (b, e), and alveolar (c, f) regions of hematoxylin and eosin-stained lung sections of mice after 5-day exposure to combined LPS and glyphosate (LPS + Gly). Group representative images showing infiltration of polymorphonuclear (arrow) and monomorphonuclear (arrowhead) leukocytes, perivascular space increase (double arrow), alveolar septa thickness increase (bent up arrow), and sloughing of bronchial epithelial surfaces (lightning bolt). Magnification: × 400 (a–c); × 1000 (d–f). Scale bar: 50 μm (a–f); PA pulmonary artery, B bronchus
High magnification images of the alveolar region of hematoxylin and eosin-stained lung sections of mice from control (a–c), LPS (d–f) or combined LPS and glyphosate (LPS + Gly; g–i) exposure groups for 1 day, 5 days, and 10 days. Group representative images showing infiltration of polymorphonuclear (arrow) and monomorphonuclear (arrowhead) leukocytes and increase of alveolar septal thickness (bent up arrow). Magnification: × 1000 (a–i). Scale bar: 50 μm (a–i)
After 10 days of exposure to the combination of LPS and glyphosate, there was increasing inflammation across all measurements with an average score of 3 (Table 3; Fig. 1) indicating continuous perivascular (Fig. 2l) and peribronchial cell accumulation (Fig. 2l), numerous cells in the alveolar septa (Figs. 2l and 4i), thickened walls throughout the alveolar region (Figs. 2l and 4i), and large increases in the perivascular spaces (Fig. 2l). In addition, exposure to the combination of LPS and glyphosate for 1 day, 5 days, and 10 days resulted in blood vessel congestion (Fig. 2j–l) and sloughing of bronchial epithelium (Fig. 3b, e). Intraluminal leukocytes were not detected in the pulmonary arteries of the treated lung sections (Fig. 5a–b). The scores for the combined LPS and glyphosate were higher than the scores for the inflammation in the LPS alone and glyphosate alone treatment groups (Table 3; Fig. 1), in which there were small increases in inflammation between the 5-day and 10-day exposures (Figs. 2f, i; 4f); however, the inflammation at 10 days continued to be less than that of the combined LPS and glyphosate exposure.
ICAM-1 expression
The cell specific expression of ICAM-1 in the perivascular region, alveolar region, and pulmonary arteries was scored to assess relationships between ICAM-1 expression and cellular inflammation. The ICAM-1 protein expressions revealed that a 5-day exposure to the combination of LPS and glyphosate resulted in higher ICAM-1 expression scores in the perivascular region (Fig. 6a) revealing intense staining as compared to the controls (Fig. 7). ICAM-1 scoring in the alveolar region was slightly higher in the exposure to LPS and glyphosate as compared to the other exposures (Fig. 6b). ICAM-1 scoring in the pulmonary arteries was similar for all groups and across all treatments with none or little staining present (Fig. 6c). Figure 7 illustrates the intense ICAM-1 staining in the perivascular regions of the lungs in the LPS and glyphosate exposed mice (1 day (Figs. 7d, g), 5 days (Figs. 7e, h), and 10 days (Figs. 7f, i)) as compared to the control group (Figs. 7 a–c). ICAM-1 staining was strong in the perivascular (Figs. 7e, h) and alveolar (Figs. 7d, f, g, i) areas in the mice exposed to the combination of LPS and glyphosate with minimal staining in the pulmonary arteries (Figs. 7e, h). We have previously shown in these same mice that exposure to the combination of LPS and glyphosate for 5 days resulted in significantly higher ICAM-1 mRNA expression as compared to 5-day individual LPS, glyphosate, and control exposures and the expression reduced after 10 days of exposure (Pandher et al, 2021).
Scoring of ICAM-1 expression in perivascular region (a), alveolar region (b), and pulmonary arteries (c) of mice lung sections from control, LPS, glyphosate (Gly), or combined LPS and glyphosate (LPS + Gly) exposure groups for 1 day, 5 days, and 10 days. Data presented as mean ± SD (N = 5 mice per group; 5 fields per section of each mouse in a group). “a” indicates a significant difference (p < 0.05) compared with the control group
Immunohistochemical expression of ICAM-1 in mice lung sections after 1-day, 5-day, and 10-day exposure to control (a–c) or combined LPS and glyphosate (LPS + Gly; d–i). Group representative images showing ICAM-1 expression in the perivascular region (square) and alveolar region (diamond). Magnification: × 400 (a–f); × 1000 (g–i). Scale bar: 50 μm (a–i). PA pulmonary artery, B bronchus
Discussion
Exposure to the combination of LPS and glyphosate resulted in greater lung inflammation as compared to individual exposures to LPS or glyphosate. Repeated exposure to the combination of LPS and glyphosate resulted in robust infiltration of inflammatory cells in the perivascular, peribronchiolar, and alveolar regions, and increases of alveolar septal thicknesses and perivascular spaces in the lungs. Intraluminal leukocytes were not detected in the pulmonary arteries of the treated lung sections. Exposures to the combination of LPS and glyphosate showed intense ICAM-1 staining in the perivascular and alveolar regions of the lungs with minimal staining in the pulmonary arteries and significantly higher ICAM-1 mRNA in the lung tissue as compared to exposure to individual LPS or glyphosate. After 10 days of repeated exposure to the combination of LPS and glyphosate, ICAM-1 expression waned as compared to the 5-day exposure to the combination of LPS and glyphosate.
Exposure to the combination of LPS and glyphosate induced greater recruitment of inflammatory cells in lungs as compared to the individual LPS or glyphosate exposures. Furthermore, repeated exposure to a combination of LPS and glyphosate resulted in robust infiltration of inflammatory cells in the perivascular, peribronchiolar, and alveolar regions, and increases of alveolar septal thicknesses and perivascular spaces in the lungs. Migration of leukocytes is mediated by the release of proinflammatory cytokines from stimulated cells. Our previous work showed a synergistic release of TNF-α, IL-6, and KC cytokines with repeated exposure to the combination of LPS and glyphosate as compared to individual LPS or glyphosate exposures (Pandher et al. 2021). Exposure to the same dose of glyphosate as used in our work, combined with the allergen, ovalbumin, did not result in greater lung inflammatory response than the ovalbumin or glyphosate alone treatments (Kumar et al. 2014). This suggests that when there is more than a single inflammatory agent in the pulmonary exposure, the combined exposures may potentiate the inflammatory response. This agrees with previous work showing that individual LPS exposure, at agricultural relevant levels, results in only a fraction of the inflammatory lung response as compared to exposures to the complex agricultural environment (Charavaryamath et al. 2008; Sundblad et al. 2009).
Repeated exposure to the combination of LPS and glyphosate, for both 5 days and 10 days, induced robust recruitment of inflammatory cells in the perivascular region as compared to the alveolar and peribronchiolar regions. Previous work has shown that multiple oral exposures to the pesticides ethion or imidacloprid, followed by single intranasal LPS exposure, resulted in perivascular, alveolar, and peribronchiolar infiltration of leukocytes (Pandit et al. 2016; Verma et al. 2019). Currently, the relationship between peribronchiolar and perivascular recruitment of inflammatory cells remains unknown; however, our results show a concomitant increase in the perivascular space around pulmonary arteries with both 5 days and 10 days of exposure to the combination of LPS and glyphosate, suggesting an active role for the perivascular region in the recruitment of inflammatory cells into the lungs in intranasal LPS and glyphosate exposures.
The characteristic feature of inflammation is the recruitment of inflammatory cells into tissue (Mizgerd 2002). Inflammatory cells are blood-borne and recruited to the site of stimulus through a series of cellular and molecular events (Kolaczkowska and Kubes 2013). Recruitment of inflammatory cells is facilitated by a variety of adhesion molecules (Schmidt et al. 2016). One of the best-known adhesion molecules for inflammation is the intercellular adhesion molecule-1 (Long 2011). Intercellular adhesion molecule-1 (ICAM-1) is a cell surface glycoprotein, constitutively expressed on endothelial cells, and its expression increases during inflammation. ICAM-1 mediates firm binding of inflammatory cells with the endothelial cells before their transmigration (Long 2011). Studies have shown upregulation of ICAM-1 on the pulmonary capillary endothelium and its important contribution for the recruitment of inflammatory cells into lungs (Burns et al. 1994; Hallahan and Virudachalam 1997; Beck-Schimmer et al. 1997; Romberger et al. 2002). In lungs, the cellular adhesion and transmigration process primarily occur in the capillaries (Kuebler et al. 1994; Doerschuk 2001). Similar to other studies (Burns et al. 1994; Hallahan and Virudachalam 1997), in our study, vascular ICAM-1 expression was observed in lungs from control mice. Further, we have previously shown that ICAM-1 mRNA was significantly increased in the lungs after repeated exposure to the combination of LPS and glyphosate (Pandher et al 2021). The results herein further show that ICAM-1 expression intensely increased, predominantly in the perivascular region of the lungs, with repeated exposure to the combination of LPS and glyphosate. Our data show that there commonality between the expression of ICAM-1 and the extent of perivascular recruitment of inflammatory cells in lungs. Typically, pulmonary migration of leukocytes occurs in capillaries and is mediated by cytokines and adhesion molecules located on endothelial cells (Doerschuk 2001). Therefore, accumulation of inflammatory cells around thick-walled pulmonary arteries (perivascular region) as observed in our study is intriguing. It is possible that in response to adhesion molecules and cytokines, inflammatory cells migrate across the thick-walled pulmonary arteries (Wang et al. 2011). However, this possibility seems very unlikely in our study as we did not observe transmigrating inflammatory cells in the pulmonary arteries of inflamed lungs, and we did not observe an increase of ICAM-1 expression in the endothelium of the pulmonary arteries in inflamed lung sections. The perivascular region also possesses capillaries (Guntheroth et al. 1982; Pabst and Tschernig 2002) which is where we saw a predominant expression of ICAM-1. It is possible capillaries in the perivascular region of the lungs may be the important site for inflammatory cell recruitment in these complex exposures. In this work, we did not elucidate the function of the perivascular leukocytes but it is an important aspect to address in future studies.
Our results further showed that with repeated exposures to the combination of LPS and glyphosate leukocyte infiltration persisted in the lungs. However, we have previously shown that ICAM-1 mRNA expression waned and there was a dampening of TNF-α, IL-6, and KC cytokines between 5 and 10 days of exposure to the combination of LPS and glyphosate (Pandher et al. 2021). There can be several reasons for these findings. Exposure to the combination of LPS and glyphosate may impair the functional ability of macrophages to clear/phagocytose the inflammatory cells. Murine macrophages treated with LPS have shown a reduction in their ability to clear inflammatory cells through an imbalance of pro- and anti-inflammatory cytokines (Feng et al. 2011). Secondly, the combination of LPS and glyphosate may have stronger direct chemotactic activity for leukocytes. Grain dust extract has been shown to have direct chemotactic activity as an indirect mechanism of neutrophil recruitment (Von Essen et al. 1988; Von Essen 1997). Thirdly, exposure to multiple inflammatory agents such as LPS and glyphosate may induce ongoing low-grade chronic inflammation. Our previously published work showed that after 10 days of exposure to the combination of LPS and glyphosate, the inflammatory mediators TNF-α, KC, and IL-6 were reduced but not completely diminished (Pandher et al. 2021). These low levels of inflammatory mediators may prevent full recovery with the repeated exposures and contribute to the progression of effects in lung tissue. This agrees with epidemiologic evidence of chronic respiratory symptoms in agricultural workers after long-term agricultural exposures (Senthilselvan et al. 1997, 2010; Kirychuk et al. 2003).
There are several strengths and limitations to this study. This study examined pulmonary inflammation after early (1 day) and repeated exposures (5 and 10 days) to common agricultural agents which is important in furthering our understanding the pathogenesis of common agricultural exposures. Our treatment doses were similar to levels measured in airborne agricultural environments and to that used by others (Kumar et al. 2014), and treatment dosing mimicked agricultural worker exposure patterns. The inflammatory scoring was blinded and carried out by four technicians well versed in histology, and agreement was very high between scorers. However, we did not undertake stereological analysis of the septal thickness; rather, we undertook qualitative visual analysis. Stereological analysis of the septal thickness would provide quantitative evidence of the differences between the groups. Our analyses were limited to immunohistochemistry as lung tissues were fixed with paraformaldehyde and sectioned from paraffin blocks, and therefore, we cannot perform electron microscopy which would more clearly visualize the impact on capillaries. Future research to investigate the inflammatory processes from complex exposures is important to furthering our understanding of the pathogenesis of common agricultural exposures.
Taken together the results reveal that exposure to the combination of inflammatory agents LPS and glyphosate results in greater cellular lung inflammation as compared to individual exposures to LPS or glyphosate. Repeated exposure to the combination of LPS and glyphosate resulted in robust infiltration of inflammatory cells in the perivascular, peribronchiolar, and alveolar regions; increases of alveolar septal thicknesses and perivascular spaces in the lungs; and intense ICAM-1 expression in the perivascular region; however, intraluminal leukocytes were not detected in the pulmonary arteries. Capillaries in the perivascular region may be important to the recruitment of inflammatory cells in complex agricultural exposures. Complementary chemotactic activity induced by complex pulmonary inflammatory exposures may be important to understanding effects. These findings are important to further our understanding of the pathogenesis from exposure to the complex inflammatory contaminants in the agricultural work environment.
References
Beck-Schimmer B, Madjdpour C, Kneller S, Ziegler U, Pasch T, Wuthrich RP, Ward PA, Schimmer RC (2002) Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. Eur Respir J 19:1142–1150
Beck-Schimmer B, Schimmer RC, Warner RL, Schmal H, Nordblom G, Flory CM, Lesch ME, Friedl HP, Schrier DJ, Ward PA (1997) Expression of lung vascular and airway ICAM-1 after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol 17:344–352
Burns AR, Takei F, Doerschuk CM (1994) Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol 153:3189–31898
Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B (2008) Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 34:19–35
Cormier Y, Duchaine C, Israël-Assayag E, Bédard G, Laviolette M, Dosman J (1997) Effects of repeated swine building exposures on normal naive subjects. Eur Respir J 10:1516–1522
Doerschuk CM (2001) Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation 8:71–88
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Management Science 64(4) 319-325. https://doi.org/10.1002/ps.1518
Feng X, Deng T, Zhang Y, Su S, Wei C, Han D (2011) Lipopolysaccharide inhibits macrophage phagocytosis of apoptotic neutrophils by regulating the production of tumour necrosis factor α and growth arrest-specific gene 6. Immunology 132:287–295
Guntheroth WG, Luchtel DL, Kawabori I (1982) Pulmonary microcirculation: tubules rather than sheet and post. J Appl Physiol Respir Environ Exerc Physiol 53:510–515
Hallahan DE, Virudachalam S (1997) Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A 94:6432–6437
Hoppin JA, Umbach DM, Long S, London SJ, Henneberger PK, Blair A, Alavanja M, Beane Freeman LE, Sandler DP (2017) Pesticides are Associated with Allergic and Non-Allergic Wheeze among Male Farmers. Environmental Health Perspectives 125(4) 535-543. https://doi.org/10.1289/EHP315
Janardhan KS, McIsaac M, Fowlie J, Shrivastav A, Caldwell S, Sharma RK, Singh B (2006) Toll like receptor-4 expression in lipopolysaccharide induced lung inflammation. Histol Histopathol 21:687–696
Kirychuk SP, Senthilselvan A, Dosman JA, Juorio V, Feddes JJR, Willson P, Classen H, Reynolds SJ, Guenter W, Hurst TS (2003) Respiratory symptoms and lung function in poultry confinement workers in Western Canada. Can Respir J 10:375–380
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175
Kuebler WM, Kuhnle GE, Groh J, Goetz AE (1994) Leukocyte kinetics in pulmonary microcirculation: intravital fluorescence microscopic study. J Appl Physiol 76:65–71
Kumar S, Khodoun M, Kettleson EM, McKnight C, Reponen T, Grinshpun SA, Adhikari A (2014) Glyphosate-rich air samples induce IL-33, TSLP and generate IL-13 dependent airway inflammation. Toxicology 325:42–51
Larsson BM, Larsson K, Malmberg P, Martensson L, Palmberg L (1999) Airway responses in naive subjects to exposure in poultry houses: comparison between cage rearing system and alternative rearing system for laying hens. Am J Ind Med 35:142–149
Larsson KA, Eklund AG, Hansson LO, Isaksson BM, Malmberg PO (1994) Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med 150:973–977
Long EO (2011) ICAM-1: Getting a grip on leukocyte adhesion. J Immunol 186:5021–5023
Mizgerd JP (2002) Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol 14:123–132
Muzio M, Polentarutti N, Bosisio D, Manoj Kumar PP, Mantovani A (2000) Toll-like receptor family and signalling pathway. Biochem Soc Trans 28:563–566
Pabst R, Tschernig T (2002) Perivascular capillaries in the lung: an important but neglected vascular bed in immune reactions? J Allergy Clin Immunol 110:209–214
Pandher U, Kirychuk S, Schneberger D, Thompson B, Aulakh G, Sethi RS, Singh B (2021) Pulmonary inflammatory response from co-exposure to LPS and glyphosate. Environ Toxicol Pharmacol 86:103651
Pandit AA, Choudhary S, Ramneek SB, Sethi RS (2016) Imidacloprid induced histomorphological changes and expression of TLR-4 and TNFα in lung. Pestic Biochem Physiol 131:9–17
Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA (2002) Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 93(1):289–296
Schmidt EP, Kuebler WM, Lee WL, Downey GP (2016) Adhesion molecules: master controllers of the circulatory system. Comprehensive physiology. John Wiley & Sons Inc, Hoboken, NJ, USA, pp 945–973
Schwartz DA, Landas SK, Lassise DL, Burmeister LF, Hunninghake GW, Merchant JA (1992) Airway injury in swine confinement workers. Ann Intern Med 116:630–635
Senthilselvan A, Chénard L, Grover V, Kirychuk SPSP, Hagel L, Ulmer K, Hurst TSTS, Dosman JAJA (2010) Excess longitudinal decline in lung function in grain farmers. J Agromedicine 15:157–165
Senthilselvan A, Dosman JA, Kirychuk SP, Barber EM, Rhodes CS, Zhang Y, Hurst TS (1997) Accelerated lung function decline in swine confinement workers. Chest 111:1733–1741
Singh B, Shinagawa K, Taube C, Gelfand EW, Pabst R, Kazuhiko, (2005) Strain-specific differences in perivascular inflammation in lungs in two murine models of allergic airway inflammation. Clin Exp Immunol 141:223–229
Sundblad BM, Von Scheele I, Palmberg L, Olsson M, Larsson K (2009) Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J 34:80–88
Tschernig T, Janardhan KS, Pabst R, Singh B (2008) Lipopolysaccharide induced inflammation in the perivascular space in lungs. J Occup Med Toxicol 3:1–5
Verma G, Mukhopadhyay CS, Verma R, Singh B, Sethi RS (2019) Long-term exposures to ethion and endotoxin cause lung inflammation and induce genotoxicity in mice. Cell Tissue Res 375:493–505
Vernooy JHJ, Dentener MA, Van Suylen RJ, Buurman WA, Wouters EFM (2002) Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol 26:152–159
Von Essen S (1997) The role of endotoxin in grain dust exposure and airway obstruction. Curr Opin Pulm Med 3:198–202
Von Essen SG, Robbins RA, Thompson AB, Ertl RF, Linder J, Rennard S (1988) Mechanisms of neutrophil recruitment to the lung by grain dust exposure. Am Rev Respir Dis 138:921–927
Wang PM, Kachel DL, Cesta MF, Martin WJ (2011) Direct leukocyte migration across pulmonary arterioles and venules into the perivascular interstitium of murine lungs during bleomycin injury and repair. Am J Pathol 178:2560–2572
Zejda JE, Dosman JA (1993) Respiratory disorders in agriculture. Tuber Lung Dis 74:74–86
Acknowledgements
The authors thank Dr. Aditya Manek, Heather Neufeld, and the Vivarium staff from the University of Saskatchewan for their assistance during the project. The authors pay tribute to the laboratory animals for their tremendous contribution in this project.
Funding
This study was funded by a Discovery Grant from Natural Sciences and Engineering Research Council (NSERC). Mr. Upkardeep Pandher received support from Integrated Graduate Training Program in Infectious Diseases, Food Safety and Public Policy (ITraP) funded by NSERC/CREATE, and from the University of Saskatchewan’s University Graduate Scholarship (UGS), Respiratory Research Centre Graduate Student Award (RRCGSA) from Respiratory Research Centre, Founding Chairs Fellowship from CCHSA, and Health Sciences Graduate Scholarship (HSGS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study design was approved by the Animal Research Ethics Board of the University of Saskatchewan (AUP 20160106), and animal research was conducted according to Canadian Council on Animal Care guidelines for the care and use of animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pandher, U., Kirychuk, S., Schneberger, D. et al. Lung inflammation from repeated exposure to LPS and glyphosate. Cell Tissue Res 386, 637–648 (2021). https://doi.org/10.1007/s00441-021-03531-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03531-7