Abstract
The chemical composition of the luminal content is now accepted to have a profound influence on the performance of chemosensory receptors. Gustatory and intestinal chemoreceptors have in common their expression of molecules involved in taste sensing and signal transduction pathways. The recent finding that enterocytes of the duodenal epithelium are capable of expressing luminal pancreatic amylase suggests that taste cells of the gustatory epithelium might, in the same way, express salivary amylase in the oral cavity. Therefore, we investigated amylase expression in rat circumvallate papillae by using analyses involving immunohistochemistry, Western blot, and reverse transcription with the polymerase chain reaction. In addition, we used double-labeling confocal laser microscopy to compare amylase immunolabeling with that of the following markers: protein gene product 9.5 (PGP 9.5) and chromogranin A (CgA) for endocrine cells, α-gustducin and phospholipase C beta 2 (PLCβ2) as taste-signaling molecules, and cystic fibrosis transmembrane regulator (CFTR) and Clara-cell-specific secretory protein of 10-kDa (CC10) as secretory markers. The results showed that amylase was present in some taste bud cells; its immunoreactivity was observed in subsets of cells that expressed CgA, α-gustducin, PLCβ2, CFTR, or CC10. PGP 9.5 immunoreactivity was never colocalized with amylase. The data suggest that amylase-positive cells constitute an additional subset of taste receptor cells also associated with chemoreceptorial and/or secretory molecules, confirming the occurrence of various pathways in taste buds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Von Ebner’s glands (VEGs), also called posterior deep lingual glands, are minor salivary glands located beneath the circumvallate papillae (CP). They are almost purely serous glands that secrete a variety of proteins under both sympathetic and parasympathetic control. Their major secretory product is amylase, a digestive enzyme that begins the hydrolysis of starch components, glycogen, and various oligosaccharides in the mouth, breaking them down into monosaccharides, i.e., mostly glucose, galactose, and fructose (Tremblay and Charest 1968; Field et al. 1989). VEGs drain their secretions directly into the bottom of the grooves surrounding the CP (Azzali et al. 1989; Riva et al. 1999) with a constant flow of fluid on the gustatory mucosa. This flow supplies the microenvironment (peri-receptor milieu) of taste receptor cells (TRCs) and is also maintained by secretions of the taste bud cells. The peri-receptor milieu of TRCs removes food, permits the solubility of taste substances and their diffusion to taste receptors, and provides a continuously renewed aqueous milieu (Mese and Matsuo 2007). Their morphological and secretory characteristics and their anatomical relationship with taste buds make VEGs important in taste-reception mechanisms (Gurkan and Bradley 1987, 1988; Spielman 1990; Matsuo 2000). CP and VEGs have also been hypothesized to constitute a single functional unit of the peripheral taste mechanism, in which TRCs and VEGs represent the chemoreceptive and secretory parts, respectively (Sbarbati et al. 1999), a structural organization resembling that of other tissues, such as pancreatic and biliary ducts (Höfer and Drenckhahn 1998).
The TRCs of the CP are sensory receptors involved in taste-transduction mechanisms. They are clustered in taste buds and extend from the base to the apex of the bud. The apical surface protrudes with microvilli or club-shaped apical processes into the taste pore, which communicates with the oral cavity. The apical membrane is the location of receptors or ion channels involved in taste transduction, which interact directly with gustatory stimuli (Kinnamon and Margolskee 1996).
The assumption is now generally accepted that epithelia exposed to the luminal environment possess chemoreceptors capable of sensing the chemical components of the luminal contents and of releasing molecules that can act through mechanisms resembling those operating in the gustatory epithelium (Höfer et al. 1996; Wu et al. 2002; Dockray 2003). On this basis, several immunohistochemical similiarities have been identified between TRCs and epithelial cells of various structures. Many recent studies have revealed that the expression of receptors for tastants, e.g., T1R and T2R, and of molecules that mediate gustatory signals, e.g., α-gustducin, phospholipase C beta 2 (PLCβ2), and inositol 1, 4, 5-trisphosphate receptor) is not restricted to TRCs but is also detected in the respiratory (Zancanaro et al. 1999; Finger et al. 2003; Sbarbati et al. 2004; Merigo et al. 2005, 2007; Tizzano et al. 2006) and in the gastrointestinal (Höfer et al. 1996, 1999; Wu et al. 2002; Dyer et al. 2005, Rozengurt et al. 2006; Bezencon et al. 2007; Hass et al. 2007; Mace et al. 2007; Margolskee et al. 2007; Sternini 2007; Sutherland et al. 2007) structures. In particular, in the rodent and human gastrointestinal tracts, the expression of taste-signaling elements of the gustatory epithelium has been interpreted as clear evidence for the ability of intestinal cells to activate chemosensory responses through mechanisms dependent on α-gustducin and PLCβ2 (Jang et al. 2007; Margolskee et al. 2007) and on chromogranin A (CgA; Rozengurt 2006; Rozengurt et al. 2006; Wu et al. 2002; Sternini 2007; Sternini et al. 2008). Various epithelial cell populations have been identified as chemosensory cell types in the gastrointestinal tract. They include chemosensory cells (i.e., brush cells, solitary chemosensory cells, enteroendocrine cells) and enterocytes and Paneth cells, which have the cytological characteristics of absorptive cells or serous /zimogenic cells, respectively (Höfer and Drenckhahn 1998; Sbarbati and Osculati 2005; Bezencon et al. 2007; Hass et al. 2007; Mace et al. 2007).
Current lines of evidence suggest that, in the gastrointestinal tract, the chemical composition of the luminal content has a profound influence on the performance of chemosensory cells, which can influence, by means of their signaling cascades, several physiological functions, including secretion, absorption, and protective mechanisms (Hass et al. 2007). This assumption makes it possible that the localization of certain proteins in various cellular compartments is cell-specific and dependent on the content of the luminal milieu. In this regard, a recent report has shown that rat enterocytes are capable of absorbing pancreatic amylase from the duodenal lumen and transfering it to the connective tissue by a transcytosis pathway that appears to be related to levels of pancreatic amylase in the duodenal lumen (Cloutier et al. 2006). The duodenal lumen has an anatomical relationship with the exocrine pancreas, which, via the pancreatic duct, drains pancreatic amylase onto the surface of the duodenal epithelium. This structural organization resembles that existing in the oral cavity, where the ducts of VEGs open onto the base of the trench wall of the CP and release salivary amylase into the groove surrounding the CP. Thus, given the occurrence of amylase in both the duodenal lumen and the oral cavity and the evident immunophenotypical similiarities between the gustatory and intestinal epithelia, the TRCs could be capable, like enterocytes, of expressing amylase.
To test this hypothesis, we have investigated the presence of amylase in TRCs of rat CP by using immunohistochemistry, Western blot, and reverse transcription with the polymerase chain reaction (RT-PCR). Subsequently, in order to characterize further the phenotype of amylase-immunoreactive cells, we have assessed the colocalization of amylase with other known markers of TRCs by using confocal laser scanning microscopy. We have selected, as markers, the following proteins: protein gene product 9.5 (PGP 9.5) and CgA as markers of endocrine cells; α-gustducin (an α subunit of the heterotrimeric G protein complex) and PLCβ2 (an effector enzyme of the taste-signaling pathway) as chemoreceptor markers, and cystic fibrosis transmembrane regulator (CFTR) and Clara-cell-specific secretory protein of 10-kDa (CC10) as secretory markers recently identified by us in TRCs (Merigo et al. 2008).
Materials and methods
Tissue preparation
The study was conducted on 14 adult Wistar rats of both sexes (150-200 g; Morini Company, Reggio Emilia, Italy) kept at the departmental animal facility. The rats were handled in accordance with the guidelines for animal experimentation as laid down by Italian law. The animals were anesthetized with ether and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The tongue of each rat was removed and further fixed by immersion in the same fixative for 2 h at 4° C. After being rinsed in 0.1 M PB, the tissues were placed into 30% sucrose overnight and cut (40 μm thickness) on a freezing microtome (Reichert-Jung, Vienna, Austria). Sections from nine animals were processed both for immunoperoxidase and single immunofluorescence labeling, and sections from five animals for double-immunofluorescence labeling. Two or three sections per animal were used for each immunohistochemical experiment.
Primary antibodies
For this study, we selected two anti-amylase antibodies, both directed against salivary amylase of human origin and raised in different animal species: a polyclonal anti-amylase raised in rabbit (Sigma-Aldrich, Milan, Italy; cat. no. A 8273) and a monoclonal anti-amylase raised in mouse (Santa Cruz Biotechnology, Heidelberg, Germany; cat. no. sc-46657). These antibodies showed comparable staining.
The following specific markers were used in double-labeled staining: anti-CC10 (Santa Cruz Biotechnology; cat. no. sc-25555); anti-CFTR (Alomone Labs, Jerusalem, Israel; cat. no. ACL-006); anti-CgA (Thermo Fisher Scientific, Fremont, Calif., USA; cat. no. RB-9003); anti-α-gustducin (Santa Cruz Biotechnology; cat. no. sc-395 ); anti-PGP 9.5 (UltraClone, Isle of White, UK); anti-PLCβ2 (Santa Cruz Biotechnology; cat. no. sc-206).
Immunohistochemistry
Sections were blocked for 1 h in blocking solution consisting of 0.3% Triton X-100, 1% bovine serum albumin (BSA), and 1% normal swine serum in 0.1 M phosphate-buffered saline (PBS); the same solution was used to dilute the antibodies. Immunohistochemical staining was performed by using the avidin-biotin complex (ABC) technique. Briefly, endogenous peroxidase was quenched by immersion in a solution of 0.3% hydrogen peroxide in methanol for 30 min. After being washed in 0.05 M TRIS-HCL buffer pH 7.6, the sections were treated with 5% normal rabbit serum for 20 min. Subsequently, sections were incubated overnight at 4°C with rabbit anti-amylase (1:1000) or mouse anti-amylase (1:1000) and then reacted with biotinylated swine anti-rabbit or rabbit anti-mouse immunoglobulins (DAKO, Milan, Italy; 1:400), respectively, for 2 h. Immunoreaction was detected by using a Vectastain Elite ABC kit (Vector, Burlingame, Calif., USA) and then visualized with 3.3’-diaminobenzidine tetrahydrocloride (DAKO) for 5-10 min. Finally, sections were collected on poly-lysine-coated slides and mounted with DAKO Faramount Aqueous Mounting Medium. Control sections were prepared by pre-absorbing the anti-amylase antibody with an excess of corresponding peptide (Sigma-Aldrich; cat. no. A0521; or Santa Cruz Biotechnology; cat. no. sc-31869P) or by omitting the primary antibody. The controls did not exhibit immunolabeling.
Sections were observed under an Olympus BX51 photomicroscope equipped with a KY-F58 CCD camera (JVC). Electronic images were analyzed and stored by using Image-ProPlus software (Media Cybernetics, Silver Springs, Md., USA).
Immunofluorescence
Single-labeling
Free-floating sections were blocked for 1 h in blocking solution. Subsequently, they were incubated overnight in rabbit or mouse anti-amylase antiserum at 4°C and, after washes, reacted with a secondary tetramethylrhodamine-isothiocyanate-conjugated goat anti-rabbit or anti-mouse IgG (Jackson Laboratories, West Grove, Pa., USA; 1:200), for 1 h at room temperature. Control sections were prepared by adding the corresponding peptide to the amylase antibody or by omitting the primary antibody.
Double-labeling
Double-labeled immunohistochemistry was used to determine any overlap between amylase and well-known taste cell markers, including PGP 9.5, CgA, α-gustducin, PLCβ2, CFTR, and CC10. Because these antibodies were all raised in rabbit, the double-labeling experiments were performed by using amylase antibody raised in mouse in order to avoid cross-reactivity of secondary antibodies. Free-floating sections were washed in PBS at room temperature and permeabilized for 1 h in blocking solution. Subsequently, sections were incubated overnight in a mixture of mouse monoclonal anti-amylase with rabbit polyclonal anti-PGP 9.5 (1:1000), anti-CgA (1:100), anti-α-gustducin (1:100), anti-PLCβ2 (1:100), anti-CFTR (1:200), or anti-CC10 (1:200). After washes, sections were then incubated in Cy3-conjugated affinity purified goat anti-mouse IgG (Jackson Laboratories; 1:200) and fluorescein-isothiocyanate-conjugated affinity purified goat anti-rabbit IgG (Jackson Laboratories; 1:200) for 2 h at room temperature. Finally, sections were mounted with fluorescent mounting medium (DAKO) and observed by using a Zeiss LSM 510 confocal microscope equipped with argon (488 nm) and helium/neon (543 nm) excitation beams. Sequential acquisition, i.e., one color at a time, was utilized on double-labeled tissues to avoid side-band excitation of the inappropriate fluorophore. All images for publication were composed in Adobe Photoshop software (version 6.0; Adobe Systems, Mountain View, Calif., USA) with adjustment of only brightness and contrast.
Control sections were prepared by one of the following methods: 1) addition of the corresponding peptide to the amylase antibody; 2) omission of the primary antibody; 3) replacement of the second primary antibody with normal rabbit serum; or 4) exchange of the fluorophore of the secondary antibodies. No controls exhibited immunolabeling.
Western blot analysis
A pool of four rats were used. The specificity of both amylase antibodies was assessed by Western blot analysis of proteins extracted from the CP and VEGs in the tongue. The areas of CP and VEGs were removed microscopically to assure that CP blocks did not contain VEGs. Tissue extracts from pancreas and kidney served as positive and negative controls, respectively. The protein extracts were quantified by using the Bradford method, denatured with sodium dodecyl sulfate (SDS) and 2-mercaptoethanol, and subsequently transferred to nitrocellulose membrane. The membrane was blocked in 5% BSA and 0.1% Tween 20 in 0.1 M TRIS-buffered saline (pH 7.4) and incubated for 24 h at 4°C with the mouse anti-amylase (Santa Cruz Biotechnology) or rabbit anti-amylase (Sigma-Aldrich) antibodies, both diluted 1:1000 in blocking solution. Horseradish-peroxidase-labeled goat anti-mouse or goat anti-rabbit IgGs (Vector Laboratories) were used as secondary antibodies at a working concentration of 0.5-5.0 μg/ml.
Bound immunoglobulins were visualized by means of the chemiluminescence reagent Luminol (Santa Cruz Biotechnology). Control experiments were performed with antibody pre-absorbed with the antigen peptide.
Total RNA isolation and RT-PCR
Total RNA was isolated from the same homogenate tissues utilized for the protein extraction by using Trizol reagent (Invitrogen, Life Technologies, Milan, Italy). Following spectrophotometric determination of total RNA content, samples of RNA (about 1 μg total RNA) from each tissue were digested with RNase-free DNase I Amp Grade (Invitrogen), reverse-transcribed to cDNA, and amplified with gene-specific primers by using the SuperScript First-Strand Synthesis System for a RT-PCR kit (Invitrogen). The following primer sequences were used: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-ACTGGCGTCTTCACCACCAT-3′ (forward) and 5′-ATCCACAGTCTTCTGGGTGG-3′ (reverse), product size: 273 bp; amylase, 5′-TGAAGCTGGACAAAGCAGTACATGC-3′ (forward) and 5’-GTGTCCGGATTTATGTGGATGCTG-3’(reverse), product size, 446 bp. The expression of GAPDH was used as the internal standard. PCR amplification was performed in an Eppendorf Mastercycler gradient at 95°C for 30 s, 60°C for 30 s, and 72°C for 15–30 s over 36 cycles. PCR products were identified on the basis of their size as determined by gel electrophoresis in 1.5% agarose gels.
Results
Amylase expression in TRC
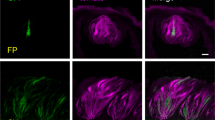
Immnunoperoxidase and immunofluorescence techniques, with both mouse and rabbit anti-amylase antibodies, were used to examine tissue sections containing CP and VEGs. The latter being a well-known site of amylase localization were employed here as positive controls. As expected, amylase immunoreactivity was detected in acinar cells of the salivary VEGs with a granular pattern; a similar pattern of staining was observed with both the anti-amylase antibodies used (Fig. 1a, b). Similar results were obtained in CP, where amylase immunoreactivity was observed in some bipolar or pear-shaped cells of taste buds (Fig. 1c-g), with the staining being homogeneously distributed throughout the cytoplasm of the entire cell. Amylase immunoreactivity was also present in the taste pore (Fig. 1d, g), the epithelium lining the top of the taste buds (Fig. 1e), and often inside the groove surrounding the CP (Fig. 1a, f). Immunoreactivity was not observed when the amylase antibody was preincubated with excess control peptide (Fig. 1h). Omission of the primary antibody also resulted in a complete loss of staining.
Immunoperoxidase (b-d, g, h) and immunofluorescent (a, e, f) staining showing amylase immunoreactivity in taste buds of rat circumvallate papillae (CP) and secretory acini of von Ebner’s glands (VEGs). Nuclei are stained with toluidine blue (g). The taste pore lies right in micrographs d-g. a, c, e, g Sections immunoreacted with mouse anti-amylase antibody. b, d, f Sections immunostained with rabbit anti-amylase antibody. h No labeling was observed when the anti-amylase antibody was pre-absorbed with its specific peptide. Bars 5 μm (g), 10 μm (d–f), 15 μm (c), 50 μm (a), 60 μm (b, h)
The specificity of the amylase antibodies was confirmed by Western blot analysis of membrane fractions from CP, VEGs, and pancreas. A band of approximately 53 kDa was observed in all the tissues, consistent with the reported molecular weight of the amylase expressed in pancreatic tissue extracts. On kidney tissue, the anti-amylase antibodies led to an absence of specific labeling (Fig. 2a). Preincubation of the amylase antibodies with their corresponding antigens also resulted in a complete absence of staining (Fig. 2b).
Detection of amylase protein by Western blotting. a Cellular proteins were probed with rabbit anti-amylase antibody. b Anti-amylase antibody was pre-incubated with the control peptide antigen. The gels were loaded with extracts of circumvallate papillae (CP), Von Ebner’s glands (VEGs), pancreas, and kidney (arrow in a 53-kDa amylase protein)
RT-PCR analysis indicated that a single band of the expected size for amylase mRNA, corresponding to a 446-bp product, was present in RNA extracted from rat CP, VEGs, and pancreas. In contrast, RT-PCR with RNA isolated from kidney did not detect any product (Fig. 3).
Colocalization of amylase with endocrine or taste-signaling markers in TRCs
Using double-immunostaining experiments and confocal laser scanner microscopy, we examined the expression pattern of amylase compared with that of PGP 9.5, CgA , α-gustducin, or PLCβ2. In addition, we counted the number of colocalized cells in two or three 40-μm-thick sections from each of three animals. Although a rigorous morphometric count was not carried out, the cell count was performed in longitudinally sectioned taste buds having a visible taste pore and in immunoreactive cells with a visible cell nucleus, in order to ensure that differences in cell counts were not attributable to different numbers of cells in the taste buds. We counted the number of cells that were immunoreactive for each antibody and those showing colocalization of both antigens.
Using anti-amylase and anti-PGP 9.5 antibodies, we found PGP 9.5 immunoreactivity in taste bud cells and in nerve fibers, which were distributed within and at the bottom of the taste buds. In no case was overlapping observed between amylase and PGP 9.5 staining (Fig. 4a-c).
Double-immunofluorescent confocal microscopy showing expression of amylase with protein gene product 9.5 (a-c PGP 9.5), chromogranin A (d-f CgA), α-gustducin (g-i), or phospholipase C beta 2 (j-l PLCβ2) in taste buds of rat circumvallate papillae. The co-expression pattern (yellow) is shown in the overlay (right). The taste pore is toward top in all micrographs. Bars 10 μm
Antibodies to amylase and CgA revealed that CgA immunoreactivity was present in taste bud cells, and that CgA-positive cells appeared more numerous than amylase-positive cells. The majority of amylase-positive cells colocalized CgA expression, but occasional cells were observed that were only amylase-positive. In a total of 42 taste buds, we counted 188 cells immunoreactive for CgA, 74 cells positive for amylase, and 71 cells positive for both the antigens. We estimated that the double-labeled cells (i.e., amylase+ CgA+) represented approximately 96% of all amylase-expressing cells and 38% of all CgA-expressing cells (Fig. 4d-f, Table 1).
Double-labeling experiments with amylase and α-gustducin showed more cells labeled with anti-α-gustducin antibody than with anti-amylase (175 and 110 cells, respectively). Some α-gustducin-immunoreactive cells also exhibited immunoreactivity to amylase, with an almost complete coincidence of labeling. Other amylase-positive cells lacked α-gustducin expression. In a total of 71 taste buds, 29% of all amylase-expressing cells displayed α-gustducin immunoreactivity, and 18% of all α-gustducin-positive cells expressed amylase (Fig. 4g-i, Table 1).
Further evidence for the association of amylase with taste-signaling elements was obtained with PLCβ2 antibody. Immunoreactive cells in 54 taste buds were counted, revealing that 47% of all amylase-expressing cells were also PLCβ2-positive. Colocalized staining was mainly observed in the perinuclear cytoplasm. A substantial number of PLCβ2-positive cells (83%) lacked amylase expression (Fig. 4j-l, Table 1).
Colocalization of amylase with CFTR and Clara cell secretory protein in TRCs
Since we had demonstrated in a previous study (Merigo et al. 2008) that TRCs expressed CFTR and CC10, here we compared their expression with that of amylase.
Comparison of amylase with CFTR revealed that, in 58 taste buds counted, more cells were labeled with antibody to CFTR than to with antibody against amylase (223 and 109 cells, respectively). Of all amylase-positive cells, 45% also displayed labeling for CFTR, which was mainly co-expressed in the perinuclear cytoplasm. A substantial number (78%) of CFTR-immunoreactive cells lacked amylase staining (Fig. 5a-c, Table 1).
Double-immunofluorescent confocal microscopy showing expression of amylase with cystic fibrosis transmembrane regulator (a-c CFTR) or Clara-cell-specific secretory protein of 10-kDa (d-f CC10). The co-expression pattern (yellow) is shown in the overlay (right). The specificity of the double-labeling procedure is demonstrated by the absence of labeling when the second primary antiserum was replaced with normal rabbit serum (g-i nrs). The taste pore is toward the right in a-f and toward the top in g-i. Bars 10 μm
Combining anti-amylase with anti-CC10 antibodies, we observed that, in 86 taste buds, cells expressing CC10 were more numerous than amylase-positive cells (346 and 153 cells, respectively). CC10 and amylase were colocalized in 86% of all amylase-expressing cells and 38% of all CC10-expressing cells, with the co-expression of labeling mainly being observed throughout the cytoplasm or in the perinuclear region (Fig. 5d-f, Table 1).
In dual-immunofluorescent staining, no specific double-labeling was seen when the second primary antibody was replaced with normal rabbit serum (Fig. 5g-i).
Discussion
Our immunohistochemistry, Western blot, and RT-PCR results provide the first evidence that amylase is expressed in TRCs of rat CP. In particular, the double-immunolabeling experiments have shown that subsets of amylase-immunoreactive cells display staining for molecules of endocrine marker (e.g., CgA), the taste-signaling cascade (e.g., α-gustducin, PLCβ2), or secretory markers (e.g., CFTR, CC10), suggesting that amylase localization occurs in heterogeneous populations of TRCs.
Amylase expression in TRCs
Sweet taste is stimulated by artificial sweeteners, certain peptides and proteins, and a variety of sweet compounds including mono-, di-, and tri-saccharides, and oligosaccharides derived from the partial hydrolysis of starch (Roper 2007). Rats are strongly attracted to the taste of starch-derived oligosaccharides and polysaccharides (Sclafani et al. 1987). The ability to detect starch-rich foods is regarded as an additional sense typical of rodents and appears to be innate, since it is manifest in newborn animals (Vigorito and Sclafani 1988). A behavioral test has demonstrated that rats are able to detect low concentrations of starch and to discriminate between different concentrations of starch molecules (Ramirez 1991). Surprisingly, the same study has revealed that desalivated rats do not lose their capacity to choose starch, suggesting that some mechanisms other than salivary amylase must be involved in starch detection.
Behavioral and genetic studies have demonstrated that polysaccharide taste differs from the sweet taste of sugar and is probably mediated by different receptors (Sclafani and Abrams 1986; Sclafani and Mann 1987; Sclafani et al. 1987; Sunderland and Sclafani 1988; Giza et al. 1991). Rats have been proposed to possess two types of carbohydrate taste receptors: one for sugar and the sweet taste and another for the polysaccharide taste (Sclafani and Mann 1987; Sclafani et al. 1987). Unfortunately, the hypothetical polysaccharide taste receptor has yet to be discovered, and the mechanism by which the cell senses starch-rich food has not been established (Sclafani 2004). Only recently, a behavioral assay in knockout (KO) mice lacking α-gustducin or TRPM5 (transient receptor potential ion channel 5) has demonstrated the involvement of α-gustducin and TRPM5 in the polysaccharide transduction pathway, indicating signaling elements common to sugar- and polysaccharide-signaling pathways (Sclafani et al. 2007).
Amylase is a metalloenzyme that requires calcium ions for its structural integrity (Steer and Levitzki 1973) and chloride ions for activity (Levitzki and Steer 1974). In animals, it is the major secretory enzyme of the pancreas and salivary glands (i.e., parotid and VEGs), but it is also present in blood, liver, and enterocytes, with a functional significance that has not been well defined (Rohr and Scheele 1983; Field et al. 2001; Cloutier et al. 2006).
In our study, some cells within each taste bud of CP have been found to be immunohistochemically positive for amylase, with intense immunoreactivity throughout the cytoplasm of the cell. Similarly, Western blot and RT-PCR analyses have shown that amylase is expressed in the CP and in VEGs and the pancreas, which are well-known sites of amylase localization. Our finding generates a number of important questions about the functional role of amylase in taste bud cells. However, an understanding of the significance of its presence in taste receptors requires functional and molecular studies and is beyond the scope of the present work. We can only suggest possible roles; this might prompt further investigation.
The first intriguing suggestion is that TRCs themselves might secrete amylase and collaborate in carbohydrate digestion with the concerted action of salivary amylase. This idea is supported by the finding of amylase immunoreactivity in the epithelium lining the top of the taste buds and in the taste pore. A local release of amylase by taste cells might bring about changes in the local environment of the taste pore because of chemical interaction between the amylase and taste substances. As the initial events in taste perception are affected by the external milieu of TRCs, the environmental changes perceived by the apical region of TRCs might in turn modulate taste perception by acting intracellularly and/or by cell-to-cell comunication with neighboring cells (Roper 2006).
Alternatively, amylase expression in taste buds might occur through an absorptive mechanism analogous to that observed in enterocytes (Cloutier et al. 2006). In this context, taste buds may be able to internalize amylase secreted from the salivary VEGs and parotid glands. The gustatory epithelium has been shown to possess a secretory capacity but has never been described as an absorptive epithelium. Nevertheless, the taste pore, considered to be the site at which TRC receptors interact with tastants, is a small environment filled with material that, in some way, hinders the extensive diffusion of saliva inside it. The substances most likely to build up inside it (albeit in small amounts) are those locally secreted by taste bud cells.
The possibility that the chemical components in the taste pore environment differ from those of saliva is also supported by experimental findings. In rabbit taste bud cells, the taste pore content has been observed to have a different composition and origin compared with that found throughout or at the top of the epithelial cells (Brouwer and Wiersma 1980). In addition, in taste pores of the foliate and CP, a different carbohydrate composition has been found compared with that of the fungiform papillae (Witt and Miller 1992).
Colocalization of amylase with endocrine or taste-signaling markers in TRCs
CgA is the major acid secretory protein in cells of the diffuse neuroendocrine system (i.e., endocrine, neuroendocrine, and neuronal cells; Helle 2004). Once secreted, chromogranin (or its derived peptides) activates other intracellular or extracellular targets through endocrine, autocrine, and paracrine interaction. In the gastro-entero-pancreatic endocrine system, CgA is considered a marker of enteroendocrine cells (Fischer-Colbrie et al. 1985; Hagn et al. 1986; Sternini et al. 2008), which have been found to be important in the chemosensory process of the gastrointestinal tract for their expression of taste-signaling molecules such as α-gustducin, transducin, and T1R2 and T1R3 sweet receptors (Höfer and Drenckhahn 1996; Rozengurt et al. 2006; Margolskee et al 2007; Jang et al. 2007). Recent studies have shown the expression of CgA in mouse taste buds (Dvoryanchikov et al. 2007; Kataoka et al. 2008); it is also colocalized with taste-signaling elements (i.e., α-gustducin, PKD2L1).
In the present study, CgA has been observed in several taste bud cells of CP; it is also co-expressed with amylase in the majority of cells expressing this enzyme (96%). In addition, amylase immunoreactivity has been colocalized with α-gustducin and PLCβ2, well-established markers of the taste-signaling mechanism, even though approximately two-thirds and one-half of the cells that are amylase-positive do not display α-gustducin or PLCβ2, respectively.
Taken together, our findings indicate that amylase is expressed in taste bud cells capable of propagating chemosensory information to downstream signaling molecules. One obvious explanation might be that amylase in the taste pore environment is able to break down the starch contained in ingested food so that it is more readily sensed by TRCs. In other words, the chemosensory information might be generated by a local build-up of sugar during carbohydrate hydrolysis; when directly exposed to a high sugar concentration, amylase-expressing cells and/or neighboring cells might operate as sensors for sweetness and/or starch in the peri-receptor milieu content and convey the detected taste signals either to intracellular targets or to neighboring cells, which, in turn, might act as effector cell types. The latter might be the PGP-9.5-expressing cells, which are considered to be able to make synapses with intragemmal nerves (Yee et al. 2001; Huang et al. 2003).
Colocalization of amylase with CFTR and Clara cell secretory protein in TRCs
CFTR and CC10 are secretory markers that have mostly been studied in the airways but have also identified in a wide variety of tissues. CFTR is a phoshorylation- and ATP-dependent chloride channel that regulates salt and water movement across many epithelia, including the intestinal and alveolar epithelia (Pilewski and Frizzell 1999; Quinton 1999). CC10 has been identified as a secretory product of Clara cells and has been studied for its protective effect against the inflammatory response and oxidative stress (Johnston et al. 1997; Mango et al. 1998)
We have previously demonstrated the expression of CFTR and CC10 in taste buds of the rat CP and their association, in some cells, with the expression pattern of α-gustducin (Merigo et al. 2008). We now show that a subset of CFTR- or CC10-positive cells is also amylase-immunoreactive (22% and 38%, respectively). Thus, taken together, our findings indicate that many taste cells are immunoreactive for CFTR and CC10, but only a subset of CFTR- and CC10-positive cells express amylase or α-gustducin. Possibly, α-gustducin and amylase are co-expressed in the same subset of immunoreactive cells, suggesting that they are involved in the same processes. However, the large number of CFTR- and CC10-positive cells implies additional roles in taste cells for these proteins.
CC10 and CFTR are involved in a variety of functions, which have been elucidated to some extent in our previous studies (Merigo et al. 2007; 2008). We have also tried to suggest that their role in TRCs is no different from their action in other tissues. Moreover, our finding that secretory cells of the rat airway epithelium express taste-transduction molecules (i.e., α-gustducin, PLCβ2), which are colocalized with CC10 and CFTR expression, suggests a link between secretory cell function and chemoreceptive processes. In the same way, the possible existence of a coupled mechanism between amylase and CFTR in chemoreceptor cells has implications for other functions, such as carbohydrate digestion and absorption.
Interestingly, a recent study has also shown the involvement of CC10 in paracrine signaling between secretory cells of the conducting airway and cells of the innate immune system (Reynolds et al. 2007). The connection between functionally distinct cell types suggests a molecular link between various processes, a link that could be critical in pathological events (Reynolds et al. 2007). Similarly, CC10 expression in taste cells might provide an important link in paracrine signaling networks, reinforcing defense capacity.
Concluding remarks
The presence of amylase in TRCs, whether it represents the expression of a secretory event or an absorptive mechanism, can be considered as a way to propagate chemosensory information from the peri-receptor milieu to other intracellular or extracellular targets, reinforcing the influence of the oral cavity content on taste receptors.
Amylase-positive cells constitute additional subsets of TRCs that are associated in different ways with chemoreceptorial and/or secretory molecules, confirming the concept that various pathways act in taste buds. Possible cross-talk between the diverse subsets might mediate different physiological functions in response to external tastants in the mouth. The heterogeneity of TRCs emphasizes the role of the taste bud as a sensor-effector organ and accounts for its complexity, strengthening the analogy with other epithelia.
Amylase in taste buds might constitute an orosensory mechanism with an important role in food intake and implications for the prevention and/or treatment of diet-related disorders.
References
Azzali G, Bucci G, Gatti R, Orlandini G, Ferrari G (1989) Fine structure of the excretory system of the deep posterior (Ebner’s) salivary glands of the human tongue. Acta Anat 136:257–268
Bezencon C, Coutre J le, Damak S (2007) Taste signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32:41–49
Brouwer JN, Wiersma A (1980) Stimulus-induced appearance of proteinaceous material in the taste pore. In: Starre H van der (ed) Olfaction and taste, vol VII. IRL, London, pp 179–182
Cloutier M, Gingras D, Bendayan M (2006) Internalization and transcytosis of pancreatic enzymes by the intestinal mucosa. J Histochem Cytochem 54:781–794
Dockray GJ (2003) Luminal sensing in the gut: an overview. J Physiol Pharmacol 54:9–17
Dvoryanchikov G, Tomchick SM, Chaudhari N (2007) Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol 505:302–313
Dyer J, Salmon KSH, Zibrik L, Shirazi-Beechey SP (2005) Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33:302–305
Field RB, Spielman AI, Hand AR (1989) Purification of lingual amylase from serous glands of rat tongue and characterization of rat lingual amylase and lingual lipase. J Dent Res 68:139–145
Field RB, Kruse DH, Redman RS (2001) Immunohistochemical localization and mRNA detection of Rab3D and /or Rab3B in rat von Ebner’s glands, parotid gland, pancreas, and liver. Histochem J 33:71–77
Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL (2003) Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA 100:8981–8986
Fischer-Colbrie R, Lassmann H, Hagn C, Winkler H (1985) Immunological studies on the distribution of chromogranin A and B in endocrine and nervous tissue. Neuroscience 16:547–555
Giza BK, Scott TR, Sclafani A, Antonucci RF (1991) Polysaccharides as taste stimuli: their effect in the nucleus tractus solitarius of the rat. Brain Res 555:1–9
Gurkan S, Bradley RM (1987) Autonomic control of von Ebner’s lingual salivary glands and implication for taste sensation. Brain Res 419:287–293
Gurkan S, Bradley RM (1988) Secretion of von Ebner’s glands influence responses from taste buds in rat circumvallate papilla. Chem Senses 13:655–661
Hagn C, Schmid KW, Fisher-Colbrie R, Winkler H (1986) Chromogranin A and B in adrenal medulla and endocrine tissues. Lab Invest 55:405–411
Hass N, Schwarzenbacher K, Breer H (2007) A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol 128:457–471
Helle KB (2004) The granin family of uniquely acid proteins of the diffuse neuroendocrine system: comparative and functional aspect. Biol Rev 79:769–794
Höfer D, Puschel B, Drenckhahn D (1996) Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci USA 93:6631–6634
Höfer D, Drenckhahn D (1996) Cytoskeletal markers allowing discrimination between brush cells and other epithelial cells of the gut including enteroendocrine cells. Histochem Cell Biol 105:405–412
Höfer D, Drenckhahn D (1998) Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol 110:303–309
Höfer D, Asan E, Drenckhahn D (1999) Chemosensory perception in gut. New Physiol Sci 14:18–23
Huang YJ, Wu YH, Lu KS (2003) Immunoelectron microscopic studies on protein gene product 9.5 and calcitonin gene-related peptide in vallate taste cells and related nerves in the guinea pig. Microsc Res Tech 62:383–395
Kinnamon SC, Margolskee RF (1996) Mechanisms of taste transduction. Curr Opin Neurobiol 6:506–513
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104:15069–15074
Johnston CJ, Mango GW, Finkelstein JN, Stripp BR (1997) Altered pulmonary response to hyperoxia in Clara cell secretory protein deficient mice. Am J Respir Cell Mol Biol 17:147–155
Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sévigny J, Kinnamon JC, Finger TE (2008) The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses 33:243–254
Levitzki A, Steer ML (1974) The allosteric activation of mammalian alpha-amylase by chloride. Eur J Biochem 41:171–180
Mace OJ, Affleck J, Patel N, Kellett GL (2007) Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol (Lond) 582:379–392
Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR (1998) Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol 275:348–356
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP (2007) T1R3 and gustducin in gut sense sugar to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104:15075–15080
Matsuo R (2000) Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med 11:216–229
Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A (2005) Alpha-gustducin immunoreactivity in the airways. Cell Tissue Res 319:211–219
Merigo F, Benati D, Di Chio M, Osculati F, Sbarbati A (2007) Secretory cells of the airway express molecules of the chemoreceptive cascade. Cell Tissue Res 327:231–247
Merigo F, Benati D, Galiè M, Crescimanno C, Osculati F, Sbarbati A (2008) Immunohistochemical localization of cystic fibrosis transmembrane regulator and Clara cell secretory protein in taste receptor cells of rat circumvallate papillae. Chem Senses 33:231–241
Mese H, Matsuo R (2007) Salivary secretion, taste and hyposalivation. J Oral Rehabil 34:711–723
Pilewski JM, Frizzell RA (1999) Role of CFTR in airway disease. Physiol Rev 79:S215–S255
Quinton PM (1999) Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 79:S3–S22
Ramirez I (1991) Chemoreception for an insoluble non-volatile substance: starch taste. Am J Physiol Regul Integr Comp Physiol 260:192–199
Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR (2007) CCSP regulates cross-talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am J Physiol Lung Cell Mol Physiol 293:L114–L123
Riva A, Loffredo F, Puxeddu R, Testa Riva F (1999) A scanning and transmission electron microscope study of the human minor salivary glands. Arch Oral Biol 44:S27–S31
Rohr G, Scheele G (1983) Fate of radioactive exocrine pancreatic proteins injected into the blood circulation of the rat. Tissue uptake and transepithelial excretion. Gastroenterology 85:991–1002
Roper SD (2006) Cell communication in taste buds. Cell Mol Life Sci 63:1494–1500
Roper SD (2007) Signal transduction and information processing in mammalian taste buds. Pflügers Arch 454:759–776
Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E (2006) Colocalization of the alfa-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291:G792–G802
Rozengurt E (2006) Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 291:G171–G177
Sbarbati A, Crescimanno C, Osculati F (1999) The anatomy and functional role of the circumvallate papilla/von Ebner gland complex. Med Hypotheses 53:40–44
Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Crescimanno C, Osculati F (2004) Identification and characterization of a specific epithelium in the rat larynx. J Comp Neurol 475:188–201
Sbarbati A, Osculati F (2005) The taste cell-related diffuse chemosensory system. Prog Neurobiol 75:295–307
Sclafani A, Abrams M (1986) Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav 37:253–256
Sclafani A, Mann S (1987) Carbohydrate taste preferences in rats: glucose, sucrose, maltose, fructose, and Polycose compared. Physiol Behav 40:563–568
Sclafani A, Nissenbaum JW, Vigorito M (1987) Starch preference in rats. Neurosci Biobehav Rev 11:253–262
Sclafani A (2004) The sixth taste? Appetite 43:1–3
Sclafani A, Zukerman S, Glendinning JI, Margolskee RF (2007) Fat and carbohydrate preferences in mice: the contribution of α-gustducin and Trpm5 taste signaling proteins. Am J Physiol Regul Integr Comp Physiol 293:1504–1513
Spielman AI (1990) Interaction of saliva and taste. J Dent Res 69:838–843
Steer ML, Levitzki A (1973) The metal specificity of mammalian-amylase as revealed by enzyme activity and structural probes. FEBS Lett 31:89–92
Sternini C (2007) Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol 292:G457–G461
Sternini C, Anselmi L, Rozengurt E (2008) Enteroendocrine cells: a site of “taste” in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes 15:73–78
Sunderland G, Sclafani A (1988) Taste preference of squirrel monkey and bonnet macaques for polycose, maltose and sucrose. Physiol Behav 43:685–690
Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA (2007) Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 292:G1420–G1428
Tizzano M, Merigo F, Sbarbati A (2006) Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J Anat 209:333–337
Tremblay G, Charest J (1968) Modified starch film method for the histochemical localization of amylase activity. J Histochem Cytochem 16:147–148
Vigorito M, Sclafani A (1988) Ontogeny of polycose and sucrose appetite in neonatal rats. Dev Psychobiol 21:457–465
Witt M, Miller IJ Jr (1992) Comparative lectin histochemistry on taste buds in foliate, circumvallate and fungiform papillae of the rabbit tongue. Histochemistry 98:173–182
Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99:2392–2397
Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC (2001) “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 440:97–108
Zancanaro C, Mucignat Caretta C, Merigo F, Cavaggioni A, Osculati F (1999) Alpha-gustducin expression in the vomeronasal organ of the mouse. Eur J Neurosci 11:4473–4475
Acknowledgement
The authors thank Christine Harris for revising the manuscript, and Marzia Di Chio for technical assistance with the confocal microscope.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merigo, F., Benati, D., Cecchini, M.P. et al. Amylase expression in taste receptor cells of rat circumvallate papillae. Cell Tissue Res 336, 411–421 (2009). https://doi.org/10.1007/s00441-009-0789-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0789-7