Abstract
This mini-review examines the role of the pro-inflammatory cytokine interleukin (IL)-1β in the interaction of Pseudomonas aeruginosa and the host immune system during lung infection. Different studies show that the reduction of the inflammatory response, especially a decrease in IL-1β, leads to a better outcome in acute lung infection with this bacterium. This includes a higher survival rate, reduced damage to the lung tissue and, in particular, a better clearance of the airways and the tissue of the lungs from P. aeruginosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Origin of Pseudomonas aeruginosa
Pseudomonas aeruginosa is an opportunistic human pathogen and a major cause of nosocomial infections (Gaynes and Edwards 2005). The natural habitat of this Gram-negative bacterium is moist soil and water but it is also found as a human commensal on the skin and in the intestine. The bacterium only rarely causes disease in healthy humans but is a great threat for patients with a compromised immune system, especially for those with cystic fibrosis (Burns et al. 1998). It is readily found in chronic wounds and the lungs of cystic fibrosis patients and as a contaminant on medical devices. Although humans are not the preferred habitat of P. aeruginosa, the pathogen is nonetheless equipped with a number of factors providing an advantage against the human immune system and giving the ability to survive even in patients receiving an intensified antimicrobial therapy (Gellatly and Hancock 2013). Probably the most important immune evasion mechanism utilized by P. aeruginosa is its ability to form a biofilm, which largely protects the biopolymer-embedded bacterial cells against phagocytosis by host immune cells and provides a certain degree of protection against antimicrobials (Alhede et al. 2014). Additional important immune evasion factors produced by this bacterium are rhamnolipids, glycolipidic bio-surfactants that were shown to induce the lysis of a number of different host cells including polymorph nuclear leukocytes (PMNs), macrophages and erythrocytes (Alhede et al. 2014). Another important feature that renders it particularly dangerous is its natural resistance against many antibiotics and its ability to acquire resistance against an even broader spectrum of antibiotics (Poole 2011). In addition, most strains of P. aeruginosa possess an injectisome-type III secretion system (injectisome-T3SS, also known as non-flagellar T3SS). This is a special protein export apparatus allowing the bacterium to inject effector molecules into the cytosol of eukaryotic cells (Cornelis 2006). The injectisome-T3SS has evolved from flagella (Abby and Rocha 2012) and has spread through horizontal gene transfer into numerous Gram-negative bacteria (Brown and Finlay 2011). Several components of the flagellum and the injectisome-T3SS act as major pathogen-associated molecular patterns (PAMPS). As these induce the production and release of IL-1ß by the host immune system (discussed below) their interaction with the immune system may not be regarded per se as being beneficial to the bacteria. In its natural habitat, P. aeruginosa is confronted, among others, with predatory amoeba, with many species of them feeding on this bacterium (Sherr and Sherr 2002). However, the bacterium has also evolved a number of strategies to counteract this process in order to resist phagocytosis by and/or survive inside these protozoa (Michel 1997) or even to kill them (Matz et al. 2008). P. aeruginosa, for instance, uses the injectisome-T3SS against Acanthamoeba castellanii to pass different exotoxins such as ExoU, ExoS, ExoY and ExoT into the cytosol of the amoeba in order to kill the protist, with ExoU displaying the greatest effect (Matz et al. 2008). Besides its relevance for survival in its natural habitat, intracellular persistence of P. aeruginosa in amoeba is also considered to be its advantage in the hospital setting as it provides an additional dissemination mechanism and, for the most part, protects the bacterium against hospital disinfection agents (Cateau et al. 2014). Additionally, the intracellular persistence of P. aeruginosa in amoeba may prime the opportunistic pathogen to better bypass the host innate immune system, as bacteria are thought to use similar mechanisms to resist phagocytosis by amoeba and macrophages, respectively (Molmeret et al. 2005).
IL-1β and the inflammasome
The pro-inflammatory cytokine IL-1β plays an important role in the inflammation caused by P. aeruginosa . Two different signals are needed to produce active IL-1β (Fig. 1). The first signal is the activation of the Toll-like-receptors, like TLR-5 that detects flagellin from both Gram-negative and Gram-positive bacteria (Hayashi et al. 2001). Via the MyD88 (myeloid differentiation primary response gene 88) signalling pathway this leads to the production of pro-IL-1β, a precursor of the active form (Szatmary 2012). The second signal is the activation of the inflammasome. This is a multiprotein complex that consists of multiple NLRC4 [nucleotide-binding and oligomerization domain-like receptor (NLR) family, caspase activation and recruitment domain (CARD) containing 4] proteins and NAIPs (NLR family apoptosis inhibitory proteins, aka neuronal apoptosis inhibitory proteins), which form a wheel-like structure (Broz 2015). The NLRC4 inflammasome is activated via NAIPs, which are the actual sensors for the PAMPs (Kofoed and Vance 2011). However, there are important differences between mice and men with regard to the NAIPs. There is just one full-length Naip gene in the human genome but there are four functional Naip genes in the mouse genome of strain C57BL/6J (Allam et al. 2015; Growney and Dietrich 2000). Moreover, human NAIP responds only to the T3SS needle protein, which is one reason why the human-derived macrophage-like cell line U937 does not react to flagellin (Zhao et al. 2011). The mouse NAIP paralogs, on the other hand, respond to different components of the T3SS and flagella, with NAIP1 reacting with the T3SS needle protein, NAIP2 reacting with the T3SS rod protein and NAIP5 and NAIP6 responding to flagellin, respectively (Tenthorey et al. 2014).
The production of IL-1β through Pseudomonas aeruginosa needs two signals. 1 The first signal consists in the activation of the TLR MyD88 pathway through PAMPs like flagellin. This leads to the production of pro-IL-1β. 2 The second signal is the intracellular recognition of PAMPs by NAIP(s) and the following assembly of the NLRC4 inflammasome. The NLRC4 inflammasome finally cuts the pro-IL-1β to its active form that is exported to the extracellular space. 3 The released mature IL-1β leads to higher inflammation. 4 The inflammation increases the survival rate of the P. aeruginosa
P. aeruginosa and chronic infection
Chronic infection of the human host leads to specific pathoadaptive changes in P. aeruginosa that include mainly alterations in regulatory networks and central metabolism, the acquisition of antibiotic resistance determinants and the loss of extracellular virulence factors (Marvig et al. 2015). Notably, genes coding for factors involved in motility and attachment were found being mutated in particular high numbers (Marvig et al. 2015), in line with previous findings reporting a significantly higher rate of immobile P. aeruginosa isolates in chronically colonized CF patients (Mahenthiralingam et al. 1994). Although of importance for the pathogenesis of P. aeruginosa in the establishment of an infection (Feldman et al. 1998), the flagella appear to be a disadvantage for the bacterium in later stages of infection/colonization, as flagellated P. aeruginosa isolates are more efficiently cleared by the host than isogenic derivatives lacking flagella (Feldman et al. 1998; Cohen and Prince 2013), suggesting that the down-regulation of this feature is beneficial to the bacterium during adaptation to its new ecologic niche within the mammalian host.
Pseudomonas aeruginosa and acute infection
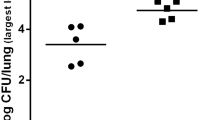
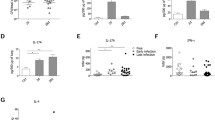
There are numerous studies on mice showing that a reduced inflammation can lead to a better outcome in a P. aeruginosa infection of the lung (Cohen and Prince 2013; Sawa et al. 1997; Skerrett et al. 1999; Veliz Rodriguez et al. 2012). TIR-8, for example, inhibits IL-R and TLR signalling and TIR-8 KO mice are more susceptible to a P. aeruginosa infection (Veliz Rodriguez et al. 2012). Reduced TNF-α signalling also seems to lead to a better clearing of the infection, as bacterial clearance was augmented in TNFR1−/− and TNFR1−/− TNFR2−/− knock-out mice, respectively (Skerrett et al. 1999). IFN-γ R−/− mice infected with a low dose of P. aeruginosa (1 × 105 CFU) have significantly less surviving bacteria in the lung homogenate 24 h post-infection. Administration of the anti-inflammatory cytokine IL-10 increases the survival rate of the infected mice and reduces the damage to the lung (Sawa et al. 1997). Intervention in the signalling of the pro-inflammatory cytokine IL-1β seems to have particularly beneficial consequences in P. aeruginosa-induced lung infection: P. aeruginosa-infected mice lacking the IL-1 receptor type 1 (IL-1R −/−) display a significantly decreased amount of IL-1β in the lungs 24 h after the bacterial challenge and contain significantly lower numbers of viable bacteria in the lung 24 h post-infection when compared with P. aeruginosa-infected wild-type mice (Schultz et al. 2002). An intraperitoneal application of neutralizing IL-1β antibodies into wild-type mice prior to infection with P. aeruginosa also markedly reduces the bacterial load in the lungs 24 h post-infection and decreases the inflammatory response (Palomo et al. 2014). Depletion of alveolar macrophages in mice prior to infection with P. aeruginosa again significantly decreases IL-1β signalling and improves bacterial clearance in the infected lung tissue. A similar effect can be provoked by reducing the IL-1β production through caspase-1 inhibition (Cohen and Prince 2013). The knock-down of NLRC4 also improves the ability of mice to clear a P. aeruginosa-induced lung infection (Cohen and Prince 2013; Faure et al. 2014). However, this effect appears to be dose-dependent, as NLRC4−/− mice challenged with a low dose of P. aeruginosa (5 × 105 CFU) displayed no significant differences in the survival rate compared to wild-type mice, except for a profound reduction in the production of IL-1ß in the NLRC4−/− mouse (Tolle et al. 2015).
Of course, the situation in other organs other than the lung might be quite different. For example, the intraperitoneal infection of NLRC4−/− mice with P. aeruginosa resulted in a significant decrease in IL-1β serum levels that was, however, accompanied by an enhanced bacterial burden in the peritoneal lavage in comparison to the bacterially challenged wild-type mice (Sutterwala et al. 2007).
Open questions and conclusion
Reduction of inflammation might be a promising way to improve the clearing of P. aeruginosa in pneumonia. However, the application of mice studies to human patients is problematic, especially in view of the discrepancy with regard to the inflammasomes and the fact that the mice models mainly focus on acute infections being induced by high bacterial loads. Nevertheless, it would be worthwhile to further study the described effects concerning the clearing of the infection in mice as well as in patients.
References
Abby SS, Rocha EP (2012) The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLoS Genet 8:e1002983
Alhede M, Bjarnsholt T, Givskov M, Alhede M (2014) Pseudomonas aeruginosa biofilms: mechanisms of immune evasion. Adv Appl Microbiol 86:1–40
Allam R, Maillard MH, Tardivel A, Chennupati V, Bega H, Yu CW, Velin D, Schneider P, Maslowski KM (2015) Epithelial NAIPs protect against colonic tumorigenesis. J Exp Med 212:369–383
Brown NF, Finlay BB (2011) Potential origins and horizontal transfer of type III secretion systems and effectors. Mob Genet Elem 1:118–121
Broz P (2015) Inflammasome assembly: the wheels are turning. Cell Res 25:1277–1278
Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR (1998) Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 27:158–163
Cateau E, Delafont V, Hechard Y, Rodier MH (2014) Free-living amoebae: what part do they play in healthcare-associated infections? J Hosp Infect 87:131–140
Cohen TS, Prince AS (2013) Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest 123:1630–1637
Cornelis GR (2006) The type III secretion injectisome. Nat Rev Microbiol 4:811–825
Faure E, Mear JB, Faure K, Normand S, Couturier-Maillard A, Grandjean T, Balloy V, Ryffel B, Dessein R, Chignard M, Uyttenhove C, Guery B, Gosset P, Chamaillard M, Kipnis E (2014) Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am J Respir Crit Care Med 189:799–811
Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A (1998) Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun 66:43–51
Gaynes R, Edwards JR (2005) Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41:848–854
Gellatly SL, Hancock RE (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173
Growney JD, Dietrich WF (2000) High-resolution genetic and physical map of the Lgn1 interval in C57BL/6J implicates Naip2 or Naip5 in Legionella pneumophila pathogenesis. Genome Res 10:1158–1171
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099–1103
Kofoed EM, Vance RE (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477:592–595
Mahenthiralingam E, Campbell ME, Speert DP (1994) Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 62:596–605
Marvig RL, Sommer LM, Molin S, Johansen HK (2015) Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet 47:57–64
Matz C, Moreno AM, Alhede M, Manefield M, Hauser AR, Givskov M, Kjelleberg S (2008) Pseudomonas aeruginosa uses type III secretion system to kill biofilm-associated amoebae. ISME J 2:843–852
Michel R (1997) Freilebende Amöben als Wirte und Vehikel von Mikroorganismen. Mitt Österr Ges Tropenmed Parasitol 19:11–20
Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y (2005) Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol 71:20–28
Palomo J, Marchiol T, Piotet J, Fauconnier L, Robinet M, Reverchon F, Le Bert M, Togbe D, Buijs-Offerman R, Stolarczyk M, Quesniaux VF, Scholte BJ, Ryffel B (2014) Role of IL-1beta in experimental cystic fibrosis upon P. aeruginosa infection. PLoS ONE 9:e114884
Poole K (2011) Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2:65
Sawa T, Corry DB, Gropper MA, Ohara M, Kurahashi K, Wiener-Kronish JP (1997) IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol 159:2858–2866
Schultz MJ, Rijneveld AW, Florquin S, Edwards CK, Dinarello CA, van der Poll T (2002) Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 282:L285–L290
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81:293–308
Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB (1999) Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol 276:L715–L727
Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA (2007) Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 204:3235–3245
Szatmary Z (2012) Molecular biology of toll-like receptors. Gen Physiol Biophys 31:357–366
Tenthorey JL, Kofoed EM, Daugherty MD, Malik HS, Vance RE (2014) Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol Cell 54:17–29
Tolle L, Yu FS, Kovach MA, Ballinger MN, Newstead MW, Zeng X, Nunez G, Standiford TJ (2015) Redundant and cooperative interactions between TLR5 and NLRC4 in protective lung mucosal immunity against Pseudomonas aeruginosa. J Innate Immun 7:177–186
Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C (2012) Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection. Infect Immun 80:100–109
Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600
Acknowledgment
The authors thank Ann Söther for editing help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wonnenberg, B., Bischoff, M., Beisswenger, C. et al. The role of IL-1β in Pseudomonas aeruginosa in lung infection. Cell Tissue Res 364, 225–229 (2016). https://doi.org/10.1007/s00441-016-2387-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2387-9