Abstract
The sense of taste facilitates the recognition of beneficial or potentially harmful food constituents prior to ingestion. For the detection of tastants, epithelial specializations in the oral cavity are equipped with taste receptor molecules that interact with sweet, umami (the taste of l-amino acids), salty, sour, and bitter-tasting substances. Over the past years, numerous tissues in addition to gustatory sensory tissue have been identified to express taste receptor molecules. These findings bear important implications for the roles taste receptors fulfill in vertebrates, which are currently envisioned much broader than thought previously. Taste receptive molecules are present in the brain, respiratory and gastrointestinal tracts, heart, male reproductive tissue, as well as other areas of the body just beginning to emerge. This review summarizes current knowledge on the occurrence and functional implications of taste receptive molecules outside the oral cavity.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The need of animals to feed their bodies constantly with energy-rich food items belongs to the most fundamental prerequisites to maintain life and well-being. As food sources are complex and consist of variable amounts of life-maintaining (macro-)nutrients as well as potentially harmful substances, a mechanism has evolved enabling animals evaluating food items prior to their consumption. The taste system present in the oral cavity furnishes animals with receptive proteins devoted to detect the building blocks of carbohydrates, proteins, and critical electrolytes as well as with sensors that identify potentially harmful substances. The activation of the oral taste receptors does not only provide information on the chemicals present in consumed food items, but it also evokes hedonic tones, e.g., perceived pleasantness or unpleasantness directly affecting consumption. Whereas sweet, umami, and low concentrations of sodium salts represent generally attractive taste stimuli promoting consumption, high salt concentrations and sour and bitter stimuli tend to elicit rejection.
In recent years, numerous reports on the extragustatory expression of taste receptors clearly suggested that their role is not limited to taste perception. Taste receptors have meanwhile been identified in the gastrointestinal (e.g., [1–26]) and respiratory tracts of mammals (e.g., [27–37]), in the male reproductive system [36, 38–43], as well as in the brain [44–49] and heart [50] to name just the areas having received the most attention during the recent years. Similar to the oral cavity, also the alimentary canal and the respiratory system represent epithelia exposed to potentially noxious substances originating from the environment. Hence, it appears conceivable that bitter taste receptors, devoted to protect organisms from the uptake of potentially toxic substances, play similar roles in these extragustatory organ systems as well. Another important activity of the intestinal mucosa is the monitoring of the nutritional value of the ingested food. This nutrient sensing allows for an efficient digestion and absorption of nutrients and allows fine tuning of metabolic parameters in response to demands and available resources. Thus, the occurrence of taste receptors, devoted to the detection of sweet and umami stimuli in the GI tract, is not surprising.
More surprising than finding taste receptors in the respiratory and gastrointestinal epithelia was the detection of these molecules in other organs such as the brain and heart. Since these organs are protected from direct contact to the outside world, the expression of TAS1R as well as TAS2Rs suggests that they are involved in the surveillance of molecules present in internal body fluids such as blood plasma or cerebrospinal fluid. It will be highly interesting to see in the future how rather low-affinity receptors, developed to detect concentrated taste stimuli in the oral cavity, fulfill their yet unknown roles in the brain and heart.
Another class of chemosensory receptors, the odorant receptors (ORs), has already been detected in sperm cells and associated with sperm motility and chemotaxis [51, 52]. The identification of taste receptors in the testis and sperm cells underscores the importance of chemoreceptor signaling for reproduction. However, in contrast to odorant receptors, which may actually serve a role in spermatozoa guidance, none of the recently published studies on taste receptors in the male reproductive tract put forward mechanisms indicating a similar function of these receptors [36, 38–43].
After a brief introduction into taste receptors and their associated signaling components, we discuss the expression of taste receptors and their functional implications in the respiratory and alimentary tract, the brain, the heart, and the reproductive system. For space constraints, we will not extensively discuss some so far rather isolated reports on taste receptor gene expression in additional tissues such as the bladder [53], adipose tissue [54, 55], and bone marrow stromal and vascular smooth muscle cells [56], as well as their emerging role in the regulation of autophagy [57] (Table 1).
2 Taste Receptors and Signaling Components
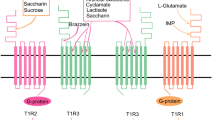
The gustatory system of mammals is equipped with taste receptors devoted to the detection of the five basic taste qualities: sweet, sour, salty, umami, and bitter. In addition to these well-accepted basic taste qualities, the perception of further stimuli such as fatty or metallic is discussed (for a recent review, see [58]). The recent generation of knockout mice, lacking the α-subunit of the epithelial sodium channel, ENaC, confirmed the role of this channel as receptor for attractive low concentrations of sodium in rodents [59]. Although numerous candidate molecules for sour taste receptors such as members of the polycystic-kidney-disease-like ion channels (PKD) had been proposed in the past (e.g., [60–62], but cf. [63]), the molecular identification of this receptor is still pending. The best characterized taste receptors are the G protein-coupled receptors responding to sweet [64–69], umami [70, 71], and bitter compounds [72–74], which belong to two different gene families (Fig. 1). The TAS1R family of taste receptors has only three members named TAS1R1, TAS1R2, and TAS1R3. Similar to other class C G protein-coupled receptors such as metabotropic glutamate receptors (mGluRs) [75] or γ-aminobutyric acid type B receptors (GABAB) [76–79], TAS1Rs form oligomeric complexes. Whereas the two subunits TAS1R1 and TAS1R3 form the main receptor for the detection of umami-tasting l-amino acids (in human specifically l-glutamate and to a lesser degree l-aspartic acid) [70, 71], the subunits TAS1R2 and TAS1R3 combine to the universal sweet taste receptor [70, 80]. The bitter taste receptors belong to the TAS2R gene family. Vertebrates possess a highly variable number of putative functional TAS2R genes ranging from 2 to 3 in some bird species to more than 50 in some reptilian and amphibian species [81]. In mammals, an average number of 15 receptors in dogs to 36 in rats have been identified [81]. So far, the best characterized bitter taste receptor repertoire is represented by the 25 functional human TAS2Rs. For 21 of the 25 human TAS2Rs, activators have been identified by heterologous expression assays. It was shown that TAS2Rs differ remarkably in their breadth of tuning with few extremely broadly tuned “generalist” receptors, several narrowly tuned “specialist” receptors, as well as numerous receptors exhibiting intermediate tuning properties [82, 83].
Schematic of the G protein-coupled taste receptors. Bitter taste receptors (left) do not possess long extracellular amino termini and function as monomers (or homooligomers), whereas sweet (middle) and umami (right) receptors exhibit large extracellular amino termini and form obligatory heterooligomers. Both receptors consist of a common subunit, TAS1R3, and a specific subunit, TAS1R2 and TAS1R1, for the sweet and umami receptor, respectively
Within the oral cavity, the G protein-coupled receptors are expressed in non-overlapping subsets of taste receptor cells dedicated to detect sweet, umami, or bitter stimuli [84]. Despite this segregation into specialized sweet, umami, and bitter receptive cells, they share common downstream signaling components. These include heterotrimeric G proteins consisting of several alternative Gα-subunits, Gβ3 (Gβ1) and Gγ13 [85, 86]. Of these, the first identified and best characterized Gα-subunit is α-gustducin [87]. Upon receptor activation, the GTP-bound heterotrimeric G protein dissociates and transmits the signal to the membrane-bound phospholipase Cβ2 [88, 89] which, in turn, results in the generation of the second messenger molecule inositol-1,4,5-trisphosphate (IP3). Next, IP3 facilitates opening of the type III IP3 receptor located in the membrane of the endoplasmatic reticulum of taste receptor cells [90–92]. The subsequent release of calcium ions into the cytoplasm activates the cation channel TRPM5 [89, 93–95] leading to membrane depolarization. Finally, the activated taste receptor cell devoid of synaptic contacts releases the neurotransmitter ATP [96] through channels [97–99] into the taste bud, where it eventually activates afferent nerve fibers. Signal termination after taste stimulation may involve RGS21 [100, 101], a protein accelerating the GTP hydrolysis of activated Gα-subunits found in taste receptor cells (Fig. 2).
G protein-coupled taste receptor linked signal transduction. The activation of a taste GPCR by a taste stimulus (red triangle) leads to the activation of a heterotrimeric G protein complex which includes α-gustducin. The subsequent activation of phospholipase Cβ2 results in the generation of IP3, which, in turn, causes release of calcium ions from intracellular stores. The elevated intracellular calcium ion level triggers the opening of the cation channel TRPM5 resulting in cell depolarization
Some of the signaling proteins, involved in taste signal transduction such as α-gustducin [102], have been identified prior to the taste receptors in non-gustatory tissues and served, therefore, as surrogate markers for taste-specific signaling events outside the oral cavity (Fig. 3).
Expression of bitter taste receptors and taste-related signaling components in the GI tract. The left panel shows single cells in mouse colonic mucosa expressing a red fluorescent protein under the control of the bitter taste receptor Tas2r131 promoter. The right panel shows cells expressing α-gustducin, a component of the canonical taste transduction cascade. Scale bar; 20 μm
3 Brain
Perhaps, one of the most surprising tissues, reported to express taste receptor genes, is the brain. The brain is a highly vulnerable organ utterly safeguarded from direct contact to the environment and even only selectively accessible to substances present in the circulation by its protective blood-brain barrier [103]. One would think the brain might be the least place to find receptors tuned to recognize concentrated tastants present in the oral cavity. Nevertheless, recent reports indicate expression of genes coding for the sweet taste receptor subunits and several bitter taste receptors in human and rodent brains [44, 45, 47, 49]. The first report by Ren et al. [48] used RT-PCR expression profiling, in situ hybridization, and immunohistochemical detection methods to localize the Tas1r2 and Tas1r3 subunits necessary to form a functional sweet taste receptor in distinct areas of the mouse brain. Prominent expression was detected in the hippocampus, hypothalamus, cortex, and the paraventricular nucleus of the thalamus. Moreover, periventricular areas surrounding the third ventricle were found rich in presumably sweet-sensing cells as well. These brain areas do not only exhibit sweet taste receptors but also co-express components of the canonical taste-signaling cascade such as α-gustducin, Gβ3, and Gγ13. In vivo experiments, using food-deprived, hyperglycemic ob/ob mice and control mice fed ad libitum, demonstrated that the umami receptor-specific gene Tas1r1, but even more pronounced the sweet taste receptor-specific Tas1r2 gene, is induced by starvation as well as in hyperglycemic mice as indicated by elevated corresponding mRNAs. This effect was restricted to hypothalamic tissue and not observed for cortical tissue suggesting a modulation of hunger/satiety regulatory circuits located in the hypothalamus. Since a selective decrease in Tas1r2 mRNA by elevated glucose levels in the culture medium was also observed in a mouse hypothalamic cell line, the authors concluded that the dynamic regulation of Tas1r1 and Tas1r2 mRNA might be a direct effect caused by local receptor activation rather than indirectly regulated by circulating hormones reporting the nutritional state of animals. Most importantly, the authors observed a similar effect on Tas1r2 gene regulation also, if the non-metabolizable artificial sweetener sucralose is used instead of glucose excluding contributions by sweet taste receptor-independent glucose-sensing pathways.
A recent report on the effects of chronic treatment of mice with the artificial sweetener acesulfame K demonstrated a downregulation of the Tas1r3 subunit, common to both the sweet and the umami taste receptor, as well as a downregulation of sweet receptor-specific Tas1r2 transcripts in the hippocampus along with alterations in neurometabolic functions of the experimental animals [46]. Compared to wild-type mice, Tas1r3 knockout animals were protected against negative cognitive effects of chronic acesulfame K treatment attesting to a putative role of Tas1r genes in learning tasks.
While taste receptors, devoted to the detection of sweet and umami compounds, may indicate an important endogenous role in energy surveillance of these receptors, the expression of bitter taste receptors in the brain is even more surprising. Nevertheless, Singh et al. [49] recently reported the expression of several Tas2r genes in the brainstem, cerebellum, cortex, and nucleus accumbens of rat brain. By RT-PCR, transcripts specific for the receptors Tas2r10, Tas2r4, and Tas2r38 were detected. Using a non-validated Tas2r4 antiserum, labeling was reported in neurons of the nucleus tractus solitarius (NTS) and the molecular layer of the cerebellum. Despite the apparent absence of bitter receptor gene expression in glial cells in vivo, the authors were able to detect several Tas2r-specific transcripts in cultured C6 glial cells, originating from experimentally induced rat gliomas [104], as well as in cultured primary neuronal cells. Tas2r4 may represent a functional ortholog of mouse Tas2r108, which is a low-sensitivity denatonium benzoate receptor [73], and/or of human TAS2R4, which has been reported to respond to a variety of bitter compounds, including denatonium benzoate [73, 83] and quinine [83, 105]. Based on this assumption functional calcium imaging experiments were performed. It turned out that, indeed, both cell lines responded in a dose-dependent fashion to rather high concentrations of the two tested stimuli. In another study performed in rat, the rat bitter taste receptor Tas2r1, along with signaling components involved in taste transduction such as α-gustducin, PLCβ2, and TRPM5, was detected in the brainstem tissue by RT-PCR experiments [47]. In contrast to the study by Singh et al [49], bitter receptor gene expression seemed to be rather restricted to few receptors in the brainstem and did not extend to the cerebellum. Further studies, using antisera specific for taste-signaling components, located these molecules in a subset of serotonergic neurons (=tryptophan hydroxylase immunoreactive) within the medullary raphe known to contain chemosensorically active cells [106]. Recent experimental evidence, obtained from human patients suffering from degenerative brain diseases, suggests that TAS2R gene expression as determined by quantitative RT-PCR analyses can be altered in cases of Parkinson’s disease, Alzheimer’s disease, progressive supranuclear palsy, and Creutzfeldt-Jakob disease [44, 45]. Whereas these data underscore the occurrence and possible functional role of brain-expressed bitter taste receptor genes, it is not known if the observed alterations in bitter taste receptor gene expression levels represent merely secondary events during disease progression or play a more active role in these processes.
4 Gastrointestinal Tract
The gastrointestinal (GI) system has received considerable attention over the past years, as it turned out to express components of the taste-signaling cascade. Early reports already suggested that cells with chemosensory ability might exist in the epithelial lining of the GI tract. Indeed, the presence of taste-signaling components was confirmed in many GI tissues, whereas the detection of G protein-coupled taste receptors on a cellular level was, depending on the receptor type, more difficult. These initial findings triggered the search for the physiological processes controlled by GI taste receptors.
4.1 Taste Receptors in the Gastrointestinal Tract
The first G protein-coupled receptors described in the GI tract were 11 members of the Tas2r family, whose transcripts were detected by RT-PCR in gastric and duodenal mucosa of rodents [25]. Subsequent reports have described the identification of further bitter taste receptor transcripts in GI tract tissues of rat and mouse origin [3, 13, 14, 24] as well as in human cecum, colon [5, 14], and ileum [107]. From these reports, it appears that different GI organs and even functionally distinct parts of the same organ host different Tas2r subsets pointing to a complex role for Tas2rs in the GI system. A recent qRT-PCR study on the distribution and expression levels of a set of Tas2r along the mouse GI tract confirmed this by showing that (1) Tas2r distribution is rather heterogeneous, with some receptors being present in all GI organs analyzed and others being restricted to only few or single organs, and (2) Tas2rs in mouse GI tract are expressed at a very low level compared to gustatory tissue [19].
Also members of the TAS1R gene family of G protein-coupled receptors were identified in the GI system. Regarding the alimentary canal, it was found that mouse small intestine harbors all three subunits (TAS1R1, TAS1R2, and TAS1R3) of the TAS1R family as shown by both RT-PCR analyses and Western blotting [6]. Along the anteroposterior axis, the site of the highest expression is the jejunum in case of the sweet taste receptor subunits TAS1R2 and TAS1R3 (the latter being the most abundant). On the contrary, the umami-specific receptor subunit TAS1R1 is found mostly in the ileum [6]. Further studies took advantage of the available immunological tools for histological techniques to reveal the localization of TAS1R receptors. Sweet and umami taste receptor subunit expression was indeed shown mostly in mouse proximal small intestine in the mucosal layer and at all levels along the crypt-villus axis [2, 4, 16, 17, 108, 109].
In human, similar data to those from rodent models were obtained. In one study, an approximately tenfold higher expression of TAS1R3 with respect to TAS1R2 in small intestinal tissues was reported [26, 110]. Another study described an approximately 1,500 times higher expression of TAS1R3 compared with TAS1R2 in the same tissues [2]. In the same report, the authors measured the amount of TAS1R transcripts also in the large intestine, in which lower levels of expression compared with the small intestine were observed. The presence of TAS1R subunits in man has also been confirmed at the protein level using specific antibodies [11, 110].
In the upper GI tract, Tas1r3 was found in the corpus mucosa of the mouse stomach, both in adult [111, 112] and young postnatal mice [113]. The distribution of the Tas1r3-expressing cells is highly restricted, with the highest number in the region of the limiting ridge, at the border between the corpus and fundus regions [112].
Also other animal models were employed to investigate TAS1R expression in the GI tract: expression was revealed in the small intestine and stomach of pig [114, 115], dog, cat, and horse [116, 117] indicating a conserved and presumably essential role in the GI physiology of mammals for taste receptors. The identification of Tas1r2 and Tas1r3 gene expression in explanted pancreatic islets of mouse [18] and in human pancreas and liver tissues [118] suggests the conserved role of these taste receptors is not limited to those GI tissues that come into direct contact to the luminal food constituents.
4.2 Taste-Signaling Components in the GI Tract
All main components of the canonical taste transduction cascade (see Sect. 2) have been investigated in GI tissues. The first identified taste-related signaling molecule was α-gustducin in mucosal cells of the stomach and duodenum in rat [102] as well as in the pancreatic duct system [119]. That also other elements of the sensorial taste system are expressed in the GI tract became clear, when α-transducin, which is closely related to and can functionally replace α-gustducin [120], was found in intestinal epithelium and in gastric fundus and antrum [25, 121]. Recently, both G protein α-subunits were found to be expressed in ghrelin-producing cells of the oxyntic glands of the stomach [12]. Numerous reports confirmed and extended the initial findings of taste-signaling elements in the GI tract to most regions of the alimentary canal [2, 4, 16, 17, 23, 108, 117, 122], with the colon harboring the highest number of α-gustducin-positive cells [123]. Noteworthy is the finding of a numerically important α-gustducin-expressing cell population confined to a relative restricted region of the stomach, the limiting ridge [112]. In human, the presence of α-gustducin was demonstrated in the small intestine and in the colon [11, 20], both at mRNA and protein level.
The notion that taste-like mechanisms might work in specialized GI cells was further corroborated by the presence of PLCβ2. Single PLCβ2-positive cells in the small intestine and in colonic mucosa were demonstrated in rodent [2, 19, 122] and human tissue samples [11]. In the stomach, PLCβ2-expressing cells are found in separated populations in two distinct anatomical and functional regions of the organ, the corpus mucosa and the limiting ridge [112, 124].
Another taste-signaling element, the transient receptor potential subfamily M member 5 (TRPM5) channel, is expressed along the entire length of the GI tract from the stomach to colon [2, 11, 26, 110, 122, 125, 126]. In the gastric organ, as it is the case for other taste-related proteins, it is mostly expressed in the region of the limiting ridge [112, 113, 115, 124].
Taste transduction cascade elements are considered as surrogate markers when studying the occurrence of cells outside the sensorial systems with potential chemoresponsive abilities. By analogy with their role in taste cells, it is assumed that they would fulfill the same task also in non-taste cells. This would consequently imply the simultaneous presence of other components of the taste transduction cascade as well as upstream taste receptors. The good availability of experimental tools such as specific antisera and genetically modified mouse lines explains their extensive use as markers for potentially tastant-responsive cells. However, available data indicate that in GI tissues the link between the various taste-signaling components is not as tight as in the gustatory system. In fact, depending on the different regions of the GI tract and even within the same region, the degree of colocalization of the various transducing elements varies considerably. The entire population of TRPM5-GFP-labeled cells in duodenal villi and the colon co-expresses α-gustducin, while only one-third of TRPM5-positive duodenal cells show also PLCβ2 expression [2]. In the colon, PLCβ2-positive cells even represent a completely separated population with respect to TRMP5-labeled cells [2]. In the stomach, the situation is again different: within the gastric groove, all α-gustducin- and TRPM5-positive cells also express PLCβ2 [124]. Regarding the co-expression of taste transduction elements with taste receptors, it turned out that TRPM5-positive cells in mouse small intestine do neither express Tas1rs nor Tas2rs [2], while in the same regions α-gustducin exhibits a certain degree of colocalization with the Tas1r subunits [4, 11, 17]. Moreover, in the GI tract taste-related signaling elements may facilitate signal propagation of G protein-coupled receptors currently not considered as bona fide taste receptors such as fatty acid receptors and oleoylethanolamide and bile acid receptors which are co-expressed with α-gustducin in mouse colon [123].
4.3 Cell Types
The numerous studies performed so far have demonstrated that the GI tract contains a heterogeneous cell population expressing taste-related molecules. One of these identified cell types are brush cells (or tuft cells or caveolated cells) representing a minor differentiated cell type that manifest as single cells scattered throughout the mucosa. In mouse, they represent 0.4% of the intestinal epithelium and they possess a tapering cell body with a tuft of microvilli on top protruding into the intestinal lumen [127]. The structural features of brush cells have led to the speculation that they might sense the chemical content in the gut lumen through their apical pole [128]. The finding that brush cells express α-gustducin represented the first molecular clue that this cell type may indeed participate in intestinal chemoreception [102, 119, 121]. Subsequent studies have shown that mouse stomach brush cells, lining the border between fundus and corpus, the so-called limiting ridge, express also other taste transduction components like TRPM5, PLCβ2, and especially Tas1r3 [10, 124]. In the mucosa of the small and large intestine, brush cells express with a variable degree of overlap TRPM5, α-gustducin, PLCβ2, and Tas1rs [2, 122].
Another intestinal cell population, discovered to express taste-related molecules, are the enteroendocrine cells (1% of the lining epithelium) [6]. In their entirety, they express up to 20 gastrointestinal hormones that, together with their localization within the mucosa, define a complex class of enteroendocrine cells that function as important metabolic regulators [129]. Indeed, it was shown that human and mouse duodenal enteroendocrine L (expressing GLP-1, GLP-2, and PYY) and K (expressing GIP) cells harbor the sweet taste receptor-specific subunit Tas1r2, together with other taste-signaling components [11, 17]. In accordance with these findings, Tas1r2 was never found to be expressed in enteroendocrine I cells identified by the expression of CCK. On the contrary, the specific umami receptor subunit Tas1r1 is consistently expressed in I cells [4], indicating the segregation of the sweet and umami receptors in two different enteroendocrine cell populations that secrete different hormones and thus exert various functions such as induction of satiety, gastric emptying, intestinal motility, and stimulation of insulin secretion. Tas1r3 is expressed in both cell types, consistent with its role as binding partner in the formation of both functional sweet and umami taste receptors as well as in ghrelin-producing cells of the duodenum [4, 10]. In mouse jejunum and in human ileum, enterochromaffin (EC) cells producing serotonin were found to express α-gustducin [23], TAS1R3, and TAS2R1 [107]. In the colon, α-gustducin is expressed again in L and K cells but not in EC cells [20, 123]. Also in the stomach of mouse, the Tas1r3 subunit was found in gastrin-positive G cells [111]. Moreover, the Tas1r3 subunit was found to be expressed in the corpus of mouse stomach in ghrelin-positive cells [10] (Fig. 4).
Model of the sweet receptor mediated pathway leading elevated intestinal glucose absorption. Activated sweet taste receptors localized on enteroendocrine L cells trigger the elevation of intracellular calcium levels via a pathway involving α-gustducin. The L cells secrete the peptide hormones GLP-1 and GLP-2. Secreted GLP-2 interacts with GLP-2 receptors located on neighboring enteric neurons, which by means of a so far unidentified neuropeptide stimulate enterocytes. Within the enterocytes, this signal results in an upregulation of SGLT1 mRNA and, finally, the incorporation of additional SGLT1 into the apical membrane. The elevation of SGLT-1 gene transcription is mediated by the cAMP-regulated protein kinase A (PKA). Redrawn and modified from Shirazi-Beechey et al. [21]
Regarding the identification of cell type(s) expressing Tas2rs in situ, the data are much more limited. The main reasons for the scarcity of data might be a lack of reliable antisera as well as rather low expression levels of bitter taste receptor mRNAs in GI tissues, which limits the use of other histological methods such as in situ hybridization [19]. To date, only two reports directly addressed the in situ expression pattern of bitter taste receptors in the rodent GI tract. One study identified enteroendocrine cells in mouse duodenum, expressing bitter taste receptor genes by immunohistochemical experiments using a non-validated antiserum raised against the receptor Tas2r138 [13]. More recently, the difficulties to demonstrate the in situ expression of Tas2rs were overcome by using a knock-in mouse model, in which a fluorescent marker protein reports the activation of the Tas2r131 locus with high sensitivity and reliability. It was shown that the Tas2r131 gene is expressed in the GI tract with a proximal-to-distal gradient from the jejunum to colon and the Tas2r131-positive cells in the colonic mucosa were identified as a subset of goblet cells [19]. Curiously, these cells do not express α-gustducin or PLCβ2, so other signal transduction elements are likely to relay the signal downstream of the receptor [19].
4.4 Model Cell Lines
Over the years, several working groups used cell lines derived from gastrointestinal tissues to study taste receptor expression and function. GI taste receptors in enteroendocrine cell lines were firstly identified in the mouse STC-1 cell line [25], which was shown to express several bitter taste receptors as well as components of the canonical taste transduction cascade [3, 24, 25]. The same cell line also expresses the subunits Tas1r1 and Tas1r3 [4], and it is able to respond to tastants by releasing GI hormones such as CCK [3, 4]. Other enteroendocrine cell lines of gastrointestinal origin were used: the HuTu-80 cell line from the human intestine expresses TAS2Rs [20, 130], and the NCI-H716 of human origin is equipped with both sweet taste receptors [11] and bitter taste receptors [5, 20] that, upon activation, can trigger the secretion of GLP-1 [5, 11]. Despite these findings, the functional significance of the simultaneous presence of sweet and bitter taste receptors in the same cells still needs further clarification, as it appears counterintuitive that sweet and bitter taste receptors should trigger the same physiological responses.
4.5 Physiological Functions of GI Taste Receptors
There is an increasing body of evidence pointing at a role of sweet taste receptors in GI organs as regulators of glucose metabolism. It is well known that administration of glucose per os is more effective than a direct injection of glucose into the blood circulation to stimulate insulin release from the pancreas, which in turn promotes the cellular uptake of the circulating glucose [131]. This phenomenon is caused by the presence of sugars in the intestinal lumen triggering the secretion of potent insulin secretagogues, the incretin hormones GLP-1 and GIP, from enteroendocrine cells of the gut wall [132]. However, the sensor molecules, detecting luminal sugars and triggering the physiological responses, have been rather elusive. Jang et al. demonstrated that sweet taste receptors are expressed together with all components of taste transduction cascade in GLP-1- and GIP-secreting enteroendocrine L and K cells, respectively [11]. They observed that excised duodenal villi treated with glucose release GLP-1 and that in α-gustducin knockout animals upon glucose gavage the plasma levels of GLP-1, GIP, and insulin were lower than in wild-type littermates. They concluded that gustducin-coupled sweet taste receptors are the luminal sensors that detect sugars in the gut and trigger incretin hormone release from enteroendocrine cells. The physiological consequences of sweet taste receptor activation in gut enteroendocrine cells are not limited to the so-called incretin effect. Prolonged exposure to a carbohydrate-rich diet or to a diet enriched with artificial sweeteners causes an increase in the expression of the Na+-glucose cotransporter 1 (SGLT-1) in the brush border membrane of enterocytes resulting in higher absorption of carbohydrates from the small intestine. This effect is abolished in α-gustducin and Tas1r3 knockout mice, suggesting that the sweet taste receptor in enteroendocrine cells senses luminal sugars to adjust the intestinal uptake capacity to the diet [17]. The authors proposed that a paracrine signal is released by the enteroendocrine cells resulting in an upregulation of the expression of SGLT-1 by enterocytes. The responsible signaling molecule might be GLP-2, which, after being secreted by enteroendocrine L cells [117, 133], activates enteric neurons expressing the receptor GLP-2R. This in turn causes the release of a yet unidentified neuropeptide that stimulates the basolateral membrane of enterocytes to upregulate SGLT-1 expression [21]. Also studies performed in human describe a situation in which sweet taste receptors are actively involved in the release of GLP-1 and PYY hormones from enteroendocrine cells of the gut upon intragastric and intraduodenal glucose infusions, as lactisole, a potent inhibitor of the human sweet taste receptor, decreased the quantity of measured hormones [22, 134]. Some in vivo studies involving animals and humans show conflicting results with regard to the specific involvement of sweet taste receptors in the measured response and pointed out that other carbohydrate sensors might operate in the gut. In some of these studies, artificial sweeteners did not increase incretin hormone levels from the gut of human volunteers and laboratory animals [135–137]. A recent study indeed showed that there are more mechanisms governing GLP-1 release upon glucose stimulation in the alimentary canal, and they differ depending on the localization along the GI tract [7]. Using Tas1r3 and Tas1r2 knockout mice, the authors showed that in an oral glucose tolerance test, the Tas1r3 subunit is fundamental for the control of plasma glucose and insulin levels, whereas Tas1r2 null mice do not show any difference with respect to WT animals. Experiments with small intestinal explants treated with glucose, fructose, and sucralose revealed that the Tas1r2 subunit is less effective in stimulating GLP-1 release than the Tas1r3 subunit, which can partially compensate for the absence of Tas1r2. Using colon explants, it was demonstrated that the main control mechanism of GLP-1 release is KATP channel dependent and glucose specific, whereas taste-like mechanisms do not seem to be fundamental for eliciting incretin release from this part of the gut [7]. The observed phenotypical differences in Tas1r2 knockout versus Tas1r3 knockout mice, which both should render the sweet taste receptor nonfunctional, have been observed in other studies as well (e.g., see below [138, 139]). Whether this might be due to a residual function of homooligomeric Tas1r3 as low-affinity receptor for natural sweet compounds [140] and/or compensatory effects of other members of the class C GPCR family such as Tas1r1 [71], the calcium-sensing receptor [141], CaSR, and the amino acid-sensitive GPRC6A [142, 143], perhaps by the formation of alternative heterooligomers, remains to be determined.
There is evidence that other mechanisms in addition to the glucose metabolic pathway fine-regulate the release of insulin from pancreatic β-cells [144]. Indeed, it was observed that artificial sweeteners provoke both insulin release from isolated pancreatic islets and an augmented insulin secretion from MIN6 cells treated with glucose. A similar potentiation effect was demonstrated for fructose to increase insulin secretion from mouse and human islets [15, 18]. Some data suggest that pancreatic β-cells may sense sweet molecules mostly by means of a TAS1R3 homodimer, because (1) the TAS1R2 subunit is expressed at a very low level compared to TAS1R3 and (2) TAS1R2 mRNA knockdown does not affect the cellular response to artificial sweeteners, as it is the case with a knockdown of TAS1R3 [138, 139].
Concerning umami taste receptor function in the gut, fewer data are available. However, one study showed that the mouse STC-1 enteroendocrine cell line is able to release CCK in response to amino acid stimulation similar to small intestinal tissue. The response was shown to be umami taste receptor dependent as demonstrated by mRNA knockdown experiments with STC-1 cells and by treating intestinal explants with gurmarin, an inhibitor of rodent sweet [145, 146] and umami [147, 148] responses [117]. In particular in the gastric mucosa, l-amino acid sensing might be facilitated by mechanisms different from umami taste perception [149].
Also the potential role of bitter taste receptors in GI physiology is less well characterized compared to that of the sweet taste receptor. This seems to be mostly due to the limited knowledge on the cell type(s) expressing bitter taste receptors as well as the high pharmacological activity of bitter compounds, which, on top of that, possess an incredible variety of chemical structures [83]. A good example for the fact that GI responses must not necessarily involve (bitter) taste-related signaling elements has been obtained in rat pancreatic tissue. Here, Straub et al. [150] used the bitter substance denatonium benzoate to stimulate insulin release from clonal HIT-T15 β-cells as well as from isolated rat pancreatic islets. In the presence of denatonium, islets bathed in glucose solution start secreting insulin through a mechanism that does not involve gustducin or transducin as assessed in a trypsin digestion assay. It rather inhibits KATP channel activity, leading to cell membrane depolarization that, in turn, opens the voltage-gated calcium channels allowing the influx of calcium from the extracellular milieu [150].
One of the hypothesized functions for Tas2r in murine GI tract is the delaying of gastric emptying. It is indeed intuitive that bitter compounds, which are frequently rather harmful for the organism [151], would trigger defensive reactions in case of accidental ingestion to prevent them from spreading throughout the body. The first reports on this issue showed contrasting evidences in experiments conducted in human subjects [152, 153]. Nevertheless, results obtained with rodent models pointed indeed to a modulatory role of ingested bitter compounds in the regulation of gastric motility. Intragastric infusions of 10 mmol/L denatonium significantly delayed the speed of gastric emptying in rats [8]. In another study, a mouse model was used to show that intragastric gavage of a mixture of bitter compounds provokes the secretion of ghrelin into the blood circulation leading to a short-term increase in food intake as assessed in GHS-R null mice [12]. Both ghrelin secretion and increase in food intake were proven to be dependent on α-gustducin. However, 4 h after the administration of the bitter mixture, a decrease in both gastric emptying and consequently in food intake was observed. These effects are independent of CCK and GLP-1 release from the small intestine but rather controlled by an unknown effect of bitter compounds on the smooth muscles of the stomach wall [12]. In view of these findings and due to the fact that rodents lack the vomiting reflex, the delay in gastric emptying appears to be an important adaptive mechanism that would slow down the rate of ingestion of further noxious compounds. In agreement with this, a report from Kaji et al. [14] showed that application of 6-n-propyl-2-thiouracil onto explanted colonic mucosa of human and rat elicits ion and fluid secretion. This would in turn be important to flush out harmful compounds that have reached the colon lumen. Jeon and coworkers suggest that murine Tas2r activity in the small intestine is regulated by diet composition presumably to prepare the intestinal lumen to a defensive response involving the xenobiotic transporter ABCB1 against ingested noxious compound [13, 154].
Part of the GI bitter-sensing mechanism may involve vagal nerve fibers, and it was proven that intragastric administration of bitter compounds in mice increases the number of activated neurons of the mid-NTS, the terminal station of the vagal afferents from the GI tract, through activation of CCK1 and Y2 receptors. This effect was shown by c-fos expression and it is abolished by subdiaphragmatic vagotomy. It was proposed that Tas2rs expressed in enteroendocrine cells trigger the release of CCK and PYY, which in turn activate the adjacent vagal fibers to relay the signal to the brain [9]. Further experiments with rats showed that other brain areas exhibit augmented c-fos expression after gavage with bitter compounds and that this correlates with an avoidance behavior upon stimulation with flavors previously paired with intragastrically administered bitter substances [155].
5 Respiratory System
Soon after the discovery of bitter taste receptor expression in the gastrointestinal tract [25], the respiratory system, another extraoral epithelium that is constantly challenged by potentially harmful substances present in the environment of organisms, was shown to express Tas2r genes [28]. The cells, which express not only Tas2r genes but also α-gustducin, PLCβ2 [28], and TRPM5 [125], show distinct morphological features identifying them as solitary chemosensory cells [28]. Within the respiratory epithelium of the rodents’ nasal cavity, these bitter taste receptor-expressing cells are ideally suited to detect irritating noxious chemicals entering the organism through the inhaled air. Moreover, synaptic contact sites between solitary chemosensory cells in the nasal cavity with trigeminal nerve fibers have been identified [28]. Indeed, it was shown that stimulation of the cells with cognate bitter substances results in the activation of trigeminal nerve responses, which mediate depression of the respiratory rate [28]. More recently, bacterial quorum sensing molecules such as acyl-homoserine lactones were found to activate nasal solitary chemosensory cells as well suggesting that these cells not only serve a role in minimizing the amount of inhaled harmful xenobiotics but also are involved in defense mechanisms against pathogenic microorganisms [35, 156]. Recently, the presence of solitary chemosensory cells that express bitter receptors and corresponding signal transduction elements was demonstrated in human nasal respiratory epithelium as well suggesting the existence of a conserved protective mechanism [157].
Bitter taste receptor expression is not restricted to the upper airways but extends into the lower airways. Intriguingly, Shah et al. [32] demonstrated that ciliated cells of human airway epithelia express bitter taste receptors and respond to stimulation with bitter compounds with changes in their ciliary beat frequency. The authors believe that this mechanism allows the rapid elimination of noxious compounds in a cell-autonomous fashion. Recently, the direct involvement of the human bitter taste receptor TAS2R38 in the detection of bacterial quorum sensing molecules was proposed [30]. The authors found that human TAS2R38 is expressed in ciliated epithelial cells of the upper airways and that activation of this receptor by acyl-homoserine lactone results in an elevated ciliary beat frequency leading to improved mucociliary clearance as well as direct antibacterial effects [30]. Strikingly, a common genetic polymorphism of the TAS2R38 gene, which furnishes carriers of this genetic variant with a nonfunctional receptor protein, was shown to be associated with a higher incidence of sinonasal gram-negative bacterial infections [30]. A subsequent study, performed in knockout mice lacking taste-related signaling components such as PLCβ2, Trpm5, and α-gustducin, confirmed a role of bitter taste receptor signaling in the protection against airway infection caused by gram-negative bacteria. It was shown that nasal epithelial cells of mice signal in response to stimulation with acyl-homoserine lactone and that this response requires PLCβ2 and Trpm5 but not α-gustducin [158]. It should be noted that another study, performed in genetically modified mice expressing TRPM5-GFP, failed to identify taste-related cell types other than solitary chemosensory cells in the airways of mice; it remains to be seen how these contrasting results resolve [33].
Another site of bitter taste receptor expression in mammalian airways is smooth muscle cells of human airways [27]. Apparently counterintuitive, the stimulation of airway smooth muscle cells with bitter agonists did not result in bronchoconstriction consistent with a protective role, but rather resulted in the dilation of airways [27]. This report, although not undisputed (cf. [33, 159, 160]), generated considerable interest in the applicability of bitter tastants for the treatment of obstructive lung diseases. The bronchodilatory effect of bitter agonists observed by Deshpande et al. has been confirmed by a recent study performed by Zhang et al. [37] on cultured mouse airway smooth muscle cells and airway explants. Deshpande et al. [27] observed bitter tastant-evoked calcium increases in cultured airway smooth muscle cells, similar to the activity of established bronchoconstrictors, such as bradykinin or histamine, and suggested that local calcium increases via BKca channels could be responsible for the observed smooth muscle relaxation. In contrast, Zhang et al. suggested a different mechanism underlying this effect: the comparatively small increase of calcium ion levels in bitter compound-stimulated cultured airway smooth muscle cells and the failure to reproduce the local calcium events, observed by Deshpande et al. [27], led Zhang et al. to believe that bitter compound signaling exerts its bronchodilatory effect rather by antagonizing smooth muscle constriction. Indeed, the authors reported that bitter compounds inhibit the activation of voltage-dependent calcium channels by bronchoconstricting drugs and that this effect causes smooth muscle relaxation [37]. Although the exact mechanism, by which bitter taste receptor signaling causes bronchodilation, requires clarification, the finding that the large number of cognate bitter compounds may, in fact, represent a pool of potentially powerful drugs in the treatment of obstructive lung diseases is intriguing. The recent reports that TAS2R gene expression is increased in severe, therapy-resistant asthma in children and, hence, the potential target receptors for such alternative treatment strategies seem to be even upregulated [31] are promising prerequisites for a potential therapeutic value of cognate bitter substances.
Although the majority of reports about taste receptor expression and function in the airways focus on bitter taste receptors, which are believed to fulfill a protective role similar to their suspected function in the gustatory system, it should be noted that also Tas1r gene expression has been detected in the respiratory system of rodents [33]. Whether the detection of Tas1r3 in solitary chemosensory cells of the airways in rodents hints at the presence of sweet or umami receptors or both remains to be determined.
6 Heart
One of the most recent additions to the growing list of extraoral tissues expressing taste receptors is the heart [50]. By qRT-PCR analyses of rat neonatal whole heart cDNA, the two genes encoding the umami receptor subunits, Tas1r1 and Tas1r3, as well as seven bitter taste receptor genes were found to be expressed. Subsequently, samples of ventricular tissue of failing human hearts were tested and revealed the expression of more than half of all human TAS2R genes. Remarkably, the mRNA of the human bitter taste receptor TAS2R14, which is known to represent a very broadly tuned human TAS2R [83, 161], was found to be present at levels comparable to that of the β1-adrenergic receptor [50]. Interestingly, a recent report suggested that this receptor responds to numerous clinically relevant drugs and that a considerable overlap between TAS2R14 agonists and small molecules, interacting with human ether-a-go-go related gene (hERG) potassium channels, exists [162]. As hERG potassium channels play an important role in cardiac physiology (e.g., [163]), more research on the impact of bitter compounds on cardiac tissue seems to be warranted. Using primary cell cultures derived from neonatal rat hearts, taste receptor gene expression as well as taste-related signaling components such as α-gustducin, phospholipase Cβ2, and TRPM5 was confirmed in cardiomyocytes. Concerning the localization of taste receptor mRNAs in situ, Tas1r and Tas2r gene expression was demonstrated in small subsets of cardiac cells by in situ hybridization. Further, Tas1r1 promoter-driven expression of fluorescent marker protein was observed in the myocardium of a knock-in mouse line [50]. Interestingly, the investigated taste receptor genes showed distinct developmental expression patterns and were regulated by experimentally induced nutrient deprivation [50].
7 Reproductive System
For successful fertilization, mammalian spermatozoa have to find their way along the female genital tract to eventually find the egg cell and fuse with it. The idea that spermatozoa are guided on their way by chemical cues is comprehensible, and in fact, chemotactic navigation of sperm cells has been demonstrated in invertebrates and vertebrates including mammals [164]. Among the first chemoreceptors suspected to serve a function in this process have been the odorant receptors (OR). Indeed, it has been shown that the human odorant receptor OR 17-4, which is located on human spermatozoa, is responsible for the attraction of sperm cells toward the compound bourgeonal [52]. Similarly, in mouse the odorant receptor MOR23 has been shown to mediate responsiveness of sperm cells to the agonist lyral to regulate sperm motility [51]. The exact role of ORs in mammalian sperm chemotaxis and the existence of relevant OR ligands in the female reproductive tract, however, remain to be determined [165]. Even though also the expression of taste receptor genes in mouse [67, 74] and human [166] testes was recognized quite some time ago, detailed analyses of expression patterns and putative functional roles have been performed only rather recently [39]. Similar to other extragustatory tissues, the first molecule that has been investigated to identify taste-related signaling in male reproductive tissue was the Gα-subunit, α-gustducin [87]. It was shown that α-gustducin expression occurs already in differentiating spermatids and is retained in mature spermatozoa [167]. Moreover, also other components of the gustatory signaling cascade, involved in signal transduction of taste GPCRs such as Gγ13, phospholipase Cβ2, and the transient receptor potential channel TRPM5, have been identified in mouse sperm cells [40]. Indeed, expression of all 3 Tas1r genes and all 35 putatively functional Tas2r genes has been detected in mouse testes [43]. The Tas1r3, the common subunit of sweet and umami receptors, is localized on the convex side of the head and the principle piece of the sperm flagellum [41]. By using genetically modified mice, which express a fluorescent marker protein under the control of the promoter of the umami receptor-specific Tas1r1 gene, an overlapping expression pattern was observed indicating the presence of both subunits of the umami receptor. This was confirmed by double-labeling immunofluorescence experiments on human spermatozoa [41]. Intriguingly, sperm derived from Tas1r1-deficient mice showed differences in basal intracellular calcium ion as well as cyclic AMP levels suggesting a role of the umami receptor in sperm function [41]. Hence, umami receptor agonists and modulators of umami receptor responsiveness may play a, so far unanticipated, role for mammalian fertility including human. The latter may also apply for sweet-tasting substances and cognate modulators of the mammalian sweet taste receptor, as also the sweet taste receptor-specific Tas1r2 gene is expressed in spermatozoa ([38, 42], however, cf. [41]).
Intriguingly, the simultaneous absence of TAS1R3 and α-gustducin genes in genetically modified mice resulted in male-specific sterility confirming an important contribution of taste-signaling molecules for normal fertility [42]. Moreover, the authors of this study engineered mice with a humanized TAS1R3 subunit instead of the native murine Tas1r3 and combined it with an α-gustducin knockout mouse strain. This mouse line was susceptible to pharmacological induction of male sterility with the antilipid drug clofibrate, which blocks human TAS1R3-mediated signal transduction, but is not able to act via the mouse Tas1r3 ortholog [42]. As several drugs, but also environmental pollutants such as herbicides, have been identified to interfere with the activation of human TAS1R receptors, the authors indicate a potential link between some forms of male infertility and these compound classes. On the other hand the numerous TAS1R receptor agonists may show so far not anticipated treatment options for some forms of male infertility. Interestingly, in a double gene-targeted mouse line, it was shown that the umami receptor subunit in mouse sperm cells is colocalized with the bitter taste receptor Tas2r131, which is in sharp contrast to the situation in taste receptor cells [36]. As all 35 mouse bitter taste receptor genes are expressed in the testis and it was shown that mouse spermatids respond to several bitter compounds with increases of intracellular calcium levels [43], it would be highly interesting to see if also bitter taste receptor agonists and antagonists may affect male fertility. The generation of a mouse line, in which all cells that express the bitter taste receptor gene Tas2r105 are genetically ablated by the expression of diphtheria toxin A, revealed a considerable reduction in testicular size [40]. However, this mouse line still produces a reduced number of spermatids including Tas1r3-positive spermatids, suggesting a heterogeneous population of sperm cells. It would be very interesting to see whether bitter agonists and activators of Tas1rs influence sperm cell physiology in synergistic or opposing fashion or whether different subpopulations of sperm cells might be susceptible to different tastants or taste modulators.
8 Outlook
The finding of taste receptors and taste-related signaling components in non-gustatory tissues has received enormous, and ever-increasing, attention over the recent years. The investigation of taste-related signaling is no longer only relevant for researchers working in the field of chemoreception, but has attracted scientists coming from diverse areas such as respiratory, gastrointestinal, reproductive, and cardiovascular systems to name just a few. This has stimulated multidisciplinary research considerably and surely affected the way of how taste receptors are presently understood; they are clearly not only “taste” receptors anymore. However, this rapid expansion of knowledge, gained on potential roles for taste-related signaling systems outside the gustatory system, has raised numerous open questions, which need to be addressed, together with conflicting results that surfaced alongside, in the future.
One open question arises from the fact that numerous animal species have lost some, several, or even numerous taste receptor genes during evolution. For instance, cats [168] and chicken [169] lost their sweet taste receptor-specific Tas1r2 gene; the giant panda genome does not possess a functional umami taste receptor-specific Tas1r1 gene [170]; and sea lions and bottlenose dolphins [171], as well as vampire bats [172, 173], do not possess any functional Tas1r subunit, and the dolphin genome may, in addition, not even contain Tas2r genes [171]. Assuming that taste receptors indeed fulfill an integral role in, e.g., brain function and fertility, how can the ever-growing number of animal species that have been demonstrated to lack taste receptor genes compensate for the absence of these molecules? The rather “benign” phenotypes, observed in the various taste receptor knockout mouse models, suggest that even animals, which have maintained their full taste receptor gene repertoire in the course of evolution, can obviously compensate the acute loss of extraoral taste receptors quite well.
Another issue arises from the fact that taste receptors in the oral cavity are rather low-affinity receptors devoted to detect food-derived compounds at high and nutritionally relevant concentrations. For taste receptors, expressed outside the oral cavity, the question arises whether the corresponding stimuli reach concentrations relevant to modulate the receptor’s activities. For bitter receptors present in the brain, heart, and testis, this question appears most obvious: does one need to consider scenarios in which an orally consumed bitter substance reaches concentrations in the organism sufficiently high to activate Tas2rs in these tissues or would these receptors rather respond to yet undiscovered endogenous high-affinity ligands? If the latter was true, what would have been the major driving force for the development of Tas2rs during evolution, the endogenous ligand(s), or food-derived xenobiotics? Similarly, mammalian sweet taste receptors respond to natural sweet compounds in the mid- to high millimolar range [70], since blood glucose and even more so brain glucose levels are within the low millimolar concentration range [174]; under what circumstances would the sweet taste receptor will become activated?
In order to determine the physiological role of taste receptors in extragustatory tissues, it is important to identify the cell types that express the receptor gene as well as demonstrate unambiguously the involvement of the receptor in the physiological response observed upon stimulation with tastants. These two issues have rarely been addressed satisfactorily in the past. One of the reasons for the lack of data concerning the in vivo expression pattern of bitter taste receptors, e.g., in the gastrointestinal tract, is that specific antisera raised against these molecules are scarce. It would be beneficial to develop these tools in order to link the physiological activity of tastant molecules to taste receptor-dependent signaling more tightly. As it seems that some selective reagents such as an antiserum raised against the human bitter taste receptor TAS2R38 [175] start to become available, the chances that this situation will improve over time are good. Although requiring a considerable amount of effort, the generation of genetically modified mice, which strongly express marker molecules under the control of taste receptor gene promoters, represents a possible option for the identification of non-gustatory cell types producing Tas2rs [19].
Because tastants may activate cellular signals independent of taste receptors, physiological responses as a result of tastant stimulation are not necessarily sufficient evidence for the involvement of taste receptors. More convincing evidence for taste receptor-dependent activity could be obtained by knockdown/knockout approaches in suitable cellular or animal systems. Alternatively, the use of taste receptor inhibitors such as, e.g., lactisole, a selective blocker of the human sweet taste receptor, as well as the use of receptor-matched agonist sets, consisting of known activators and non-activators, should help to clarify the putative involvement of taste receptors better. This of course depends heavily on the continuing success of the in vitro characterization of taste receptor responses to identify receptor activation patterns and inhibitors for a larger panel of taste receptors.
The intriguing finding that taste receptors are expressed in numerous non-gustatory tissues and fulfill within these tissues a variety of important physiological functions does not only have implications on the way we are looking at “taste” receptors but also their agonists, the tastants. Obviously, tastants may act via the activation of extraoral taste receptors in multiple ways on the physiology of vertebrates. In particular, bitter-tasting drugs may exert many off-target effects via the activation of extraoral bitter taste receptors as pointed out in a highly recommendable recent review article [176]. In the future it will be very important to investigate such potential effects in detail in order to develop strategies to avoid adverse side effects of bitter-tasting pharmaceuticals or even to identify novel drugs based on bitter “lead” structures.
References
Behrens M, Meyerhof W (2011) Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav 105(1):4–13
Bezencon C, le Coutre J, Damak S (2007) Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32(1):41–49
Chen MC, Wu SV, Reeve JR Jr, Rozengurt E (2006) Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol 291(4):C726–C739
Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP (2013) Sensing of amino acids by the gut-expressed taste receptor T1R1–T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol 304(3):G271–G282. doi:10.1152/ajpgi.00074.2012
Dotson CD, Zhang L, Xu H, Shin YK, Vigues S, Ott SH, Elson AE, Choi HJ, Shaw H, Egan JM, Mitchell BD, Li X, Steinle NI, Munger SD (2008) Bitter taste receptors influence glucose homeostasis. PLoS One 3(12):e3974
Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP (2005) Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33(Pt 1):302–305
Geraedts MC, Takahashi T, Vigues S, Markwardt ML, Nkobena A, Cockerham RE, Hajnal A, Dotson CD, Rizzo MA, Munger SD (2012) Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab 303(4):E464–E474
Glendinning JI, Yiin YM, Ackroff K, Sclafani A (2008) Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav 93(4–5):757–765
Hao S, Sternini C, Raybould HE (2008) Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol 294(1):R33–R38
Hass N, Schwarzenbacher K, Breer H (2010) T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res 339(3):493–504
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 104:15069–15074
Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I (2011) Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A 108(5):2094–2099
Jeon TI, Zhu B, Larson JL, Osborne TF (2008) SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest 118(11):3693–3700
Kaji I, Karaki S, Fukami Y, Terasaki M, Kuwahara A (2009) Secretory effects of a luminal bitter tastant and expressions of bitter taste receptors, T2Rs, in the human and rat large intestine. Am J Physiol Gastrointest Liver Physiol 296(5):G971–G981
Kyriazis GA, Soundarapandian MM, Tyrberg B (2012) Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A 109(8):E524–E532. doi:10.1073/pnas.1115183109
Mace OJ, Affleck J, Patel N, Kellett GL (2007) Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582(Pt 1):379–392
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP (2007) T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A 104(38):15075–15080
Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I (2009) Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One 4(4):e5106. doi:10.1371/journal.pone.0005106
Prandi S, Bromke M, Hubner S, Voigt A, Boehm U, Meyerhof W, Behrens M (2013) A subset of mouse colonic goblet cells expresses the bitter taste receptor tas2r131. PLoS One 8(12):e82820. doi:10.1371/journal.pone.0082820
Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E (2006) Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291(5):G792–G802
Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M (2011) Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc 70(2):185–193. doi:10.1017/S0029665111000103
Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C (2011) The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr 30(4):524–532. doi:10.1016/j.clnu.2011.01.007
Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA (2007) Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 292(5):G1420–G1428
Wu SV, Chen MC, Rozengurt E (2005) Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics 22(2):139–149
Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A 99(4):2392–2397
Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA (2009) Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 58(3):337–346
Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB (2010) Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16(11):1299–1304
Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL (2003) Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A 100(15):8981–8986
Finger TE, Kinnamon SC (2011) Taste isn’t just for taste buds anymore. F1000 Biol Rep 3:20
Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Beauchamp GK, Doulias PT, Ischiropoulos H, Kreindler JL, Reed DR, Cohen NA (2012) T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 122(11):4145–4159. doi:10.1172/JCI64240
Orsmark-Pietras C, James A, Konradsen JR, Nordlund B, Soderhall C, Pulkkinen V, Pedroletti C, Daham K, Kupczyk M, Dahlen B, Kere J, Dahlen SE, Hedlin G, Melen E (2013) Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics. Eur Respir J 42(1):65–78. doi:10.1183/09031936.00077712
Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ (2009) Motile cilia of human airway epithelia are chemosensory. Science 325(5944):1131–1134
Tizzano M, Cristofoletti M, Sbarbati A, Finger TE (2011) Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 11:3. doi:10.1186/1471-2466-11-3
Tizzano M, Finger TE (2013) Chemosensors in the nose: guardians of the airways. Physiology (Bethesda) 28(1):51–60. doi:10.1152/physiol.00035.2012
Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE (2010) Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A 107(7):3210–3215
Voigt A, Hubner S, Lossow K, Hermans-Borgmeyer I, Boehm U, Meyerhof W (2012) Genetic labeling of Tas1r1 and Tas2r131 taste receptor cells in mice. Chem Senses 37(9):897–911
Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R (2013) The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol 11(3):e1001501. doi:10.1371/journal.pbio.1001501
Iwatsuki K, Nomura M, Shibata A, Ichikawa R, Enciso PL, Wang L, Takayanagi R, Torii K, Uneyama H (2010) Generation and characterization of T1R2-LacZ knock-in mouse. Biochem Biophys Res Commun 402(3):495–499
Li F (2013) Taste perception: from the tongue to the testis. Mol Hum Reprod 19(6):349–360. doi:10.1093/molehr/gat009
Li F, Zhou M (2012) Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol Hum Reprod 18(6):289–297
Meyer D, Voigt A, Widmayer P, Borth H, Huebner S, Breit A, Marschall S, de Angelis MH, Boehm U, Meyerhof W, Gudermann T, Boekhoff I (2012) Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca(2)(+) and cAMP concentrations in spermatozoa. PLoS One 7(2):e32354. doi:10.1371/journal.pone.0032354
Mosinger B, Redding KM, Parker MR, Yevshayeva V, Yee KK, Dyomina K, Li Y, Margolskee RF (2013) Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc Natl Acad Sci U S A 110(30):12319–12324. doi:10.1073/pnas.1302827110
Xu J, Cao J, Iguchi N, Riethmacher D, Huang L (2013) Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod 19(1):17–28. doi:10.1093/molehr/gas040
Garcia-Esparcia P, Schluter A, Carmona M, Moreno J, Ansoleaga B, Torrejon-Escribano B, Gustincich S, Pujol A, Ferrer I (2013) Functional genomics reveals dysregulation of cortical olfactory receptors in Parkinson disease: novel putative chemoreceptors in the human brain. J Neuropathol Exp Neurol 72(6):524–539. doi:10.1097/NEN.0b013e318294fd76
Ansoleaga B, Garcia-Esparcia P, Llorens F, Moreno J, Aso E, Ferrer I (2013) Dysregulation of brain olfactory and taste receptors in AD, PSP and CJD, and AD-related model. Neuroscience 248C:369–382. doi:10.1016/j.neuroscience.2013.06.034
Cong WN, Wang R, Cai H, Daimon CM, Scheibye-Knudsen M, Bohr VA, Turkin R, Wood WH 3rd, Becker KG, Moaddel R, Maudsley S, Martin B (2013) Long-term artificial sweetener acesulfame potassium treatment alters neurometabolic functions in C57BL/6J mice. PLoS One 8(8):e70257. doi:10.1371/journal.pone.0070257
Dehkordi O, Rose JE, Fatemi M, Allard JS, Balan KV, Young JK, Fatima S, Millis RM, Jayam-Trouth A (2012) Neuronal expression of bitter taste receptors and downstream signaling molecules in the rat brainstem. Brain Res 1475:1–10. doi:10.1016/j.brainres.2012.07.038
Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE (2009) Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3:12
Singh N, Vrontakis M, Parkinson F, Chelikani P (2011) Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun 406(1):146–151
Foster SR, Porrello ER, Purdue B, Chan HW, Voigt A, Frenzel S, Hannan RD, Moritz KM, Simmons DG, Molenaar P, Roura E, Boehm U, Meyerhof W, Thomas WG (2013) Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One 8(5):e64579. doi:10.1371/journal.pone.0064579
Fukuda N, Yomogida K, Okabe M, Touhara K (2004) Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci 117(Pt 24):5835–5845. doi:10.1242/jcs.01507
Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H (2003) Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299(5615):2054–2058
Elliott RA, Kapoor S, Tincello DG (2011) Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol 186(6):2455–2462. doi:10.1016/j.juro.2011.07.083
Masubuchi Y, Nakagawa Y, Ma J, Sasaki T, Kitamura T, Yamamoto Y, Kurose H, Kojima I, Shibata H (2013) A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS One 8(1):e54500. doi:10.1371/journal.pone.0054500
Simon BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, Ning X, Gallagher K, Tyrberg B, Assadi-Porter FM, Evans CR, MacDougald OA (2013) Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem 288(45):32475–32489. doi:10.1074/jbc.M113.514034
Lund TC, Kobs AJ, Kramer A, Nyquist M, Kuroki MT, Osborn J, Lidke DS, Low-Nam ST, Blazar BR, Tolar J (2013) Bone marrow stromal and vascular smooth muscle cells have chemosensory capacity via bitter taste receptor expression. PLoS One 8(3):e58945. doi:10.1371/journal.pone.0058945
Wauson EM, Zaganjor E, Lee AY, Guerra ML, Ghosh AB, Bookout AL, Chambers CP, Jivan A, McGlynn K, Hutchison MR, Deberardinis RJ, Cobb MH (2012) The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol Cell 47(6):851–862. doi:10.1016/j.molcel.2012.08.001
Chaudhari N, Roper SD (2010) The cell biology of taste. J Cell Biol 190(3):285–296
Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS (2010) The cells and peripheral representation of sodium taste in mice. Nature 464(7286):297–301
Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS (2006) The cells and logic for mammalian sour taste detection. Nature 442(7105):934–938
Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A 103(33):12569–12574
LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL (2006) Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem 98(1):68–77
Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, Ninomiya Y (2011) Sour taste responses in mice lacking PKD channels. PLoS One 6(5):e20007. doi:10.1371/journal.pone.0020007
Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK (2001) Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26(7):925–933
Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS (1999) Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 96(4):541–551
Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A (2001) Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 283(1):236–242
Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF (2001) Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28(1):58–63
Montmayeur JP, Liberles SD, Matsunami H, Buck LB (2001) A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4(5):492–498
Sainz E, Korley JN, Battey JF, Sullivan SL (2001) Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77(3):896–903
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E (2002) Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A 99(7):4692–4696
Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS (2002) An amino-acid taste receptor. Nature 416(6877):199–202
Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS (2000) A novel family of mammalian taste receptors. Cell 100(6):693–702
Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ (2000) T2Rs function as bitter taste receptors. Cell 100(6):703–711
Matsunami H, Montmayeur JP, Buck LB (2000) A family of candidate taste receptors in human and mouse. Nature 404(6778):601–604
Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407(6807):971–977. doi:10.1038/35039564
Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C (1998) GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396(6712):674–679. doi:10.1038/25348
Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B (1998) GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396(6712):683–687. doi:10.1038/25360
Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC (1999) Role of heteromer formation in GABAB receptor function. Science 283(5398):74–77
White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH (1998) Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396(6712):679–682. doi:10.1038/25354
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106(3):381–390
Dong D, Jones G, Zhang S (2009) Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evol Biol 9:12
Behrens M, Meyerhof W (2013) Bitter taste receptor research comes of age: from characterization to modulation of TAS2Rs. Semin Cell Dev Biol 24(3):215–221. doi:10.1016/j.semcdb.2012.08.006
Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M (2010) The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35(2):157–170
Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS (2006) The receptors and cells for mammalian taste. Nature 444(7117):288–294
Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999) Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2(12):1055–1062
Rossler P, Boekhoff I, Tareilus E, Beck S, Breer H, Freitag J (2000) G protein betagamma complexes in circumvallate taste cells involved in bitter transduction. Chem Senses 25(4):413–421
McLaughlin SK, McKinnon PJ, Margolskee RF (1992) Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357(6379):563–569
Rossler P, Kroner C, Freitag J, Noe J, Breer H (1998) Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol 77(3):253–261
Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112(3):293–301
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC (2001) Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci 2:6
Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K (2007) Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem 282:37225–37231
Miyoshi MA, Abe K, Emori Y (2001) IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses 26(3):259–265
Liu D, Liman ER (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A 100(25):15160–15165
Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5(11):1169–1176
Zhang Z, Zhao Z, Margolskee R, Liman E (2007) The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci 27(21):5777–5786
Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310(5753):1495–1499
Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2007) The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci U S A 104(15):6436–6441
Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS (2007) Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 26(3):657–667
Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK (2013) CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495(7440):223–226. doi:10.1038/nature11906
Cohen SP, Buckley BK, Kosloff M, Garland AL, Bosch DE, Cheng G Jr, Radhakrishna H, Brown MD, Willard FS, Arshavsky VY, Tarran R, Siderovski DP, Kimple AJ (2012) Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J Biol Chem 287(50):41706–41719. doi:10.1074/jbc.M112.423806
von Buchholtz L, Elischer A, Tareilus E, Gouka R, Kaiser C, Breer H, Conzelmann S (2004) RGS21 is a novel regulator of G protein signalling selectively expressed in subpopulations of taste bud cells. Eur J Neurosci 19(6):1535–1544
Hofer D, Puschel B, Drenckhahn D (1996) Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A 93(13):6631–6634
Ribatti D, Nico B, Crivellato E, Artico M (2006) Development of the blood-brain barrier: a historical point of view. Anat Rec B New Anat 289(1):3–8. doi:10.1002/ar.b.20087
Benda P, Lightbody J, Sato G, Levine L, Sweet W (1968) Differentiated rat glial cell strain in tissue culture. Science 161(3839):370–371
Pydi SP, Bhullar RP, Chelikani P (2012) Constitutively active mutant gives novel insights into the mechanism of bitter taste receptor activation. J Neurochem 122(3):537–544
Hodges MR, Richerson GB (2010) Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol 173(3):256–263. doi:10.1016/j.resp.2010.03.006
Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R (2008) Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 295(2):G260–G272
Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, Bronk JR, Kellett GL, Meredith D, Boyd R, Pieri M, Bailey PD, Pettcrew R, Foley D (2009) An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol 587(Pt 1):195–210
Swartz TD, Duca FA, de Wouters T, Sakar Y, Covasa M (2012) Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br J Nutr 107(5):621–630. doi:10.1017/S0007114511003412
Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, Horowitz M, Rayner CK (2013) Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes 62(10):3532–3541. doi:10.2337/db13-0581
Haid D, Widmayer P, Breer H (2011) Nutrient sensing receptors in gastric endocrine cells. J Mol Histol 42(4):355–364. doi:10.1007/s10735-011-9339-1
Hass N, Schwarzenbacher K, Breer H (2007) A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol 128(5):457–471
Sothilingam V, Hass N, Breer H (2011) Candidate chemosensory cells in the stomach mucosa of young postnatal mice during the phases of dietary changes. Cell Tissue Res 344(2):239–249. doi:10.1007/s00441-011-1137-2
Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP (2010) Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr 104(5):637–646
Widmayer P, Breer H, Hass N (2011) Candidate chemosensory cells in the porcine stomach. Histochem Cell Biol 136(1):37–45. doi:10.1007/s00418-011-0824-0
Batchelor DJ, Al-Rammahi M, Moran AW, Brand JG, Li X, Haskins M, German AJ, Shirazi-Beechey SP (2011) Sodium/glucose cotransporter-1, sweet receptor, and disaccharidase expression in the intestine of the domestic dog and cat: two species of different dietary habit. Am J Physiol Regul Integr Comp Physiol 300(1):R67–R75. doi:10.1152/ajpregu.00262.2010
Daly K, Al-Rammahi M, Arora DK, Moran AW, Proudman CJ, Ninomiya Y, Shirazi-Beechey SP (2012) Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am J Physiol Regul Integr Comp Physiol 303(2):R199–R208. doi:10.1152/ajpregu.00031.2012
Taniguchi K (2004) Expression of the sweet receptor protein, T1R3, in the human liver and pancreas. J Vet Med Sci 66(11):1311–1314
Hofer D, Drenckhahn D (1998) Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol 110(3):303–309
Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, Margolskee RF (1995) Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature 376(6535):80–85
Hofer D, Asan E, Drenckhahn D (1999) Chemosensory perception in the gut. News Physiol Sci 14:18–23
Bezencon C, Furholz A, Raymond F, Mansourian R, Metairon S, Le Coutre J, Damak S (2008) Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol 509(5):514–525. doi:10.1002/cne.21768
Li Y, Kokrashvili Z, Mosinger B, Margolskee RF (2013) Gustducin couples fatty acid receptors to GLP-1 release in colon. Am J Physiol Endocrinol Metab 304(6):E651–E660. doi:10.1152/ajpendo.00471.2012
Eberle JA, Richter P, Widmayer P, Chubanov V, Gudermann T, Breer H (2013) Band-like arrangement of taste-like sensory cells at the gastric groove: evidence for paracrine communication. Front Physiol 4:58. doi:10.3389/fphys.2013.00058
Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V (2007) TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8:49. doi:10.1186/1471-2202-8-49
Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H, Margolskee RF, Mosinger B (2009) Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology 137(2):598–606, 606 e591–592. doi:10.1053/j.gastro.2009.02.070
Gerbe F, Legraverend C, Jay P (2012) The intestinal epithelium tuft cells: specification and function. Cell Mole Life Sci 69(17):2907–2917. doi:10.1007/s00018-012-0984-7
Luciano L, Reale E, Ruska H (1968) On a glycogen containing brusc cell in the rectum of the rat. Z Zellforsch Mikrosk Anat 91(1):153–158
Sternini C, Anselmi L, Rozengurt E (2008) Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes 15(1):73–78
Le Neve B, Foltz M, Daniel H, Gouka R (2010) The steroid glycoside H.g.-12 from Hoodia gordonii activates the human bitter receptor TAS2R14 and induces CCK release from HuTu-80 cells. Am J Physiol Gastrointest Liver Physiol 299:G1368–G1375
McIntyre N, Holdsworth CD, Turner DS (1964) New interpretation of oral glucose tolerance. Lancet 2(7349):20–21
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132(6):2131–2157. doi:10.1053/j.gastro.2007.03.054
Sato S, Hokari R, Kurihara C, Sato H, Narimatsu K, Hozumi H, Ueda T, Higashiyama M, Okada Y, Watanabe C, Komoto S, Tomita K, Kawaguchi A, Nagao S, Miura S (2013) Dietary lipids and sweeteners regulate glucagon-like peptide-2 secretion. Am J Physiol Gastrointest Liver Physiol 304(8):G708–G714. doi:10.1152/ajpgi.00282.2012
Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C (2011) The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab 301(2):E317–E325. doi:10.1152/ajpendo.00077.2011
Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR (2011) Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr 65:508–513
Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, Kieffer TJ (2009) Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 296(3):E473–E479
Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL, Horowitz M, Rayner CK (2009) Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296(4):G735–G739
Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I (2013) Multimodal function of the sweet taste receptor expressed in pancreatic beta-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J 60(10):1191–1206
Nakagawa Y, Ohtsu Y, Nagasawa M, Shibata H, Kojima I (2014) Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr J 61:119–131
Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS (2003) The receptors for mammalian sweet and umami taste. Cell 115(3):255–266
Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC (1993) Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366(6455):575–580. doi:10.1038/366575a0
Wellendorph P, Brauner-Osborne H (2004) Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335:37–46. doi:10.1016/j.gene.2004.03.003
Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Brauner-Osborne H (2005) Deorphanization of GPRC6A: a promiscuous l-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol 67(3):589–597. doi:10.1124/mol.104.007559
Torres N, Noriega L, Tovar AR (2009) Nutrient modulation of insulin secretion. Vitam Horm 80:217–244. doi:10.1016/S0083-6729(08)00609-2
Imoto T, Miyasaka A, Ishima R, Akasaka K (1991) A novel peptide isolated from the leaves of Gymnema sylvestre–I. Characterization and its suppressive effect on the neural responses to sweet taste stimuli in the rat. Comp Biochem Physiol A Comp Physiol 100(2):309–314
Ninomiya Y, Imoto T (1995) Gurmarin inhibition of sweet taste responses in mice. Am J Physiol 268(4 Pt 2):R1019–R1025
Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G (2000) Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr 130(4S Suppl):950S–953S
Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T (1991) Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav 49(5):919–925
Nakamura E, Hasumura M, San Gabriel A, Uneyama H, Torii K (2010) New frontiers in gut nutrient sensor research: luminal glutamate-sensing cells in rat gastric mucosa. J Pharmacol Sci 112(1):13–18
Straub SG, Mulvaney-Musa J, Yajima H, Weiland GA, Sharp GW (2003) Stimulation of insulin secretion by denatonium, one of the most bitter-tasting substances known. Diabetes 52(2):356–364
Behrens M, Meyerhof W (2010) Oral and extraoral bitter taste receptors. In: Meyerhof W, Beisiegel U, Joost H-G (eds) Sensory and metabolic control of energy balance, vol 52. Results and problems in cell differentiation. Springer, Heidelberg, pp 87-99. doi:10.1007/978-3-642-14426-4_8
Little TJ, Gupta N, Case RM, Thompson DG, McLaughlin JT (2009) Sweetness and bitterness taste of meals per se does not mediate gastric emptying in humans. Am J Physiol Regul Integr Comp Physiol 297(3):R632–R639
Wicks D, Wright J, Rayment P, Spiller R (2005) Impact of bitter taste on gastric motility. Eur J Gastroenterol Hepatol 17(9):961–965
Jeon TI, Seo YK, Osborne TF (2011) Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J 438(1):33–37
Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L (2009) Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol 296(3):R528–R536
Sbarbati A, Tizzano M, Merigo F, Benati D, Nicolato E, Boschi F, Cecchini MP, Scambi I, Osculati F (2009) Acyl homoserine lactones induce early response in the airway. Anat Rec 292(3):439–448. doi:10.1002/ar.20866
Barham HP, Cooper SE, Anderson CB, Tizzano M, Kingdom TT, Finger TE, Kinnamon SC, Ramakrishnan VR (2013) Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol 3(6):450–457. doi:10.1002/alr.21149
Lee RJ, Chen B, Redding KM, Margolskee RF, Cohen NA (2013) Mouse nasal epithelial innate immune responses to Pseudomonas aeruginosa quorum-sensing molecules require taste signaling components. Innate Immun. doi:10.1177/1753425913503386
Belvisi MG, Dale N, Birrell MA, Canning BJ (2011) Bronchodilator activity of bitter tastants in human tissue. Nat Med 17(7):776. doi:10.1038/nm0711-776a, author reply 776–778
Morice AH, Bennett RT, Chaudhry MA, Cowen ME, Griffin SC, Loubani M (2011) Effect of bitter tastants on human bronchi. Nat Med 17(7):775. doi:10.1038/nm0711-775
Behrens M, Brockhoff A, Kuhn C, Bufe B, Winnig M, Meyerhof W (2004) The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem Biophys Res Commun 319(2):479–485
Levit A, Nowak S, Peters M, Wiener A, Meyerhof W, Behrens M, Niv MY (2013) The bitter pill: clinical drugs that activate the human bitter taste receptor TAS2R14. FASEB J. doi:10.1096/fj.13-242594
Zhou PZ, Babcock J, Liu LQ, Li M, Gao ZB (2011) Activation of human ether-a-go-go related gene (hERG) potassium channels by small molecules. Acta Pharmacol Sin 32(6):781–788. doi:10.1038/aps.2011.70
Yoshida M, Yoshida K (2011) Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol Hum Reprod 17(8):457–465. doi:10.1093/molehr/gar041
Kaupp UB, Kashikar ND, Weyand I (2008) Mechanisms of sperm chemotaxis. Annu Rev Physiol 70:93–117. doi:10.1146/annurev.physiol.70.113006.100654
Behrens M, Bartelt J, Reichling C, Winnig M, Kuhn C, Meyerhof W (2006) Members of RTP and REEP gene families influence functional bitter taste receptor expression. J Biol Chem 281(29):20650–20659
Fehr J, Meyer D, Widmayer P, Borth HC, Ackermann F, Wilhelm B, Gudermann T, Boekhoff I (2007) Expression of the G-protein alpha-subunit gustducin in mammalian spermatozoa. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 193(1):21–34
Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, Brand JG (2005) Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genet 1(1):27–35
Shi P, Zhang J (2006) Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol Biol Evol 23(2):292–300
Li R, Fan W, Tian G, Zhu H, He L, Cai J, Huang Q, Cai Q, Li B, Bai Y, Zhang Z, Zhang Y, Wang W, Li J, Wei F, Li H, Jian M, Li J, Zhang Z, Nielsen R, Li D, Gu W, Yang Z, Xuan Z, Ryder OA, Leung FC, Zhou Y, Cao J, Sun X, Fu Y, Fang X, Guo X, Wang B, Hou R, Shen F, Mu B, Ni P, Lin R, Qian W, Wang G, Yu C, Nie W, Wang J, Wu Z, Liang H, Min J, Wu Q, Cheng S, Ruan J, Wang M, Shi Z, Wen M, Liu B, Ren X, Zheng H, Dong D, Cook K, Shan G, Zhang H, Kosiol C, Xie X, Lu Z, Zheng H, Li Y, Steiner CC, Lam TT, Lin S, Zhang Q, Li G, Tian J, Gong T, Liu H, Zhang D, Fang L, Ye C, Zhang J, Hu W, Xu A, Ren Y, Zhang G, Bruford MW, Li Q, Ma L, Guo Y, An N, Hu Y, Zheng Y, Shi Y, Li Z, Liu Q, Chen Y, Zhao J, Qu N, Zhao S, Tian F, Wang X, Wang H, Xu L, Liu X, Vinar T, Wang Y, Lam TW, Yiu SM, Liu S, Zhang H, Li D, Huang Y, Wang X, Yang G, Jiang Z, Wang J, Qin N, Li L, Li J, Bolund L, Kristiansen K, Wong GK, Olson M, Zhang X, Li S, Yang H, Wang J, Wang J (2010) The sequence and de novo assembly of the giant panda genome. Nature 463(7279):311–317. doi:10.1038/nature08696
Jiang P, Josue J, Li X, Glaser D, Li W, Brand JG, Margolskee RF, Reed DR, Beauchamp GK (2012) Major taste loss in carnivorous mammals. Proc Natl Acad Sci U S A 109(13):4956–4961
Zhao H, Xu D, Zhang S, Zhang J (2012) Genomic and genetic evidence for the loss of umami taste in bats. Genome Biol Evol 4(1):73–79. doi:10.1093/gbe/evr126
Zhao H, Zhou Y, Pinto CM, Charles-Dominique P, Galindo-Gonzalez J, Zhang S, Zhang J (2010) Evolution of the sweet taste receptor gene Tas1r2 in bats. Mol Biol Evol 27(11):2642–2650. doi:10.1093/molbev/msq152
Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE (2009) Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci 29(21):7015–7022. doi:10.1523/JNEUROSCI.0334-09.2009
Behrens M, Born S, Redel U, Voigt N, Schuh V, Raguse JD, Meyerhof W (2012) Immunohistochemical detection of TAS2R38 protein in human taste cells. PLoS One 7(7):e40304
Clark AA, Liggett SB, Munger SD (2012) Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J 26(12):4827–4831. doi:10.1096/fj.12-215087
Acknowledgments
The authors thank the German Ministry of Education and Research (BMBF, #0315669, to WM and MB) for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Behrens, M., Prandi, S., Meyerhof, W. (2014). Taste Receptor Gene Expression Outside the Gustatory System. In: Krautwurst, D. (eds) Taste and Smell. Topics in Medicinal Chemistry, vol 23. Springer, Cham. https://doi.org/10.1007/7355_2014_79
Download citation
DOI: https://doi.org/10.1007/7355_2014_79
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48925-4
Online ISBN: 978-3-319-48927-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)