Abstract
Damage-associated molecular patterns (DAMPs) comprise intracellular molecules characterized by the ability to reach the extracellular environment, where they prompt inflammation and tissue repair. The high-mobility box group 1 (HMGB1) protein is a prototypic DAMP and is highly conserved in evolution. HMGB1 is released upon cell and tissue necrosis and is actively produced by immune cells. Evidence suggests that HMGB1 acts as a key molecule of innate immunity, downstream of persistent tissue injury, orchestrating inflammation, stem cell recruitment/activation, and eventual tissue remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Innate immunity encompasses an immediate and stereotypical response to diverse events that share the potential to jeopardize cell and tissue integrity. Immunologists have, until recent years, focused on the innate response elicited by microorganisms. Protection against invading microbes relies on the recognition of the molecular structures shared by pathogens, referred to as pathogen-associated molecular patterns (PAMPs; Janeway 1992). Dedicated pattern-recognition receptors (PRRs) recognize PAMPs and thus allow them to identify pathogens. Most innate immune cells express PRRs. PRR activation in turn recruits several signaling pathways. As a consequence, PAMP recognition:
-
(1)
promotes the production of soluble inflammatory molecules, including cytokines and chemokines, which recruit and locally activate inflammatory leukocytes (Nathan 2002), with the inflammatory cells acquiring the ability to terminate the pathogen and any cells that had been infected;
-
(2)
elicits an acute phase response, with the generation and release of conserved soluble pattern recognition receptors (Manfredi et al. 2008a), which locally tune and regulate the potentially noxious effects of leukocyte activation;
-
(3)
favors the migration of antigen-presenting cells, in particular dendritic cells (DCs), to secondary lymphoid organs, where they productively activate naive T cells. The clonal expansion of antigen-specific T cells is the basis of an adaptive immunological response, characterized by specificity for the pathogen and by memory; expanded clones retain the ability to react faster and more effectively in the case of a further encounter with the microbe on future occasions (Pulendran et al. 2001). The effect of PAMP recognition is not only quantitative; depending on the specific array of PRRs, antigen presentation in lymphoid organs results in the preferential expansion of lymphocytes committed toward a Th1, Th2, Th17, or regulatory T-cell fate, therefore better suited to enforce protective immunity effective against diverse microbes, to promote inflammation and autoimmunity, or to establish tolerance (Manfredi et al. 2009).
Sterile inflammation, which occurs as a consequence of tissue necrosis, even in the absence of pathogens, allows effective clearance of necrotic cells and debris, thus preventing the dangers (Matzinger 2002) associated to residual uncleared material (Munoz et al. 2010a) and enforcing effective regeneration programs, with eventual tissue healing. Inflammation, in particular, sustains the secretion of growth and survival factors by bystander tissue cells that have not been directly damaged; this in turn recruits and activates local precursor and stem cells (Shaw and Martin 2009). Novel extracellular matrix assembly, via deposition by fibroblasts and degradation by activated macrophages, further contributes to eventual healing. Complex syndromes, including generalized sterile inflammation and systemic inflammatory response syndrome on one hand, and possibly specific features of autoimmune systemic rheumatic diseases, represent part of the price for the protection that inflammation provides (Banchereau and Pascual 2006; Pisetsky et al. 2008; Hreggvidsdottir et al. 2009; Pisetsky and Ronnblom 2009). Specifically, damage-associated molecular patterns (DAMPs) or alarmins are intracellular molecules that are released during cell and tissue necrosis and are endowed with the ability to elicit inflammation and, possibly as a direct consequence, both tissue regeneration and activation of acquired T-cell-dependent protective immune (and autoimmune) responses (Oppenheim and Yang 2005; Lotze et al. 2007; Bianchi and Manfredi 2009; D. Yang et al. 2010).
In real-life, sterile injuries readily become infected. Conversely, infection is associated with cell death and therefore with the release of DAMPs, with the specific features and extent of the inflammatory response. Here, we discuss, in particular, the ability of the best-characterized endogenous DAMP, the high-mobility group box 1 protein (HMGB1), to contribute to the innate responses in injured and/or infected peripheral tissues. HMGB1 is mostly located in the nucleus of living cells. Its structure (Fig. 1) justifies its efficacy at bending DNA, thus promoting the assembly of proteins on specific targets. Moreover, it is possibly involved in the ability of HMGB1 to form biologically active complexes with diverse substrates, which are heterogeneous in terms of physico-chemical and biological properties (see below).
The HMG family
HMGB1 is an abundantly occurring parent form of HMG proteins. Its name indicates its ability to migrate quickly in Triton-urea and polyacrylamide gels, a feature that reflects the high content of charged amino acid residues and that interestingly also reflects its “temperament” in the nucleus of living cells, where it is highly motile. Hmgb1 is located on the 13q12 human chromosome. The gene consists of six exons that encode for a 215-amino-acid polypeptide. It has a large sequence consensus in all animal species (Sessa and Bianchi 2007). Mammals have several genes, including Hmgb1, Hmgb2, and Hmgb3, which express similar (>80% identity) proteins. They code for proteins with a molecular mass of around 25 kDa, two DNA-binding domains (referred to as box A and box B), and a long acidic carboxy-terminal region. HMGB proteins are not redundant, as demonstrated by triple HMGB silencing (Yanai et al. 2009). HMGB1 proteins from all mammals are virtually identical (>99%), implying that each single residue is under selective pressure; HMGB2 and HMGB3 are also strongly conserved (Stros 2010). A shorter HMGB4 protein, devoid of the acidic tail and possibly endowed with specific nuclear roles, has also been recently identified (Catena et al. 2009).
HMGB1 has been extensively studied during the last two decades. So far, other members of the family have received less attention. Embryos express high levels of both HMGB1 and HMGB2 proteins. In contrast, HMGB1 is expressed in almost all nucleated cells of adult animals (and not only adults; see Rouhiainen et al. 2000) and HMGB2 in testis and lymphoid organs (Muller et al. 2004). Moreover, HMGB2 is expressed in the superficial zone of articular cartilage, where it plays a protective role during aging (Taniguchi et al. 2009). HMGB2 has been further characterized during the last two years as a chromatin protein (Stros 2010; Taniguchi et al. 2009; Ugrinova et al. 2009; Tian et al. 2007; Lee et al. 2010). Moreover, HMGB2 plays a role in cell death and inflammation (Pusterla et al. 2009; Krynetskaia et al. 2009; Wixted et al. 2010) thanks to its mitogenic and chemoattractant activities (Pusterla et al. 2009) and to its putative involvement in the response to oxidative stress (Lee et al. 2010). HMGB proteins 1, 2, and 3 share the ability to bind to nucleic acids and are required for type-I interferon and inflammatory cytokine induction by DNA or RNA (Yanai et al. 2009; see also below).
HMGB1: a molecule in motion
The positively charged DNA-binding domains (A and B boxes) of HMGB1 contain nuclear-localization signals. Interestingly, they seem to have distinct extra-cellular functions: the A box is an antagonist of B box pro-inflammatory activity (Li et al. 2003). The tail specifically interacts with the two boxes and influences their ability to bind to DNA (Knapp et al. 2004).
HMGB1 is ubiquitously expressed; its level and sub-cellular localization depend on the cell type and state of activation, with more differentiated cells often being characterized by a lower content of the protein (Muller et al. 2004). HMGB1 expression is mostly nuclear; however, under certain conditions, the molecule reaches the cytoplasm and thereafter the extracellular environment. Schematically, HMGB1 leaves the nucleus either because:
-
(1)
cells die via an unscheduled accidental pathway that is associated with the loss of membrane compartmentalization and with the release of intracellular components;
-
(2)
cells undergo activation and actively secrete HMGB1. During innate immune responses, leukocytes secrete intracellular components, thus artificially recreating the environment associated with cell necrosis, a primeval condition associated with immune activation (Bianchi and Manfredi 2004, 2007).
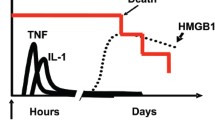
Monocytes, macrophages and immature DCs secrete HMGB1 in response to lipopolysaccharide (LPS), tumor necrosis factor α (TNFα), or interleukin-1β (IL-1β) stimulation (Wang et al. 1999; Dumitriu et al. 2005a, b, c). Post-translational modifications, including acetylation, phosphorylation, methylation, and oxidation influence the function of extracellular HMGB1 (Gardella et al. 2002; Bonaldi et al. 2003; Hoppe et al. 2006; Youn and Shin 2006; Ito et al. 2007; see also below).
Acetylated lysines at positions 2 and 11 are a feature of HMGB1 released by dying cells. In contrast, lysines throughout the entire length of actively secreted HMGB1 are acetylated (Bonaldi et al. 2003), including those within the 27–43 and 178–184 domains that behave as nuclear localization signals. Acetylation might influence the intracellular localization of the HMGB1 in activated cells, facilitating access to “secretory” lysosomes, a group of intracellular vesicles whose content is released into the extracellular environment in the presence of appropriate secretagogs (Bianchi and Manfredi 2007; Wang et al. 1999; Dumitriu et al. 2005a, b, c; Gardella et al. 2002). This pathway is apparently dominant in myeloid cells. Other cells, including neurons, also actively secrete HMGB1 in the absence of bona-fide secretory lysosomes (Rauvala and Rouhiainen 2010). Other modifications, including acetylation and phosphorylation possibly facilitate nuclear/cytoplasmic shuttling (Youn and Shin 2006). Phosphorylation is calcium-dependent and is mediated by the classical protein kinase C (Oh et al. 2009). Mono-methylation of lysine at position 42 also occurs post-translationally. Methylated HMGB1 is less effective at DNA binding and, apparently as a consequence, passively diffuses in the cytoplasm of neutrophils (Ito et al. 2007).
A redox-regulated biological function?
HMGB1 function in the environment depends on its functional integrity. In turn, environmental cues directly target and modify HMGB1. Oxidative stress is an early player during acute inflammatory response and results in the formation of reversible covalent disulfide bonds between thiols (Rubartelli and Sitia 2009; Carta et al. 2009). HMGB1 contains three cysteine residues at positions 23, 45, and 106. Upon mild oxidation, cysteines at positions 23 and 45 establish an intra-molecular disulfide bridge, which is reverted under reducing conditions (Hoppe et al. 2006). In contrast, the cysteine at position 106 contributes to the nuclear localization of the molecule (Hoppe et al. 2006). Moreover, the residue is required for binding to the Toll-like receptor 4 (TLR4) PRR on the macrophage plasma membrane and for HMGB1-elicited cytokine secretion (H. Yang et al. 2010). So far, the precise effect of the redox state on this interaction has not, to the best of our knowledge, been investigated.
The latter issue is relevant; a regulated change of the intra- and extra-cellular redox state characterizes two events in which HMGB1 plays a key role, i.e., cell death and inflammation. Necrotic cells are a primary source of HMGB1 (Scaffidi et al. 2002; Raucci et al. 2007). HMGB1 released by necrotic cells appears to be oxidized: the molecular pathway by which oxidation takes place has not so far been elucidated (Urbonaviciute et al. 2009). Eventually, HMGB1 also undergoes oxidation in cells that die via apoptosis. Apoptosis is associated with the generation of reactive oxygen species (ROS) by mitochondria, which in turn oxidize the cytosine at position 106 (Kazama et al. 2008). As a consequence, HMGB1 extracellular functions are dramatically altered. This aspect has been studied verifying, in particular, the ability of the molecule to activate or tolerate the acquired immune response (Kazama et al. 2008), a feature that primarily depends on the action of HMGB1 on DCs (Manfredi et al. 2009; Dumitriu et al. 2007; Yang et al. 2007). This is of particular importance, since HMGB1 associates with nucleosomes that are generated during apoptotic cell death and that represent a key autoantigen in systemic autoimmunity (Urbonaviciute et al. 2008; Munoz et al. 2010b). The central role of HMGB1 in autoimmune diseases has been the topic of excellent recent reviews (Pisetsky et al. 2008; Abdulahad et al. 2010; Andersson and Harris 2010; Pisetsky 2010) and will not be discussed further here.
ROS generation is a common occurrence in living cells. In particular it occurs after activation of PRRs expressed by inflammatory cells. As a consequence, antioxidant responses are activated, which contribute to limit excessive oxidation of the inflamed environment (Rubartelli and Sitia 2009; Carta et al. 2009). The net effect of oxidant and anti-oxidant events might be important, given the exquisite sensitivity of HMGB1 to oxidation. An oxidized environment by inactivation of HMGB1 has been proposed to restrict the action of the molecule both temporally and spatially, thus focusing it when and where it is needed. Conversely, a reduced environment might contribute to maintaining and prolonging HMGB1 bioactivity (Carta et al. 2009). An interesting feedback loop has been recently identified: HMGB1 promotes the survival and the activation of eosinophils, thus possibly providing a molecular explanation for their preferential recruitment within necrotic tissues. In turn, eosinophils respond to HMGB1 with an oxidative burst, and the generated gaseous species inactivate HMGB1 (Lotfi et al. 2009) possibly limiting the immunogenicity of antigens associated with necrotic tissues, including specifically tumor-associated antigens. Further support for a role of the redox potential in finely tuning the extracellular actions of HMGB1 is provided by data on the role of apurinic/apyrimidinic endonuclease 1/Redox factor-1 (APE1), a multifunctional protein that regulates the reduction-oxidation balance on HMGB1 release and on events downstream of HMGB1 recognition, including the activation of p38 and c-Jun N-terminal kinase, ROS generation, cytokine secretion, and cyclooxygenase-2 expression by monocytes and macrophage-like cells (Yuk et al. 2009).
Bound (or unbound) HMGB1
HMGB1 per se is well established as having mitogenic and chemoattractive properties (Rouhiainen et al. 2007). Moreover, HMGB1 triggers the release of cytokines from inflammatory leukocytes, although the pro-inflammatory effect of the recombinant molecule has been discussed (Rouhiainen et al. 2007). The reasons for these discrepancies have not so far been identified. Post-translational modification of the molecule, depending on the characteristics of the cells or on the environmental conditions (see above), could well yield molecules endowed with only partially overlapping extracellular functions.
The issue may be even more complex in vivo. HMGB1 is a molecule that “loves company” (Bianchi 2009) and has promiscuous habits. It forms relatively stable multi-molecular complexes with various substrates molecules. Some ligands per se interact, on cells, with receptors of the innate immunity system, including nucleic acids, PAMPs, and selected cytokines and chemokines. HMGB1-containing complexes are likely to be more the rule than an exception in inflamed tissues, being by definition characterized by the presence of cytokines, of microbial products, and of by-products of dying and activated cells. HMGB1 association stabilizes and complements the biological function of its substrate via the simultaneous or sequential activation of various PRRs (see below).
HMGB1 and LPS physically interact (Hreggvidsdottir et al. 2009; Youn et al. 2008). The complexes elicit the release of inflammatory cytokines more effectively than either molecule alone (Youn et al. 2008). HMGB1 therefore has the potential to act at inflammatory sites as a potent endogenous amplificatory signal, endowed with the ability to magnify the effects even of traces of bacterial components. Interestingly, separate A box and B box HMGB1 domains bind to LPS and enhance IL-6 production (Hreggvidsdottir et al. 2009). Inflammatory endogenous molecules also associate with HMGB1: this is the case for IL-1β (Sha et al. 2008). As described for LPS, HMGB1/IL-1β complexes are more effective than IL-1β alone and elicit a higher production of IL-6 (Hreggvidsdottir et al. 2009), of major intrinsic protein-2 and TNFα (Sha et al. 2008). The activity of HMGB1/IL-1β complexes is inhibited by adding, separately, neutralizing antibodies for the cytokine and its receptor, indicating that the complex acts through the IL-1β receptor. The actual mechanism(s) by which HMGB1 enhances and prolongs the activity of IL-1β, and possibly also of TNFα and interferon γ (Sha et al. 2008), has(have) not so far been identified.
The response to chemotactic signals is a critical issue in immunity; it requires that motile cells are recruited at inflammatory sites or reach lymphoid organs. HMGB1 is a crucial regulator of the fate and function of DCs (Dumitriu et al. 2005a, c; Andersson and Harris 2010; Rovere-Querini et al. 2004; Messmer et al. 2004; Semino et al. 2005; Ulloa and Messmer 2006; Apetoh et al. 2007a, b), professional antigen-presenting cells that connect innate and acquired immune responses. DCs, like most myeloid cells, translocate HMGB1 from the nucleus in the cytosol upon activation and eventually release the molecule into the extracellular environment (see above). Secreted HMGB1 is biologically active and required for DC maturation, a complex event by which activated DCs switch their responsiveness to chemokines from CCL5 to CCL21, thus acquiring the ability to migrate to the T cell zone of secondary lymph nodes (Randolph et al. 2005). Indeed, DCs acquire antigens in peripheral tissues and present them to T lymphocytes several hours later in the lymph nodes, i.e., at a distant site after a time-consuming journey. In the presence of inhibitors of HMGB1 or of one of its best-characterized receptors, the receptor for advanced glycated end-products (RAGE; see below), DCs activated with PAMPs or cytokines fail to mature (Dumitriu et al. 2005a, c). As a consequence, they fail to sustain T cell proliferation and survival and Th1 polarization (Dumitriu et al. 2005a, c), to migrate in response to the lymph-node chemokines CCL19 and CXCL12 (Dumitriu et al. 2007), and effectively to reach lymphoid organs in vivo (Manfredi et al. 2008b).
Conversely, maturing DCs that migrate in response to CXCL12 release HMGB1, which is required for CXCL12-dependent migration in vitro; the formation of complexes in the fluid phase between the two molecules maintains the conformation and function of CXCL12 in a reducing environment, such as that of the lymph-node. This is therefore possibly important for the attraction of antigen-presenting cells at the relevant sites at which T-cell-dependent immune responses begin (Campana et al. 2009). The regulation of the leukocyte recruitment in vivo clearly involves several steps, including the interaction between RAGE and leukocyte β2 integrins (Orlova et al. 2007). HMGB1 also up-regulates the expression and the sensitivity to TLR4 of maturing DCs; TLR4-dependent signaling on DC is required for HMGB1-mediated liver injury upon ischemia reperfusion (Klune et al. 2008; Tsung et al. 2005, 2007).
The intracellular function of HMGB1 strictly depends on its ability to bind to DNA. Therefore, unsurprisingly, nucleic acids represent a ligand for HMGB1, even outside the cell. Binding to HMGB1, as described for other substrates (see above), influences nucleic acid recognition by innate immune cells and, thus, their inflammatory properties. The chromatin of cells undergoing apoptosis undergoes extensive modifications that lead to a tight and long-lasting interaction with HMGB1, in stark contrast with the loose interaction of the molecule with the DNA of living or primary necrotic cells (Scaffidi et al. 2002). Apoptotic cells represent a source of HMGB1 and nucleosomes (Bell et al. 2006; Jiang et al. 2007; Pisetsky and Fairhurst 2007), which are released per se or as multimolecular complexes. Nucleosome/HMGB1 complexes can be traced in the blood of autoimmune patients and represent the unusual combination between an autoantigen (the nucleosome) and a natural adjuvant such as HMGB1, which is capable of conferring immunogenicity to antigenic soluble and particulate substrates (Rovere-Querini et al. 2004). Indeed, HMGB1/nucleosome complexes effectively activate innate immune cells, including DCs and macrophages in vitro, while triggering the production of anti-histone and anti-double-stranded DNA in experimental animals (Urbonaviciute et al. 2008). The complexes might therefore be involved in the original breakdown of tolerance associated with deregulated or uncleared apoptosis, which then fosters the development, in appropriate genetic backgrounds, of self-sustaining autoimmune diseases (Bondanza 2004; Mahoney and Rosen 2005; Bondanza et al. 2007; Rovere-Querini et al. 2007; Munoz et al. 2009, 2010a).
Nucleic acids have long been known to trigger the release of cytokines, including type 1 interferons and chemokines. This event is mediated by the activation of dedicated PRRs expressed either on the cell membrane or within the cell (Latz et al. 2004; Marshak-Rothstein and Rifkin 2007). The requirement of the HMGB proteins (see above) for the innate recognition of nucleic acids has been elegantly demonstrated by using genetic tools (Yanai et al. 2009), although the hierarchy of the associated molecular events needs to be characterized at molecular levels. HMGB1 effectively binds to synthetic sequences containing unmethylated cytosine-guanine (CpG) dinucleotides. These oligonucleotides mimic hypomethylated microbial DNA, which exerts inflammatory and immunostimulatory actions mostly via the activation of endosomal TLR9. CpG containing sequences trigger the secretion of HMGB1 from macrophages and DCs; in turn, HMGB1 favors the access of the molecule to the receptor (Ivanov et al. 2007), thus magnifying the cytokine release downstream of TLR9 activation (Hreggvidsdottir et al. 2009; Yanai et al. 2009). The involvement of RAGE in TLR9-MyD88-mediated cytokine production has also been clearly defined (Tian et al. 2007).
HMGB1: uno, nessuno, centomila (one, none, one hundred thousand)
The plurality of effects of HMGB1 on innate immune cells, mediated via direct or indirect actions on multiple PRRs (Hreggvidsdottir et al. 2009; Rauvala and Rouhiainen 2010; H. Yang et al. 2010; Andersson et al. 2000; Sims et al. 2010; Fig. 2) that converge on the activation of pathways dependent on mitogen-activated protein kinase and nuclear factor-κB (NF-κB; Palumbo et al. 2007, 2009; Penzo et al. 2010), provides a reason for its potent activity as a signal of necrosis (Raucci et al. 2007) triggering inflammation and tissue repair (Sims et al. 2010). HMGB1 acts on stem and precursor cells, recruiting and activating them at sites of damage and injury (Bianchi and Manfredi 2007; Palumbo et al. 2004, 2007, 2009; Limana et al. 2005; Chavakis et al. 2007; Germani et al. 2007; Lolmede et al. 2009).
The many lives of HMGB1: a molecule that shapes inflammation and tissue repair and that depends on environmental conditions and interactions with selected substrates and receptors (TM thrombomodulin, LPS lipopolysaccharide, IL-1β interleukin-1β, IFNγ interferon γ, TNFα tumor necrosis factor α, CXCL-12 a lymph-node chemokine, RAGE receptor for advanced glycation end-products, TLRs Toll-like receptors, CD24 a glycosylated glycosyl-phosphatidyl-inositol-anchored membrane protein expressed by immune and stem cells, NS nucleosomes)
This action is physiologically important for wound healing and tissue regeneration. Conversely, HMGB1 plays a major role not only in inflammatory and autoimmune diseases, but also in conditions as diverse as cancer and ictogenesis (Mittal et al. 2010; Maroso and Balosso 2010) in which persistent activation of inflammatory and reparative pathways leads to inappropriate tissue remodeling (Vakkila and Lotze 2004; Zeh and Lotze 2005). These studies highlight the potentially noxious outcomes of sustained HMGB1 release in the environment and imply that mechanisms exist that physiologically restrict the biological activity of the molecule. Two such mechanisms involve thrombomodulin (TM) and CD24.
TM is a transmembrane protein that regulates hemostasis through interactions with thrombin; it has been shown to quench HMGB1 inflammatory action by sequestering it via the N-terminal lectin-like domain (Abeyama et al. 2005; Koutsi et al. 2008) and by promoting the proteolytic cleavage of HMGB1 by thrombin (Ito et al. 2008). In vivo, the recombinant human soluble TM reduces HMGB1 levels and increases the survival of rats challenged with LPS (Nagato et al. 2009). The anti-inflammatory activity of the molecule and its ability to reduce HMGB1 levels after LPS challenge have been confirmed by using hTM transgenic mice (Crikis et al. 2010).
HMGB1 also interacts with CD24, a glycosylated glycosyl-phosphatidyl-inositol-anchored membrane protein expressed by immune and stem cells. As a consequence, Siglec 10 is recruited, which contains an immune receptor tyrosine-based inhibitory motif (ITIM); the result is the activation of a negative feedback forward loop, which prevents HMGB1-elicited inflammation by inhibiting NF-kB activation. CD24-/- and siglec 10-/- mice are exquisitely sensitive to the systemic effects of endogenous DAMPs, such as HMGB1. In contrast, they are normally resistant to the effects of PAMPs (Bianchi and Manfredi 2009; Chen et al. 2009; Liu et al. 2009). These data hint at an unusual scenario in which HMGB1 differs from PAMPs on the basis that it simultaneously activates inflammation via activatory PRRs (see above) and a CD24/Siglec-10-dependent regulatory pathway. This is possibly advantageous, restraining the ability of HMGB1 to activate inflammation and immunity under conditions of sterile tissue injuries, including vascular diseases (Maugeri et al. 2009) and ischemia/reperfusion (Chavakis et al. 2007).
A failure of these and most likely of other negative feed-back regulatory circuits underlie diseases attributable to the deregulated activation of innate immunity. Sepsis is a typical example; it is the leading cause of death in intensive care units in developed high-income countries and represents an urgent and unmet medical need. The first evidence that links HMGB1 to sepsis was obtained more than ten years ago when, in a pioneering study, HMGB1 was identified as a late mediator of lethal systemic inflammation and as being involved in the delayed lethality of endotoxin and systemic inflammation (Wang et al. 1999). Since then, we have gained a better insight into the underling mechanisms, and preclinical studies have validated the possibility of targeting HMGB1 as a therapeutic agent, by using independent approaches (Sims et al. 2010), including anti-HMGB1 antibodies and the A box fragment of HMGB1, which has antagonistic actions. Recently, encouraging results have been obtained, including the blocking of RAGE-HMGB1 signaling (Susa et al. 2009) and, as discussed above, exploiting the regulatory properties of TM (Nagato et al. 2009; Crikis et al. 2010). The identification of HMGB1 polymorphisms as significant factors associated with early and late mortality systemic inflammatory response syndrome and sepsis hints at a possible role for HMGB1 genetics in predictive medicine (Kornblit et al. 2008, 2010).
HMGB1 has also been linked also to tumor formation, progression, and metastasis and to the responses to chemotherapeutics. Its expression is elevated in several solid tumors, and HMGB1 serum levels are often associated with worse prognosis (Sims et al. 2010; Chung et al. 2009; Sparvero et al. 2009). On the other hand, HMGB1 plays a role in the immune responses against tumors elicited by conventional therapies. HMGB1 is released from irradiated and doxorubicin-treated tumor cells, and through TLR4, HMGB1 is efficient in activating DCs to cross-present tumor antigens, suggesting a dual role for the molecule (Apetoh et al. 2007a, b; Campana et al. 2008). The redox state of HMGB1 is important in this context. Reduced HMGB1 binds to RAGE, but not to TLR4, promoting tumor resistance to chemotherapeutic agents such as melphalan, paclitaxel, UV, and oxaliplatin. Oxidized HMGB1, in contrast, apparently increases the cytotoxicity of the agents, with the eventual death of tumor cells (Tang et al. 2010).
Concluding remarks
HMGB1 has many lives (Muller et al. 2001). The concentrated efforts of several groups have revealed some of them, providing hints regarding its multi-layer actions as a master regulator of innate immunity. The studies of the last few years suggest that the possible function of HMGB1 reflects the variable conditions of the extra-cellular environment, by signaling to immune cells the need for an acute and immediate response or for stem cell activation and wound repair, depending on the post-translational modifications of the molecule and/or on the array of substrates with which HMGB1 preferentially interacts. Other DAMPs are possibly more potent at immediately activating the inflammatory response to cell death (Chen et al. 2007; Rock and Kono 2008; Zhang et al. 2010; Manfredi and Rovere-Querini 2010). However, the versatility of HMGB1 makes it an intriguing molecule for unraveling the plasticity of innate immunity; it acts immediately under dangerous conditions and selects, in any given tissue and depending on the nature of injury or of the offending agent, the most appropriate (more effective and less harmful) response to be made.
References
Abdulahad DA, Westra J, Limburg PC, Kallenberg CG, Bijl M (2010) HMGB1 in systemic lupus erythematosus: its role in cutaneous lesions development. Autoimmun Rev 9:661-665
Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M et al (2005) The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 115:1267–1274
Andersson U, Harris HE (2010) The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta 1799:141–148
Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H et al (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192:565–570
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A et al (2007a) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059
Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R et al (2007b) The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 220:47–59
Banchereau J, Pascual V (2006) Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25:383–392
Bell CW, Jiang W, Reich CF 3rd, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291:C1318–C1325
Bianchi ME (2009) HMGB1 loves company. J Leukoc Biol 86:573–576
Bianchi ME, Manfredi A (2004) Chromatin and cell death. Biochim Biophys Acta 1677:181–186
Bianchi ME, Manfredi AA (2007) High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 220:35–46
Bianchi ME, Manfredi AA (2009) Immunology. Dangers in and out. Science 323:1683–1684
Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A et al (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22:5551–5560
Bondanza A, Zimmermann VS, Dell'Antonio G, Dal Cin E, Balestrieri G, Tincani A et al (2004) Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum 50:1549–1560
Bondanza A, Rovere-Querini P, Zimmermann VS, Balestrieri G, Tincani A, Sabbadini MG et al (2007) Requirement for dendritic cells in the establishment of anti-phospholipid antibodies. Autoimmunity 40:302–306
Campana L, Bosurgi L, Rovere-Querini P (2008) HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol 20:518–523
Campana L, Bosurgi L, Bianchi ME, Manfredi AA, Rovere-Querini P (2009) Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol 86:609–615
Carta S, Castellani P, Delfino L, Tassi S, Vene R, Rubartelli A (2009) DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol 86:549–555
Catena R, Escoffier E, Caron C, Khochbin S, Martianov I, Davidson I (2009) HMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatids. Biol Reprod 80:358–366
Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P et al (2007) Highmobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res 100:204–212
Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL (2007) Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 13:851–856
Chen GY, Tang J, Zheng P, Liu Y (2009) CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323:1722–1725
Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, Lim JB (2009) Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med 7:38
Crikis S, Zhang XM, Dezfouli S, Dwyer KM, Murray-Segal LM, Salvaris E et al (2010) Antiinflammatory and anticoagulant effects of transgenic expression of human thrombomodulin in mice. Am J Transplant 10:242–250
Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP et al (2005a) Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174:7506–7515
Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P (2005b) HMGB1: guiding immunity from within. Trends Immunol 26:381–387
Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P (2005c) Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol 25:25
Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P (2007) The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 81:84–91
Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME et al (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3:995–1001
Germani A, Limana F, Capogrossi MC (2007) Pivotal advances: high-mobility group box 1 protein—a cytokine with a role in cardiac repair. J Leukoc Biol 81:41–45
Hoppe G, Talcott KE, Bhattacharya SK, Crabb JW, Sears JE (2006) Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res 312:3526–3538
Hreggvidsdottir HS, Ostberg T, Wahamaa H, Schierbeck H, Aveberger AC, Klevenvall L et al (2009) The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol 86:655–662
Ito I, Fukazawa J, Yoshida M (2007) Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem 282:16336–16344
Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H et al (2008) Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol 28:1825–1830
Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G et al (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110:1970–1981
Janeway CA Jr (1992) The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 13:11–16
Jiang W, Bell CW, Pisetsky DS (2007) The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol 178:6495–6503
Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA (2008) Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high mobility group box-1 protein. Immunity 29:21–32
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A (2008) HMGB1: endogenous danger signaling. Mol Med 14:476–484
Knapp S, Muller S, Digilio G, Bonaldi T, Bianchi ME, Musco G (2004) The long acidic tail of high mobility group box 1 (HMGB1) protein forms an extended and flexible structure that interacts with specific residues within and between the HMG boxes. Biochemistry 43:11992–11997
Kornblit B, Munthe-Fog L, Madsen HO, Strøm J, Vindeløv L, Garred P (2008) Association of HMGB1 polymorphisms with outcome in patients with systemic inflammatory response syndrome. Crit Care 12:R83
Kornblit B, Masmas T, Petersen SL, Madsen HO, Heilmann C, Schejbel L, Sengeløv H, Müller K, Garred P, Vindeløv L (2010) Association of HMGB1 polymorphisms with outcome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 16:239–252
Koutsi A, Papapanagiotou A, Papavassiliou AG (2008) Thrombomodulin: from haemostasis to inflammation and tumourigenesis. Int J Biochem Cell Biol 40:1669–1673
Krynetskaia NF, Phadke MS, Jadhav SH, Krynetskiy EY (2009) Chromatin-associated proteins HMGB1/2 and PDIA3 trigger cellular response to chemotherapy-induced DNA damage. Mol Cancer Ther 8:864–872
Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF et al (2004) TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5:190–198
Lee D, Kwon JH, Kim EH, Kim ES, Choi KY (2010) HMGB2 stabilizes p53 by interfering with E6/E6AP-mediated p53 degradation in human papillomavirus-positive HeLa cells. Cancer Lett 292:125–132
Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X et al (2003) Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med 9:37–45
Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G et al (2005) Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res 97:e73–e83
Liu Y, Chen GY, Zheng P (2009) CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol 30:557–561
Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E et al (2009) Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol 85:779–787
Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A et al (2009) Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol 183:5023–5031
Lotze MT, Deisseroth A, Rubartelli A (2007) Damage associated molecular pattern molecules. Clin Immunol 124:1–4
Mahoney JA, Rosen A (2005) Apoptosis and autoimmunity. Curr Opin Immunol 17:583–588
Manfredi AA, Rovere-Querini P (2010) The mitochondrion—a Trojan horse that kicks off inflammation? N Engl J Med 362:2132–2134
Manfredi AA, Rovere-Querini P, Bottazzi B, Garlanda C, Mantovani A (2008a) Pentraxins, humoral innate immunity and tissue injury. Curr Opin Immunol 20:538–544
Manfredi AA, Capobianco A, Esposito A, De Cobelli F, Canu T, Monno A et al (2008b) Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J Immunol 180:2270–2275
Manfredi AA, Capobianco A, Bianchi ME, Rovere-Querini P (2009) Regulation of dendriticand T-cell fate by injury-associated endogenous signals. Crit Rev Immunol 29:69–86
Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM et al (2010) A causative role of Toll-like receptor 4 (TLR4) activation by high mobility group box 1 (HMGB1) protein in ictogenesis. Nat Med 16:413–419
Marshak-Rothstein A, Rifkin IR (2007) Immunologically active autoantigens: the role of tolllike receptors in the development of chronic inflammatory disease. Annu Rev Immunol 25:419–441
Matzinger P (2002) The danger model: a renewed sense of self. Science 296:301–305
Maugeri N, Rovere-Querini P, Baldini M, Sabbadini MG, Manfredi AA (2009) Translational mini-review series on immunology of vascular disease: mechanisms of vascular inflammation and remodelling in systemic vasculitis. Clin Exp Immunol 156:395–404
Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B et al (2004) High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 173:307–313
Mittal D, Saccheri F, Vénéreau E, Pusterla T, Bianchi ME, Rescigno M (2010) TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J 29:2242-2252
Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A et al (2001) New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J 20:4337–4340
Muller S, Ronfani L, Bianchi ME (2004) Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med 255:332–343
Munoz LE, Janko C, Grossmayer GE, Frey B, Voll RE, Kern P et al (2009) Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis Rheum 60:1733–1742
Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M (2010a) The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 6:280–289
Munoz L, Lauber K, Schiller M, Manfredi AA, Herrmann M (2010b) Defective clearance of apoptotic cells—role in the etiology and pathogenesis of systemic autoimmunity. Nat Rev Rheumatol 6:280–289
Nagato M, Okamoto K, Abe Y, Higure A, Yamaguchi K (2009) Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit Care Med 37:2181–2186
Nathan C (2002) Points of control in inflammation. Nature 420:846–852
Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE et al (2009) HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol 182:5800–5809
Oppenheim JJ, Yang D (2005) Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 17:359–365
Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E et al (2007) A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J 26:1129–1139
Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A et al (2004) Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 164:441–449
Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB et al (2007) Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NFkappaB activation. J Cell Biol 179:33–40
Palumbo R, De Marchis F, Pusterla T, Conti A, Alessio M, Bianchi ME (2009) Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol 86:617–623
Penzo M, Molteni R, Suda T, Samaniego S, Raucci A, Habiel DM et al (2010) Inhibitor of NF-kappa B kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis. J Immunol 184:4497–4509
Pisetsky DS (2010) HMGB1: a dangerous player in lupus pathogenesis. J Rheumatol 37:689–691
Pisetsky DS, Fairhurst AM (2007) The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity 40:281–284
Pisetsky DS, Ronnblom L (2009) Systemic lupus erythematosus: a matter of life and death. Arthritis Rheum 60:1567–1570
Pisetsky DS, Erlandsson-Harris H, Andersson U (2008) High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther 10:209
Pulendran B, Palucka K, Banchereau J (2001) Sensing pathogens and tuning immune responses. Science 293:253–256
Pusterla T, De Marchis F, Palumbo R, Bianchi ME (2009) High mobility group B2 is secreted by myeloid cells and has mitogenic and chemoattractant activities similar to high mobility group B1. Autoimmunity 42:308–310
Randolph GJ, Angeli V, Swartz MA (2005) Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5:617–628
Raucci A, Palumbo R, Bianchi ME (2007) HMGB1: a signal of necrosis. Autoimmunity 40:285–289
Rauvala H, Rouhiainen A (2010) Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta 1799:164–170
Rock KL, Kono H (2008) The inflammatory response to cell death. Annu Rev Pathol 3:99–126
Rouhiainen A, Imai S, Rauvala H, Parkkinen J (2000) Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb Haemost 84:1087–1094
Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H (2007) Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J Leukoc Biol 81:49–58
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M et al (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5:825–830
Rovere-Querini P, Castiglioni MT, Sabbadini MG, Manfredi AA (2007) Signals of cell death and tissue turnover during physiological pregnancy, pre-eclampsia, and autoimmunity. Autoimmunity 40:290–294
Rubartelli A, Sitia R (2009) Stress as an intercellular signal: the emergence of stress-associated molecular patterns (SAMP). Antioxid Redox Signal 11:2621–2629
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Semino C, Angelini G, Poggi A, Rubartelli A (2005) NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 106:609–616
Sessa L, Bianchi ME (2007) The evolution of high mobility group box (HMGB) chromatin proteins in multicellular animals. Gene 387:133–140
Sha Y, Zmijewski J, Xu Z, Abraham E (2008) HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 180:2531–2537
Shaw TJ, Martin P (2009) Wound repair at a glance. J Cell Sci 122:3209–3213
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ (2010) HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367–388
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J et al (2009) RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7:17
Stros M (2010) HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta 1799:101–113
Susa Y, Masuda Y, Imaizumi H, Namiki A (2009) Neutralization of receptor for advanced glycation end-products and high mobility group box-1 attenuates septic diaphragm dysfunction in rats with peritonitis. Crit Care Med 37:2619–2624
Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE et al (2010) HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene (in press)
Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME et al (2009) Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci USA 106:1181-1186
Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H et al (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496
Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT et al (2005) The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201:1135–1143
Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X et al (2007) HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 204(12):2913–2923
Ugrinova I, Pashev IG, Pasheva EA (2009) Nucleosome binding properties and core-modeling activities of native and in vivo acetylated HMGB-1 and HMGB-2 proteins. Biochemistry 48:6502–6507
Ulloa L, Messmer D (2006) High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 28:28
Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F et al (2008) Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 205:3007–3018
Urbonaviciute V, Meister S, Furnrohr BG, Frey B, Guckel E, Schett G et al (2009) Oxidation of the alarmin high-mobility group box 1 protein (HMGB1) during apoptosis. Autoimmunity 42:305–307
Vakkila J, Lotze MT (2004) Inflammation and necrosis promote tumour growth. Nat Rev Immunol 4:641–648
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Wixted WE, Kitson C, Colebrook JC, Roberts EJ, Fox SM, Kou JP et al (2010) A model to identify novel targets involved in oxidative stress-induced apoptosis in human lung epithelial cells by RNA interference. Toxicol In Vitro 24:310–318
Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H et al (2009) HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462:99–103
Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ (2007) High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 81:59–66
Yang D, Tewary P, Rosa G de la, Wei F, Oppenheim JJ (2010) The alarmin functions of high-mobility group proteins. Biochim Biophys Acta 1799:157–163
Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J et al (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942-11947
Youn JH, Shin JS (2006) Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol 177:7889–7897
Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS (2008) High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol 180:5067–5074
Yuk JM, Yang CS, Shin DM, Kim KK, Lee SK, Song YJ et al (2009) A dual regulatory role of apurinic/apyrimidinic endonuclease 1/redox factor-1 in HMGB1-induced inflammatory responses. Antioxid Redox Signal 11:575–588
Zeh HJ 3rd, Lotze MT (2005) Addicted to death: invasive cancer and the immune response to unscheduled cell death. J Immunother 28:1–9
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W et al (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107
Acknowledgements
The work in the authors’ laboratories is supported by the Ministero della Salute, by the AIRC (Associazione Italiana per la Ricerca sul Cancro) and by the MIUR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castiglioni, A., Canti, V., Rovere-Querini, P. et al. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res 343, 189–199 (2011). https://doi.org/10.1007/s00441-010-1033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1033-1