Abstract
The majority of 5-HT (serotonin) in the body is contained in enteroendocrine cells of the gastrointestinal mucosa. From the time of their discovery over 80 years ago, the 5-HT-containing cells have been regarded as a class of cell that is distinct from enteroendocrine cells that contain peptide hormones. However, recent studies have cast doubt on the concept of there being distinct classes of enteroendocrine cells, each containing a single hormone or occasionally more than one hormone. Instead, data are rapidly accumulating that there are complex patterns of colocalisation of hormones that identify multiple subclasses of enteroendocrine cells. In the present work, multiple labelling immunohistochemistry is used to investigate patterns of colocalisation of 5-HT with enteric peptide hormones. Over 95 % of 5-HT cells in the duodenum also contained cholecystokinin and about 40 % of them also contained secretin. In the jejunum, about 75 % of 5-HT cells contained cholecystokinin but not secretin and 25 % contained 5-HT plus both cholecystokinin and secretin. Small proportions of 5-HT cells contained gastrin or somatostatin in the stomach, PYY or GLP-1 in the small intestine and GLP-1 or somatostatin in the large intestine. Rare or very rare 5-HT cells contained ghrelin (stomach), neurotensin (small and large intestines), somatostatin (small intestine) and PYY (in the large intestine). It is concluded that 5-HT-containing enteroendocrine cells are heterogeneous in their chemical coding and by implication in their functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of 5-HT in the body is contained in or derived from enteroendocrine cells (EEC) contained in the epithelial lining of the gastrointestinal tract (Gershon 2013). 5-HT-containing EEC, commonly referred to as enterochromaffin (EC) cells, are the source of circulating 5-HT, mostly carried by platelets and for the activation of adjacent enterocytes, neurons (including the endings of extrinsic afferent neurons) and immune cells within the gut wall (Mawe et al. 2006).
EEC have been classified into about 12 types, based on the hormones that they contain (Rehfeld 2004). In most cases, each cell type has been assumed to contain one hormone, which has led to the naming of EEC by a simple letter code, for example, G cells being gastrin-containing, S cells being secretin-containing and I cells being cholecystokinin (CCK)-containing. The exception is L cells that contain glucagon gene products and peptide YY (PYY). It is now clear that the one cell–one hormone (or hormone combination) classification is no longer tenable (Helander and Fändriks 2012; Gribble and Reimann 2015). For example, when cells expressing a reporter transgene under CCK promotor control are isolated and molecularly analysed, it is found that CCK gene transcripts are commonly co-expressed with secretin, GIP, GLP-1, PYY and neurotensin transcripts and co-expression of the peptide hormones has been confirmed by mass spectrometry and immunohistochemistry (Egerod et al. 2012). Isolation of GIP-expressing and GLP-expressing EEC and correlated immunohistochemical analysis confirms overlaps in expression of GIP, GLP-1, CCK, PYY and secretin (Habib et al. 2012). Quantitative analysis of colocalisation of the K cell marker, GIP and the L cell markers, GLP-1 and PYY, in the mouse and pig gastrointestinal show that all possible combinations of these three hormones occur in EEC (Cho et al. 2015).
Amongst EEC, EC cells were originally identified as a unique cell type containing 5-HT (Vialli and Erspamer 1933; Erspamer and Asero 1952). However, Roth and Gordon (1990) reported that about 80 % of secretin immunoreactive cells in the mouse duodenum were immunoreactive for 5-HT. 5-HT and CCK are also frequently colocalised in the mouse proximal small intestine (Cho et al. 2014).
The range of effects that have been attributed to 5-HT that is released from EEC and the diversity of receptors that they express also suggest that EC cells might form subpopulations with different functions and cell biological properties. EEC-derived 5-HT influences gastrointestinal motility, with some authors arguing that it is critical for the induction of peristalsis (Bülbring and Crema 1958; Heredia et al. 2013; Smith and Gershon 2015) and others arguing that it modulates peristalsis but is not essential (Keating and Spencer 2010; Spencer et al. 2015) and yet others concluding that the role of mucosal 5-HT remains enigmatic (Bornstein 2012; Gribble and Reimann 2015). 5-HT induces changes in motility and secretion that are associated with elimination of toxins. Noxious stimuli release 5-HT from EC, which acts on mucosal vagal afferent endings to cause nausea and emesis; effects that are counteracted by 5-HT3 receptor antagonists (Andrews et al. 1998; Sanger and Andrews 2006; Hagbom et al. 2011). In the lower gut, 5-HT may contribute to secretory diarrhoea, another defence against toxins and 5HT3 antagonists aid in regularising bowel function in diarrhoea-predominant irritable bowel syndrome (Spiller and Garsed 2009). Other factors that release 5-HT from EC cells include raised oxygen tension, bile salts, sweet and umami taste receptor stimulants and the phytochemicals, thymol and eugenol (Kidd et al. 2008; Haugen et al. 2012). Luminal receptors on EC cells include Toll-like receptors, consistent with roles of 5-HT in defence against toxins and pathogens (Bogunovic et al. 2007). EC cells also release 5-HT in response to stimulation of receptors for neurotransmitters and hormones (Kidd et al. 2006; Raghupathi et al. 2013). Furthermore, gut microbes stimulate 5-HT production and release by EC cells (Reigstad et al. 2015; Yano et al. 2015).
In the present study, we investigate the chemical coding of 5HT-containing cells throughout the length of the gut using double and triple labelling immunohistochemistry.
Materials and methods
Animals and tissue preparation
All procedures were conducted according to the National Health and Medical Research Council of Australia guidelines and were approved by the University of Melbourne Animal Experimentation Ethics Committee. C57BL/6 mice, aged 8–10 weeks, were housed in the Biomedical Animal Facility at the University of Melbourne and were provided standard chow and water ad libitum. Animals were rendered unconscious with CO2 gas and killed by decapitation. Segments of gastric corpus and antrum, the first part of the duodenum, 3 cm of proximal jejunum, distal ileum 2 cm from the caecum, caecum, proximal colon just distal to the caecum and distal colon between the colonic flexure and the pelvic brim were removed. The segments were cleaned of contents, opened along the mesenteric attachment and pinned, mucosa up without stretching, to balsa wood sheets in ice-cold phosphate-buffered saline (PBS: 0.15 M NaCl in 0.01 M sodium phosphate buffer, pH 7.2). The tissue was then placed in fixative (2 % formaldehyde plus 0.2 % picric acid in 0.1 M sodium phosphate buffer, pH 7.2) overnight at 4 °C. The following day, tissues were cleared 3 times (10 min) in dimethyl sulfoxide (DMSO) and then washed 3 times (10 min) in PBS. Tissue was transferred to PBS-sucrose-azide (PBS containing 0.1 % sodium azide and 30 % sucrose as a cryoprotectant) and stored at 4 °C. The next day, the tissue samples were placed in PBS-sucrose-azide and OCT compound (Tissue Tek, Elkhart, IN, USA) in a ratio of 1:1 for a further 24 h, before being trimmed and embedded in 100 % OCT and frozen in isopentane cooled with liquid nitrogen.
Immunohistochemistry
Sections of 12 μm thickness were cut, air-dried for 1 h on microscope slides (SuperFrostPlus®; Menzel-Glaser #1.5; Thermo Fisher, Scoresby, Vic, Australia) and incubated with 10 % normal horse serum for 30 min. Sections were then incubated with mixtures of primary antibodies (Table 1) for double or triple staining at 4 °C overnight. The tissue was washed three times in PBS and incubated in secondary antibody (Table 2) for 1 h at room temperature. For staining nuclei, preparations were washed once with PBS then twice with distilled water and incubated for 5 min in Hoechst 33258 solution (Bisbenzimide–Blue, diluted to 10 μg/mL in dH2O) and then washed 3 times with distilled water before mounting with fluorescent mounting medium (Dako, Carpinteria, CA, USA).
Image analysis
Slides were examined using an AxioImager microscope (Zeiss, Sydney, Australia) and high resolution confocal microscopy (Zeiss Meta510). For quantitative analysis, images were captured using a V-Slide fluorescent slide scanner (Zeiss). Images were exported and analysed off-line using ImageJ (imagej.nih.gov/ij/). For quantitation of immunoreactive cells, cells were counted in 3 sections from each sample and this analysis was repeated in 3 animals. For counts of total 5-HT cell numbers, sections from 6 mice were used. EEC in the crypts and villi were counted separately in the small intestine. Cells were counted in the crypts in regions of sections in which the crypt lumen was clearly defined and in the villi where the villus core was obvious. Cells in ambiguous regions at the crypt–villus interface were not counted. Counts in the upper and lower villi as previously defined (Aiken et al. 1994) were combined. Images were imported into CorelDraw (Corel, Ottowa, Canada) for final preparation of figures.
Statistical analysis
Data were analysed using Prism 5.0 (GraphPad Software, San Diego, CA, USA) and presented as mean ± SEM. Differences were evaluated with 2-tailed Student’s t tests.
Results
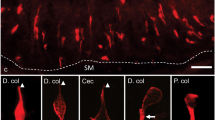
5-HT immunoreactive cells were observed throughout the length of the gastrointestinal tract, from the stomach to the distal colon (Fig. 1). There were few immunoreactive 5-HT cells in the corpus of the stomach (1.7 ± 0.4 cells/mm2, n = 6) but they were abundant in the antrum (11.9 ± 3.4 cells/mm2, n = 6). Expression of 5-HT decreased distally in the small intestine (19.7 ± 1.9 cells/mm2 in duodenum to 11.6 ± 1.3cells/mm2 in the distal ileum, n = 6) and increased again in the large intestine (28.1 ± 2.3 cells/mm2 in the proximal colon, n = 6) (Fig. 1).
Distribution of 5-HT immunoreactive enteroendocrine cell populations along the mouse gastrointestinal tract. Total 5-HT cell numbers per mm2 of mucosa, independent of whether they contained other hormones, are plotted. Data are from analysis of 4 sections in tissue samples from each of 6 mice. Cor gastric corpus; Ant gastric antrum; Duo duodenum; Jej jejunum; D.il distal ileum; Cec caecum; P.c proximal colon; D.c distal colon. Mean ± SEM, n = 6
Colocalisation of 5-HT with gastrin, ghrelin and somatostatin in the stomach
Ghrelin immunoreactive cells were abundant in the corpus and antrum of the stomach (46.2 ± 3.4 and 73.6 ± 8.5 cells/mm2, respectively; both n = 3). Previous studies have shown that gastrin is not expressed in the corpus (Schubert and Peura 2008). Somatostatin was also found in both the gastric antrum and corpus (1.4 ± 0.5 in corpus and 4.2 ± 0.9 cells/mm2 in the antrum).
Very few cells were immunoreactive for both 5-HT and either ghrelin (0.2 ± 0.05 cells/mm2, n = 3) (Fig. 3a), gastrin (2.0 ± 0.9 cells/mm2, n = 3) (Figs. 2a, 3b) or somatostatin (0.7 ± 0.1 cells/mm2, n = 3) (Fig. 3c).
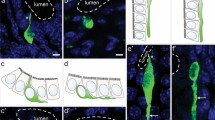
Examples of double labelling of mouse enteroendocrine cells expressing 5-HT. Colocalisation with gastrin in the gastric antrum (a), with neurotensin in the jejunum (b) and glucagon-like peptide 1 (GLP-1) in the distal colon (c) are shown. 5-HT and PYY are generally not colocalised (d). Arrows show locations of immunoreactive cells, stars cells immunoreactive for both 5-HT and a peptide hormone. Scale bars 20 μm
Quantitation of colocalization of 5-HT and peptide hormones in enteroendocrine cell populations of different areas of the gastrointestinal tract using immunohistochemistry. 5-HT and (a) ghrelin, (b) gastrin and (c) somatostatin in the stomach, (d) somatostatin in the small and large intestine and GLP-1 (e), neurotensin (f) and PYY (g) in the small and large intestine. Cor gastric corpus; Ant gastric antrum; Duo duodenum; Jej jejunum; D.il distal ileum; Cec caecum; P.c proximal colon; D.c distal colon. Mean ± SEM, n = 3 mice
Colocalisation of 5-HT, somatostatin, GLP-1, neurotensin and PYY in the intestine
Compared to the stomach, there were more somatostatin immunoreactive cells in the small and large intestins with expression increasing slightly from duodenum to distal colon (6.4 ± 3.5 cells/mm2 in the duodenum to 13.3 ± 5.1 cells/mm2, n = 3 in the distal colon) (Fig. 3d). The number of somatostatin cells colocalised with 5-HT also increased distally (0.4 ± 0.1 cells/mm2 in the duodenum to 5.1 ± 2.4 cells/mm2, n = 3 in the distal colon) (Fig. 3d).
GLP-1 expression increased distally (from 13.2 ± 3.6 in the duodenum to 43.1 ± 8.2 cells/mm2, n = 3 in the distal colon) (Fig. 3e). Small numbers of EEC that were immunoreactive for both GLP-1 and 5-HT were observed throughout the intestine (2.2 ± 0.8 cells/mm2, n = 3 in the distal colon) (Figs. 2c, 3e). Some sections from the small intestine from each mouse were triple stained for 5-HT, GLP-1 and somatostatin but no triple labelled cells were found. Only rare cells were immunoreactive for both GLP-1 and somatostatin.
Neurotensin expression increased distally thoughout the small intestine (Fig. 3f), with the maximum number of neurotensin containing cells being found in the caecum (14.9 ± 4.3 cells/mm2, n = 3); numbers of neurotensin positive cells then decreased progressively along the colon (1.9 ± 0.3 cells/mm2, n = 3 in the distal colon) (Fig. 3f). Very few cells contained both neurotensin and 5-HT (0.5 ± 0.3 cells/mm2, n = 3 in the distal colon) (Figs. 2b and 3f).
PYY expression was fairly constant in the distal ileum (8.4 ± 0.4 cells/mm2, n = 3) and large intestine (10.8 ± 0.9 cells/mm2, n = 3 in the proximal colon) (Fig. 3g). There were very few cells containing both PYY and 5-HT (0.2 ± 0.1 cells/mm2, n = 3 in the proximal colon) (Figs. 2d, 3g).
Colocalisation of 5-HT, CCK and secretin
The results showed that there were substantial populations of 5-HT/CCK/secretin cells and 5-HT/CCK cells but few cells that were immunoreactive for only 5-HT in the proximal small intestine (Figs. 4, 5). Colocalisation of 5-HT (goat antibody) and secretin (rabbit antibody) was abundant in the duodenum (9.0 ± 2.5 cells/mm2, n = 3) and jejunum (3.4 ± 0.7 cells/mm2, n = 3). In the duodenum, 48.1 ± 4.5 % of 5-HT cells were immunoreactive for secretin and 56.9 ± 4.7 % of secretin cells were immunoreactive for 5-HT.
Example of double labelling for 5-HT and secretin in the duodenal villi (a, a’, a”). All combinations are seen, cells with both hormones (stars) and cells with either hormone (arrows). An example of an enteroendocrine cell that is immunoreactive for the three hormones, 5-HT, cholecystokinin and secretin (b, b’, b”). Arrows show location of immunoreactive cells with a single hormone, stars cells immunoreactive for two or more hormones. Scale bar 20 μm
A goat secretin antibody was used to triple stain CCK (rabbit antibody) and 5-HT (rat antibody). There were few secretin immunoreactive cells in the crypts (1.4 ± 0.5 cells/mm2, n = 3) (Fig. 5b), which confirms previous observations (Aiken et al. 1994). In these cells, secretin was co-localised with CCK. In contrast, CCK cells and CCK cells containing 5-HT were abundant in the crypts (7.9 ± 1.0 cells/mm2 and 5.7 ± 1.6 cells/mm2, respectively, in the duodenum, n = 3).
In the villi, a large number of cells contained 5-HT, CCK and secretin (duodenum: 8.6 ± 0.8 cells/mm2, n = 3) (Fig. 5a). Analysis of sections triple stained for 5-HT, CCK and secretin in the duodenum indicated that 25.2 ± 3.4 % of CCK cells also contained 5-HT and secretin and 32.3 ± 3.8 % contained 5-HT but not secretin. Of secretin cells, 51.0 ± 8.2 % contained both CCK and 5-HT and 41.6 ± 6.5 % contained CCK but not 5-HT, whereas very few secretin cells contained 5-HT without CCK (1.0 ± 1.0 %) or secretin alone (6.4 ± 1.5 %, n = 3). This is consistent with our secretin/5-HT double stain results where around half of secretin cells (56.9 ± 4.7 %) contained 5-HT.
Summary of patterns of colocalisations of 5-HT throughout the GI tract
The patterns of colocalisation throughout the stomach and intestine are summarised in Fig. 6. Three types of colocalisation were encountered: substantial proportions of 5-HT cells having colocalisation with CCK and CCK/secretin in the duodenum and jejunum; small degrees of colocalisation with gastrin and with somatostatin in the stomach, with PYY or GLP1 in the small intestine and with GLP1 or somatostatin in the large intestine; and rare or very rare colocalisation with ghrelin (stomach), neurotensin (small and large intestines), PYY (in the large intestine) and somatostatin (small intestine).
Analysis of 5-HT sub-populations in the stomach, small intestine and large intestine. For each combination of hormone, the total numbers of 5-HT cells have been scaled to 100 %. In the stomach, overlap with ghrelin (Ghr) is very rare and there are small proportions of 5-HT cells containing gastrin (Gas) or somatostatin (Som). In the duodenum (Duo) and jejunum (Jej), significant proportions of 5-HT cells also contained cholecystokinin (CCK) or CCK plus secretin (Sec). Other hormones, glucagon like peptide 1 (GLP-1), somatostatin, neurotensin (NT) and peptide YY (PYY) had low frequencies of overlap with 5-HT in the small intestine. 5-HT was contained in a few cells that also contained peptide hormones in the large intestine. Error bars SEM, n = 3 mice
Discussion
The results indicate a heterogeneity of 5-HT-containing enteroendocrine cell types and confirm that the historical classification of 5-HT cells as enterochromaffin cells, a class separate from other gastrointestinal endocrine cells (Vialli and Erspamer 1933; Erspamer and Asero 1952), is outdated. Co-localisation of 5-HT with secretin (Roth and Gordon 1990) and with CCK (Cho et al. 2014) has been previously identified. Moreover, secretin has been reported in CCK cells of CCK-eGFP transgenic mice and in CCK cells of human duodenum (Egerod et al. 2012). In an earlier study, colocalisation of CCK and secretin was reported in EEC of the villi in the mouse proximal small intestine (Aiken et al. 1994). Our study expands these observations by showing that almost all 5-HT-containing EEC of the mouse duodenum contain either CCK (5-HT/CCK cells; 50–60 % of 5-HT cells) or both CCK and secretin (5-HT/CCK/secretin cells; these were 40–50 % of 5-HT cells and were confined to the villi). Very rare cells, fewer than 2 %, in these regions contained 5-HT but not either CCK or secretin or both.
Thus, it might be postulated that 5-HT, CCK and secretin act together in digestive control. 5-HT and CCK appear to have synergistic effects. Nutrients infused into the upper small intestine cause a vagus nerve-dependent increase in pancreatic enzyme release that has both CCK-mediated and 5-HT-mediated components (Li and Owyang 1996; Li et al. 2000). In conscious rats, it was found that intraluminal rodent chow evoked increases in pancreatic enzyme secretion that were reduced by 54 % when CCK receptors were blocked and 92 % when both CCK and 5-HT receptors were blocked (Li et al. 2000). Pancreatic responses to intraluminal nutrients were prevented by cutting vagal afferents close to the brain stem. In the case of secretin, its established effect to stimulate bicarbonate release (Bayliss and Starling 1902) is mimicked by 5-HT. Low doses of 5-HT (20–200 nmol/kg/h) infused into the vasculature of the rat duodenum (Säfsten et al. 2006) or direct application of 5-HT to the isolated duodenum from mice increases bicarbonate secretion (Tuo and Isenberg 2003). Thus, synergistic effects of 5-HT and secretin released from the same cells are predicted.
5-HT and CCK also have parallel effects on patterns of movement of segments of guinea-pig duodenum and jejunum. Mixing activity that was induced by luminal fat was mimicked by increasing 5-HT availability (by inhibiting 5-HT uptake) and by CCK (Ellis et al. 2013). Moreover, the stimulation of mixing movements induced by intraluminal decanoic acid was reduced by both 5-HT and by CCK receptor antagonists. This points to a physiological role of CCK and 5-HT acting together in inducing fed-state motility patterns in the proximal small intestine.
CCK and 5-HT possibly also act together in causing satiety. CCK is well established as a gastrointestinal hormone that is released by nutrients and stimulates vagal afferent endings in the gut to induce satiety (Morley 1990). 5-HT, 5-HT receptor stimulants and increased availability of 5-HT (caused by inhibiting its metabolism) all decrease food intake and antagonists of 5-HT receptors increase feeding (Blundell 1986; Cooper and Dourish 1990). Although 5-HT causes nausea (Sanger and Andrews 2006), which in itself reduces the desire for food, it has been argued that the effects that manipulating the 5-HT system have on food intake are independent of the induction of nausea (Blundell 1986). It is feasible that only when there are high levels of 5-HT release, for example after ingestion of toxins (Hagbom et al. 2011), is there induction of nausea.
In the present work, we found low degrees of overlap of 5-HT with some hormones, such as gastrin or somatostatin in the stomach and PYY in the small intestine, or very low incidence of colocalisation (e.g., ghrelin, GLP-1 or neurotensin). Other recent studies have also found low-level overlaps, for example 5 % of CCK cells express glucagon-like insulinotropic peptide (Egerod et al. 2012). It has been suggested that low-incidence hormone overlaps in the same EEC reflect that cells that express more than one hormone in an early developmental stage, will with maturation express one hormone (Roth et al. 1992). However, more mature cells, which have migrated to the villi, also show co-localisation (Egerod et al. 2012), so this cannot be the entire explanation.
It has been suggested that there are different EEC lineages. Roth et al. (1992) deduced that 5-HT cells were in one lineage and that CCK, GLP-1, PYY and neurotensin cells were in another. More recently, Egerod (2012) provided evidence for a CCK, secretin, GIP, GLP-1, PYY and neurotensin lineage that is separate from a somatostatin lineage, although if cells with gip gene expression (Venus labelled) are separated by FACS, cells with somatostatin gene (sst) are encountered (Habib et al. 2012). Manipulation of the transcription factors, Arx and Pax4, also distinguishes a somatostatin cell lineage and a 5-HT lineage from the CCK, secretin, GIP, GLP-1, PYY and neurotensin lineage (Beucher et al. 2012). Thus, the current work suggests that 5-HT is expressed by more than one EEC lineage.
In conclusion, 5-HT is colocalised with many gut hormones and has particularly frequent overlaps with CCK and secretin in the duodenum and jejunum. It is not justified to maintain the separate enterochromaffin terminology to distinguish 5-HT-containing EEC from other EEC. The roles of 5-HT-containing EEC of different gut regions are likely to be different and the lineages to which they belong also differ.
References
Aiken KD, Kisslinger JA, Roth KA (1994) Immunohistochemical studies indicate multiple enteroendocrine cell differentiation pathways in the mouse proximal small intestine. Dev Dyn 201:63–70
Andrews PLR, Naylor RJ, Joss RA (1998) Neuropharmacology of emesis and its relevance to anti-emetic therapy. Support Care Cancer 6:197–203
Bayliss WM, Starling EH (1902) The mechanism of pancreatic secretion. J Physiol Lond 28:325–353
Beucher A, Gjernes E, Collin C, Courtney M, Meunier A, Collombat P, Gradwohl G (2012) The homeodomain-containing transcription factors Arx and Pax4 control enteroendocrine subtype specification in mice. PLoS ONE 7:e36449–e36449
Blundell JE (1986) Serotonin manipulations and the structure of feeding behaviour. Appetite 7:39–56
Bogunovic M, Dave SH, Tilstra JS, Chang DTW, Harpaz N, Xiong H, Mayer LF, Plevy SE (2007) Enteroendocrine cells express functional toll-like receptors. Am J Physiol Gastrointest Liver Physiol 292:G1770–G1783
Bornstein JC (2012) Serotonin in the gut: what does it do? Frontiers in Neuroscience 6
Bülbring E, Crema A (1958) Observations concerning the action of 5-hydroxytryptamine on the peristaltic refllex. Br J Pharmacol 13:444–457
Cho H-J, Callaghan B, Bron R, Bravo DM, Furness JB (2014) Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res 356:77–82
Cho H-J, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB (2015) Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res 359:693–698
Cooper SJ, Dourish CT (1990) Multiple cholecystokinin (CCK) receptors and CCK-monoamine interactions are instrumental in the control of feeding. Physiol Behav 48:849–857
Costa M, Furness JB, Cuello AC, Verhofstad AAJ, Steinbusch HWM, Elde RP (1982) Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience 7:351–363
Egerod KL, Engelstoft MS, Grunddal KV et al (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795
Ellis M, Chambers JD, Gwynne RM, Bornstein JC (2013) Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 304:G749–G761
Erspamer V, Asero B (1952) Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169:800–801
Furness JB, Hunne B, Matsuda N, Yin L, Russo D, Kato I, Fujimiya M, Patterson M, McLeod J, Andrews ZB, Bron R (2011) Investigation of the presence of ghrelin in the central nervous system of the rat and mouse. Neuroscience 193:1–9
Gershon MD (2013) 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Op Endoc Diab Obes 20:14–21
Gribble FM, Reimann F (2015) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78 (in press)
Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065
Hagbom M, Istrate C, Engblom D et al (2011) Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 7, e1002115
Haugen M, Dammen R, Svejda B, Gustafsson BI, Pfragner R, Modlin I, Kidd M (2012) Differential signal pathway activation and 5-HT function: the role of gut enterochromaffin cells as oxygen sensors. Am J Physiol 303:G1164–G1173
Helander HF, Fändriks L (2012) The enteroendocrine “letter cells” – time for a new nomenclature? Scand J Gastroenterol 47:3–12
Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK (2013) Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol Lond 591:5939–5957
Keating DJ, Spencer NJ (2010) Release of 5-Hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138:659–670
Kidd M, Modlin IM, Eick GN, Champaneria MC (2006) Isolation, functional characterization, and transcriptome of Mastomys ileal enterochromaffin cells. Am J Physiol 291:G778–G791
Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R (2008) Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol 295:G260–G272
Li Y, Owyang C (1996) Pancreatic secretion evoked by cholecystokinin and non-cholecystokinin-dependent duodenal stimuli via vagal afferent fibes in the rat. J Physiol Lond 494:773–782
Li Y, Hao Y, Zhu J, Owyang C (2000) Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology 118:1197–1207
Mawe GM, Coates MD, Moses PL (2006) Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther 23:1067–1076
Morley JE (1990) Appetite regulation by gut peptides. Annu Rev Nutr 10:383–395
Raghupathi R, Duffield MD, Zelkas L, Meedeniya A, Brookes SJH, Sia TC, Wattchow DA, Spencer NJ, Keating DJ (2013) Identification of unique release kinetics of serotonin from guinea-pig and human enterochromaffin cells. J Physiol Lond 591:5959–5975
Rehfeld JF (2004) A centenary of gastrointestinal endocrinology. Horm Metab Res 36:735–741
Reigstad CS, Salmonson CE, Rainey JF III, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29:1395–1403
Roth KA, Gordon JI (1990) Spatial differentiation of the intestinal epithelium: analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. Proc Natl Acad Sci U S A 87:6408–6412
Roth KA, Kim S, Gordon JI (1992) Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol 263:G174–G180
Säfsten B, Sjöblom M, Flemström G (2006) Serotonin increases protective duodenal bicarbonate secretion via enteric ganglia and a 5-HT4-dependent pathway. Scand J Gastroenterol 41:1279–1289
Sanger GJ, Andrews PLR (2006) Treatment of nausea and vomiting: gaps in our knowledge. Autonom Neurosci 129:3–16
Schubert ML, Peura DA (2008) Control of gastric acid secretion in health and disease. Gastroenterology 134:1842–1860
Smith TK, Gershon MD (2015) CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol Lond 593:3225–3227
Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ (2015) CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol Lond 593:3229–3231
Spiller R, Garsed K (2009) Postinfectious irritable bowel syndrome. Gastroenterology 136:1979–1988
Tuo B-G, Isenberg JI (2003) Effect of 5-hydroxytryptamine on duodenal mucosal bicarbonate secretion in mice. Gastroenterology 125:805–814
Vialli M, Erspamer V (1933) Celluli enterocromaffini e cellule basigranulose acidofile nei vertebrati. Z Zellforsch 19:743–773
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reynaud, Y., Fakhry, J., Fothergill, L. et al. The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res 364, 489–497 (2016). https://doi.org/10.1007/s00441-015-2349-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2349-7