Abstract
Hearing loss and impaired fertility are common human disorders each with multiple genetic causes. Sometimes deafness and impaired fertility, which are the hallmarks of Perrault syndrome, co-occur in a person. Perrault syndrome is inherited as an autosomal recessive disorder characterized by bilateral mild to severe childhood sensorineural hearing loss with variable age of onset in both sexes and ovarian dysfunction in females who have a 46, XX karyotype. Since the initial clinical description of Perrault syndrome 70 years ago, the phenotype of some subjects may additionally involve developmental delay, intellectual deficit and other neurological disabilities, which can vary in severity in part dependent upon the genetic variants and the gene involved. Here, we review the molecular genetics and clinical phenotype of Perrault syndrome and focus on supporting evidence for the eight genes (CLPP, ERAL1, GGPS1, HARS2, HSD17B4, LARS2, RMND1, TWNK) associated with Perrault syndrome. Variants of these eight genes only account for approximately half of the individuals with clinical features of Perrault syndrome where the molecular genetic base remains under investigation. Additional environmental etiologies and novel Perrault disease-associated genes remain to be identified to account for unresolved cases. We also report a new genetic variant of CLPP, computational structural insight about CLPP and single cell RNAseq data for eight reported Perrault syndrome genes suggesting a common cellular pathophysiology for this disorder. Some unanswered questions are raised to kindle future research about Perrault syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seventy years ago, M. Perrault described a rare multisystem disorder of deafness and infertility in two sisters (Josso et al. 1963; Pallister and Opitz 1979; Perrault et al. 1951). Since then, more cases of Perrault syndrome have been reported and it is now recognized as a clinically and genetically heterogenous sex-influenced disorder that has an autosomal recessive mode of inheritance (Demain et al. 2017; Newman et al. 2018). Perrault syndrome is characterized in females by moderate to profound sensorineural hearing loss with variable age of onset, usually the initial finding, a range of ovarian dysfunction with the most severe being the absence of ovaries despite a normal 46,XX karyotype. When ovaries are absent, estrogens are present at significantly lower levels, secondary sex characteristics are under-developed, and gonadotropins are elevated. A usual presentation of Perrault syndrome is a bilateral hearing loss, primary amenorrhea and a hormone profile consistent with hypergonadotropic hypogonadism or primary ovarian insufficiency (POI) characterized by menses cessation before the fourth decade of life (Newman et al. 2018). In some families with females affected by Perrault syndrome, the deaf males were not tested for fertility, there were no deaf males or the males are prepubertal (Jenkinson et al. 2013; Newman et al. 2018). Perrault syndrome may not be suspected when a deaf prepubertal female is ascertained and only her hearing ability is evaluated. Males with pathogenic variants in Perrault syndrome genes, without affected female siblings, are now being ascertained due to the increased application of genetic testing, including exomes and large panels of genes associated with hearing loss. A pre-pubertal deaf female seeking an explanation for her hearing loss, but lacking a molecular diagnosis, may not have an ovarian dysfunction noticed by an astute clinician or by the patient until after the expected onset of puberty. Similarly, the increased use of genetic testing for presumed isolated deafness can identify pre-pubertal females with a possible diagnosis of Perrault syndrome before ovarian dysfunction has presented clinically.
Two clinical classifications of Perrault syndrome have been proposed (Fiumara et al. 2004; Newman et al. 2018; Pierce et al. 2010). Both Type 1 and Type 2 include the two cardinal signs of sensorineural hearing loss and POI. For the Type 2 subgroup of Perrault syndrome, there is a more complex presentation that may include intellectual disability, progressive sensory disorders or motor neuropathies. In some of the Type 2 cases, there is reluctance to necessarily assume that the additional neurological features are the direct result of the variants associated with the classical features of Perrault syndrome rather than coincidental clinical findings (Demain et al. 2018; Fiumara et al. 2004; Gottschalk et al. 1996). In both Type 1 and Type 2 Perrault syndrome, estrogen levels are decreased while the gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH) are significantly elevated. The two clinical subtypes (Type 1 and 2) of Perrault syndrome should not be confused with the consecutive number types on the Online Mendelian Inheritance of Man (OMIM) that have been assigned to each gene with variants associated with Perrault syndrome.
In this review we focus on genes where variants result in a clinical presentation of POI and SNHL as the primary features while acknowledging that more deleterious variants in these same genes can lead to more severe clinical presentations. We excluded from discussion genes where variants are associated with a broad spectrum of clinical features which include POI and SNHL. The genetic bases of Perrault syndrome involve recessive pathogenic variants of at least eight different genes described below and in Fig. 1. In many of the reported cases of Perrault syndrome the phenotype is variable within a family suggesting environmental influences, modifier variants in the genetic background or both. Seven of the eight genes with autosomal recessive variants associated with Perrault syndrome encode mitochondrial proteins while HSD17B4 is a peroxisomal protein.
The eight genes to date reported to be associated with Perrault syndrome, their pathogenic variants and locations in the exons or introns of these genes. There are two published deletions of CLPP that are in compound heterozygosity with amino acid substitutions. Deletion 1 was reported to be in trans to p.G162S while deletion 2 was in trans to p.P142L (Theunissen et al. 2016). For HARS2, # indicates variants that are associated with sensorineural hearing loss (Supplementary Table 1 for all reported variants and references). The ## for a variant of HSD17B4 indicates an association with premature ovarian failure while D/PS indicates variants associated with d-bifunctional protein deficiency/Perrault syndrome Type 1. The p.R663W variant of LARS2 was previously associated with Intellectual Disability (Cherot et al. 2018) although the same variant was reported for Perrault syndrome (Tucker et al. 2020). The homozygous N238S variant of RMND1 is associated with a Perrault-like syndrome with renal defects. Interestingly, the same variant in compound heterozygosity with p.Gln189* or c.613G > T is associated with a more severe phenotype that also includes chronic kidney disease, dilated cardiomyopathy, neurological involvement, neonatal lactic acidosis and deafness (Broenen et al. 2019; Gupta et al. 2016; Janer et al. 2015; Ravn et al. 2016; Shayota et al. 2019)
CLPP
The CLPP gene is located on human chromosome 19p13 and encodes caseinolytic peptidase proteolytic subunit (Jenkinson et al. 2013). Molecular genetic data in human and mouse models support the conclusion that recessive variants of CLPP result in Perrault syndrome Type 1 or Type 2 (Fig. 1). The primary sequence of the nascent CLPP protein has a N-terminus mitochondrial import sequence. The mature CLPP monomer multimerizes in the mitochondrial matrix to form a heptamer that docks with CLPXP (AAA + family member caseinolytic peptidase X) forming a tetradecamer (CLPP-CLPX) necessary in this organelle for proteostasis. Regulated ATP-dependent degradation of a subset of mitochondrial proteins for quality control and regulation of translation occurs when a protein is recognized by the hexameric ring of CLPX, which unfolds and translocates the protein strand through the axial pore of the tetradecamer into the barrel-shaped chamber enclosing 14 peptidase active sites (Brodie et al. 2018; Flanagan et al. 1995; Kang et al. 2004; Wang et al. 1997).
Multiple crystal structures of CLPP from several organisms reveal a conserved architecture (Gottesman 1996; Jenkinson et al. 2013; Szyk and Maurizi 2006). The structures of CLPP were then used to deduce possible functional impairments caused by pathogenic missense variants of human CLPP associated with Perrault syndrome (Brodie et al. 2018; Jenkinson et al. 2013). CLPP was first identified as a Perrault syndrome gene in three consanguineous families with profound deafness. In two of the three families, deaf females were diagnosed with POI. Genetic linkage analyses of the deafness phenotype revealed a locus on chromosome 19. Subsequently, the families were found to be segregating two different homozygous missense variants and a homozygous splice site variant. Hormonal profiles were evaluated for some of the deaf females and imaging of the ovaries were performed (Jenkinson et al. 2013). In family PDF1, one affected female had two male children. Notably she had a premature menopause at 23 years, consistent with ovarian insufficiency. One of her affected sisters had secondary amenorrhea whereas the other had streak ovaries and hypergonadotropic hypogonadism. All three sisters in this family have epilepsy, short stature, microcephaly and mild learning disability. Four affected females of a second family had primary amenorrhea, hypergonadotropic hypogonadism, rudimentary uteruses and small ovaries by ultrasonography (Jenkinson et al. 2013). To date, there are 13 different variants associated with Perrault syndrome that affect single base pairs of CLPP and two intragenic deletions of CLPP (Fig. 1) (Theunissen et al. 2016). Damaging variants of the eight genes associated with Perrault syndrome (Fig. 1) are either absent or at low frequency in the public human variant databases.
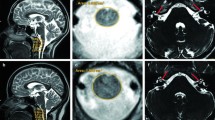
Individuals diagnosed with Perrault syndrome who have biallelic variants of CLPP (Fig. 1) are usually profoundly deaf. This hearing loss phenotype could be an ascertainment bias (Jenkinson et al. 2013) as individuals who are profoundly deaf are more likely to undergo genetic testing than individuals with more modest impairment. Here, we describe a homozygous missense variant of CLPP that results in moderate to severe hearing loss in an individual diagnosed with Perrault syndrome (Fig. S1). The homozygous p.T135S missense variant of CLPP is segregating in a consanguineous Pakistani family (HLZM05) with a proband who is an 18-year-old woman reporting childhood onset bilateral sensorineural moderate to severe hearing loss similar to the auditory phenotype of her 20-year-old brother. She had primary amenorrhea and self-reports that her secondary sexual characteristics are underdeveloped. Taken together, the p.T135S variant of CLPP segregating in family HLZM05 is associated with Type 1 Perrault syndrome with a moderate to severe hearing loss.
Variants that are null alleles or severely disable CLPP function result in more severe cases of Perrault syndrome. For instance, two severely affected siblings were homozygous for a frameshift variant (c.21delA) in exon 1 of CLPP (premature translation stop codon in exon 3) predicted to truncate 160 residues (Theunissen et al. 2016). The authors demonstrated that the c.21delA allele causes nonsense mediated decay of CLPP mRNA. The phenotype of the affected siblings included small stature, congenital sensorineural deafness, psychomotor retardation, ataxia, autism and epilepsy. Brain-MRI signal abnormalities were observed in the subcortical and deep cerebral white matter. Similar leukoencephalopathies were reported in three other patients with variants of CLPP one of whom was hemizygous for a missense mutation (G162S) predicted to destabilize and disrupt CLPP structure. This missense variant was in trans to a deletion (Fig. 1) inherited from the healthy mother that spanned exons 3 and 4 of CLPP (Theunissen et al. 2016).
CLPP protein is readily detected in mitochondria of human ovarian germ cells but was not detected in the stromal layers (Jenkinson et al. 2013) while in mouse ovaries, CLPP was immunolocalized in granulosa cells and oocytes. In the mouse inner ear, in the organ of Corti, CLPP is predominantly localized in supporting cells and weakly in adjacent sensory hair cells at postnatal day 3 (Jenkinson et al. 2013) even though hair cells have abundant mitochondria (Weaver and Schweitzer 1994). The functional significance of the cell-type restricted expression of CLPP and the limited phenotypic consequence of diminished CLPP activity is not well understood in human but has been explored in Clpp mutant mice (Gispert et al. 2013).
Three different CLPP deficient mouse models have been reported, two independent gene-traps in intron 1 and intron 2 (Gispert et al. 2013) and a conditional knockout via cre-recombination mediated excision of floxed exons 3, 4 and 5 (Szczepanowska et al. 2016). The phenotypes of these CLPP deficient mice show important differences in viability of adults for reasons not fully understood (Szczepanowska et al. 2016). One study reported some postnatal lethality (Gispert et al. 2013) while for homozygous Clpp exon 3–5 deleted mice, no difference was observed for adult survival when compared to the wild type, although there was significant embryonic lethality (Szczepanowska et al. 2016). Males and females in both CLPP deficient mouse models are hearing impaired and sterile, suggesting a more careful evaluation of fertility status is warranted for deaf human males with biallelic variants of CLPP. There is one example of a deaf male proband homozygous for the p.C144R variant of CLPP who is azoospermic (Demain et al. 2017).
We used template-based molecular modelling to analyze the possible structural and functional impact of each of the ten reported and one novel missense variants of CLPP associated with Perrault syndrome (Fig. 1). Each mutant structural model was obtained using the X-ray structure of human CLPP (hCLPP; PDB id:1tg6) (Kang et al. 2004) as template and structurally superimposed on the CLPP-CLPX complex from Neisseria meningitidis (PDB id: 6vfs) (Ripstein et al. 2020) to analyze the variant impact on the proteolytic active complex formed by two heptameric CLPP rings bound to a CLPX pentamer. Four wild type residues (p.C86, p.T135, p.C144 and p.G162), mutated in human CLPP-associated Perrault syndrome (Fig. 1), have a crucial role in different interacting networks. Their side chains establish key interactions with other residues where, or close to where, CLPX binds to CLPP. These are important contact regions as they are responsible for maintaining the 3D-architecture of the CLPX binding site. For example, p.C86 takes part in an intra- and inter-protomeric network of residue-residue interactions located deep in the CLPP cavity, which is abolished when the p.C86 is substituted with tyrosine (p.C86Y). p.T135 stabilizes the packing between two alpha-helices that face the cavity by stabilizing a set of hydrophobic contacts with its methyl group (Fig. 2). The substitution of p.T135 by a serine (p.T135S) removes this key methyl group, completely abolishing the hydrophobic interactions in this region. The p.G162 residue is located in a tightly packed region that involves the loop containing p.G162 itself and two beta-strands that shape CLPX binding site. The introduction of the larger serine residue at this position (p.G162S), induces allosteric clashes between the new side chain and the hydrophobic residues in the region in agreement with the previous work (Theunissen et al. 2016). The potential detrimental effect of these three substitutions, p.C86Y, p.G162S and p.T135S, on the shape of the CLPX binding site are predicted to impair formation of the CLPP-CLPX active complex and consequently diminishing CLPP proteolytic function (Fig. 2). In contrast to p.C86, p.G162 and p.T135 residues, which are not located directly in the CLPX binding site, p.C144 faces the cavity where CLPX binds. The p.C144R substitution introduces a large, positively charged residue directly in the CLPX binding site that not only is predicted to change the volume available for CPLX to bind to CLPP but also the electrostatic potential of the pocket itself.
Structural models of amino acid substitutions of CLPP protein associated with Perrault syndrome. a Human CLPP X-ray structure without a ligand bound (apo) and with ONC201 bound (CLPP ring1) superimposed on the CLPP-CLPX complex formed by two CLPP rings and one heptamer of CLPX. ONC201 is shown as sticks in mustard color and the CLPP heptamer corresponding to the apo structure. CLPP and CLPX are represented as cartoon where the superimposed apo CLPP (ring 1) is highlighted in a different color per protomer, while the remainder CLPP heptamer (ring2) and the CLPX pentamer (corresponding to the CLPP-CLPX structure) are gray and red colored. The C-alphas of the residues mutated in Perrault syndrome are shown as spheres only in one of the CLPP protomers (green) where the residues in or close to the CLPX binding site are dark-green colored. b Structural comparison of the interacting networks where C86, T135, C144 G162 and Y229 participate in the WT and the corresponding modelled variant. As before, the model of each mutant was structurally superimposed on the CLPP-CLPX complex. The interactions between residues are shown as dashed lines and the residues participating in each interacting network are shown as sticks
The substitutions p.P142L, p.T145P, p.C147S and p.I208M (Supplementary Fig. 2) have a more subtle impact on the structure, which is more difficult to relate to changes in CLPX binding or protease activity. We predict that p.P142L introduces a large hydrophobic residue that might change the local packing in agreement with published speculation that this may affect CLPP-CLPX binding (Theunissen et al. 2016). The p.T145P substitution completely abolishes the local interacting network that involves a loop and two helices. Yet such a change is not predicted to affect CLPX binding but may induce a toxic-gain of protease activity (Brodie et al. 2018). p.C147S has a minimal structural impact which agrees with the fact that such a mutation does not affect CLPX binding or protease activity and may explain the mild symptoms observed in patients with this mutation (Brodie et al. 2018). The substitution of p.I208 by a methionine that contains a larger side chain introduces steric clashes in a region (Brodie et al. 2018) and is close to the catalytic triad, possibly impacting the protease activity of the CLPP mutant.
While the variants p.C86Y, p.G162S, p.T135S and p.C144R misshape the CLPX binding site of CLPP, p.Y229D has severe structural impact as it affects different aspects of CLPP. p.Y229 is part of the interacting network located at the interface between CLPP protomers and is also close to the catalytic triad responsible of the protease activity of CLPP. The introduction of an aspartate at position 229 may abolish the interactions at the interface between protomers destabilizing the formation of the heptameric ring of CLPP itself but it may also affect the relative orientation of the catalytic triad. These observations agree with the experiments of Brodie and co-authors in which p.Y229D not only affects the correct formation of the heptameric CLPP complex and subsequent CLPX binding but also decreases the protease activity of CLPP (Brodie et al. 2018). Considering that p.C86Y, p.G162S, p.T135S, p.P142L and p.C144R are each predicted to impair binding of CLPX to form a CLPP proteolytic active complex, we hypothesize that the missense-mutated CLPP may, nevertheless, still be available for a CLPX-independent “chemical activation” using small molecules such as imipridones. This family of compounds, for instance ONC201, is used as chemical activators of CLPP as they induce similar structural changes in CLPP when CLPX binds to CLPP (Bonner et al. 2020). ONC201 binds in the same CLPP cavity as CLPX (Ishizawa et al. 2019) and it activates the CLPP proteolytic machinery, but without the need for CLPX and is already being used in the clinic as a cancer chemotherapeutic drug (Bonner et al. 2020). We further speculate that imipridone compounds may improve hearing and fertility in a subset of Perrault syndrome patients. One prerequisite for this potential therapeutic intervention to work in patients with an amino acid substitution of CLPP predicted to abolish the binding of CLPX to CLPP, is a lack of secondary structural degeneration of the inner ear at the time of treatment. In addition, females should have intact ovaries.
ERAL1
ERAL1 is a nuclear gene that encodes a 48 kDa mitochondrial protein that is indispensable for the assembly of the 12S ribosomal RNA subunit. Exome sequencing of a male and three females of Dutch ancestry revealed a homozygous missense variant (c.707A > T; p.N236I) of ERAL1 (Era-like 12S mitochondrial rRNA chaperone) (Fig. 1) associated with Perrault syndrome (Chatzispyrou et al. 2017). All affected individuals had variable degrees of progressive sensorineural hearing loss. There was a spectrum of ovarian insufficiency in the females from early menopause at age 27 years to complete ovarian dysgenesis. This c.707A > T is a founder variant with an allele frequency of 4.6% (49 heterozygotes of 530 individuals) in a Dutch isolate (Chatzispyrou et al. 2017). To date no other variants in ERAL1 have been reported to be associated with Perrault syndrome. However, the authors of the original discovery paper provided convincing supporting experimental data for the pathogenicity of p.N236I damaging the normal function of ERAL1 (Chatzispyrou et al. 2017). The asparagine at residue 236 is evolutionarily conserved and directly interacts with GTP. Cultured skin fibroblasts from one of the Perrault subjects displayed decreased levels of ERAL1 protein compared to control fibroblasts, impaired assembly of the small 28S ribosomal subunit and a 30 to 40% reduced level of proteins composing the small 28S mitochondrial ribosomal subunit. Excess expression of ERAL1 in these fibroblasts restores 12S ribosomal RNA levels. RNAi knockdown in the C. elegans orthologue of human ERAL1 induced reduced fecundity. Moreover, in the wild type mouse, the necessary and timely normal withdrawal of ERAL1 from the maturing mitochondrial ribosomes requires CLPX and CLPP (CLPXP) proteolytic function (Szczepanowska et al. 2016). In mouse, the absence of CLPP proteolysis of ERAL1 results in its increased level and ERAL1 remains abnormally associated with mitochondrial ribosomes preventing maturation to functional mitoribosomes. Both male and female CLPP deficient mice have respiratory chain deficiency, are reduced in size and are sterile (Gispert et al. 2013; Szczepanowska et al. 2016). When CLPP function is deficient, there is excess ERAL1 expression resulting in reduced mitochondrial protein synthesis. Collectively, these observations point to a breakdown in mitochondrial protein homeostasis as the predominant pathogenic hypothesis that connects a spectrum of variants of ERAL1, CLPP, LARS2 and HARS2 with the Perrault syndrome phenotype (Szczepanowska et al. 2016).
GGPS1
GGPS1 encodes a cytosolic and perhaps mitochondrial, geranylgeranyl diphosphate (GGPP) synthase. There appears to be some colocalization of GGPS1 with ATP synthase beta, a marker for mitochondria (Foley et al. 2020). In 11 patients of six unrelated families, Foley and coauthors described a new syndrome manifesting with early-onset, progressive muscular dystrophy, likely congenital deafness and POI with a menopausal level of FSH (Foley et al. 2020). The fertility status of five deaf males in these families is unknown. GGPS1 is expressed in testes and reduced expression of GGPS1 is associated with infertility in men (Bae et al. 2019; Foley et al. 2020; Wang et al. 2013). Eight of the 11 affected subjects had short stature and a failure to thrive. Exome sequencing revealed biallelic missense variants in GGPS1 (Fig. 1). Two unrelated individuals with Perrault syndrome were reported as homozygous for missense variants (R261H and N90S) of GGPS1 (Tucker et al. 2020). The substitutions of GGPS1 associated with Perrault syndrome are not located in the catalytic domain and are suggested to alter interactions with predicted, although presently unreported, binding partners important for function of GGPS1 in ovaries, ears and muscle (Foley et al. 2020).
LARS2
LARS2 and HARS2 are nuclear genes that encode mitochondrial amino acyl tRNA synthetases. To date, nine variants of LARS2 have been reported associated with Perrault syndrome (Fig. 1). LARS2 is located on human chromosome 3p21.31 and encodes mitochondrial leucyl aminoacyl tRNA synthetase (Konovalova and Tyynismaa 2013). In the discovery paper, the female probands had hearing loss and POI with normal 46, XX karyotypes. After exome sequencing, a homozygous missense variant (p.T522N) was segregating in one consanguineous family. The affected individuals in the other family have a compound heterozygous genotype for a p.T629M substitution and, in trans, a frameshift allele of LARS2 (c.1077delT; p.I360Ffs*15) (Fig. 1). The three variants of LARS2 are predicted to be damaging (Pierce et al. 2013). At the age of 8 years, one of the probands presented with mild to moderate hearing loss, which was more severe at low frequencies (upsloping audiogram) and rapidly progressed from severe to profound deafness by 17 years of age at which time she was diagnosed with primary amenorrhea, post-menopausal high levels of LH and FSH. By ultrasound, her ovaries were not visualized (Pierce et al. 2013). When a truncated LARS2 protein is expressed in C. elegans, the worms are sterile due to lack of functional germ cells (Lee et al. 2003; Pierce et al. 2013).
A non‐consanguineous British family presenting with hearing loss and infertility was ascertained and found to be segregating compound heterozygous variants of LARS2 (c.1565C > A, p.T522N and a novel LARS2: c.351G > C p.M117I) (Demain et al. 2017). The proband and an affected male sibling had bilateral severe to profound hearing loss. The proband presented with oligomenorrhea and a 46, XX karyotype. Her ovaries and uterus were structurally normal but small. Hormone levels of the affected male were in the normal range, but he presented with hypospadias without other urogenital abnormalities. Both siblings had mild dysmorphic features, low set ears but without other neurological features (Demain et al. 2017). Additionally, two variants of LARS2 (c.899C > T, p.T300M and c.1912G > A, p.E638K) in compound heterozygosity were reported in two affected siblings. The family was ascertained in Italy and the proband had congenital profound hearing loss, but a normal menarche at age 13 and premature menopause at the age of 28 years (Solda et al. 2016).
HARS2
The HARS1 gene encodes the cytoplasmic histidyl aminoacyl tRNA synthetase while the closely linked head-to-head HARS2 gene encodes the mitochondrial histidyl aminoacyl tRNA synthetase. A family was ascertained with five siblings, three females presenting with Type 1 Perrault syndrome and two deaf males. Genome-wide linkage analysis, followed by positional cloning and Sanger sequence screening of 58 candidate genes in a 4.1 Mb interval on human chromosome 5q31 identified two missense variants (p.V368L and p.L200V) of HARS2 (Pierce et al. 2011). Lymphoblasts mRNA was evaluated from one of the affected females. The p.L200V substitution created an alternative splice site deleting 12 amino acid residues in-frame (Pierce et al. 2011). Both wild type residues of human HARS2 are conserved in the yeast orthologue. In comparison to wild type, HARS2 purified protein with either the leucine-368 or valine-200 have reduced enzymatic aminoacylation activity (Pierce et al. 2011). Together with additional experimental data, a convincing case was made that the variants of HARS2 are associated with Perrault syndrome. A total of 13 variants of HARS2 associated with Perrault syndrome have been reported (Fig. 1).
HSD17B4
The nascent HSD17B4 protein is an 80 kDa 736-amino acid polypeptide that is imported into the peroxisome and proteolytically cleaved into 35 kD and 45 kDa monomers. The monomers then assemble into two distinct homodimeric enzymes, respectively, a 17 beta-hydroxysteroid dehydrogenase catalyzing the beta-oxidation of very long-chain fatty acids (VLCFA) and a fatty acyl-CoA-hydratase (Baes et al. 2000; Leenders et al. 1996). Two deaf sisters were reported to have short stature, intellectual disability, peripheral motor neuropathy and ovarian dysgenesis (Fiumara et al. 2004). By exome sequencing, the authors identified compound heterozygous variants (p.Y217C and p.Y568X) of HSD17B4, which was the first gene associated with Perrault syndrome (Pierce et al. 2010). HSD17B4 expresses multiple spliced transcripts. Prior to post-translation proteolytic cleavage, the longest HSD17B4 protein has two independent enzymatic active domains that catalyze two steps in the beta-oxidation of fatty acids (de Launoit and Adamski 1999) and at the carboxy-terminus there is a peroxisome targeting signal (alanine-lysine-isoleucine). Peroxisomes are organelles specialized for catabolism of hydrogen peroxide and oxidation of fatty acids (Fujiki et al. 2020; Violante et al. 2019).
There are other cases of Type 2 Perrault syndrome associated with variants of HSD17B4 (Chen et al. 2017; Demain et al. 2017; Kim et al. 2013) for a total of 10 variants (Fig. 1). One or both of the Perrault syndrome-associated HSD17B4 variants are located in 9 of its 24 exons and at least one of the two alleles results in an amino acid substitution in one of the two catalytic domains of the two HSD17B4 proteins. However, the majority of pathogenic variants of HSD17B4 are not associated with Perrault syndrome, but rather with DBP deficiency (D-bifunctional protein), characterized by a severe spectrum of life-limiting neurological symptoms usually fatal in the first few years after birth. DBP deficiency was first described in two patients (Suzuki et al. 1997) exhibiting reduced fertility, seizures, hypotonia, loss of vision due to retinal atrophy and mild to severe hearing loss. Variants of HSD17B4 associated with Perrault syndrome (Fig. 1) are on the mild side of the continuum with more severe DBP-deficiency peroxisomal disorders.
TWNK
TWINKLE is a nuclear encoded hexameric mitochondrial protein with primase and helicase domains essential for replication of the mitochondrial chromosome (Morino et al. 2014). Two unrelated families of Japanese ancestry were reported to be segregating hearing loss and ovarian dysgenesis as well as multiple neurological impairment, including progressive ataxia, axonal neuropathy, hyporeflexia, and abnormal eye movements. Exome sequencing of the two affected females in each family identified a total of four different pathogenic variants of TWNK (c10orf2). Only 12 variants of TWNK have been reported to date which are responsible for Perrault syndrome (Fig. 1) (Gotta et al. 2020; Oldak et al. 2017). There are additional recessive variants of TWNK that are associated with mtDNA depletion syndrome 7 (infantile-onset spinocerebellar ataxia) while progressive external ophthalmoplegia type 3 (PEOA3, OMIM 609286) is due to a different set of dominant variants of TWNK (Tyynismaa et al. 2005).
RNA-Seq of Perrault syndrome genes demonstrates prominent expression in spiral ganglion neurons
Heatmaps of gene expression across cochlear cell types were generated by utilizing single-nucleus RNA-Seq datasets from the P30 adult mouse SV (Gu et al. 2020), single-cell RNA-Seq datasets from P7 mouse organ of Corti (Kolla et al. 2020) and P25-P27 mouse spiral ganglion neurons (SGN) (Shrestha et al. 2018) (Fig. 3a–c). In mouse, hair cells exist in a postmitotic state by P0. By postnatal day seven (P7), hair cells are at an early stage of maturity. The onset of hearing in mouse occurs at approximately P12. Datasets were normalized using a previously published analysis pipeline (Gu et al. 2020). All data were scaled between 0 and 1 using min–max scaling as described (Gu et al. 2020). Low levels of expression of Perrault syndrome-associated genes can be seen in the stria vascularis (SV) cell types including marginal, intermediate, basal and spindle cells as well as the adjacent root cells and Reissner’s cell type (Fig. 3a). Similarly, from the P7 organ of Corti single-cell RNA-Seq dataset, Perrault syndrome-associated gene expression in inner and outer hair cells, pillar and Deiters cells show relatively low levels of expression of these genes except for Clpp, which exhibits broad low-level expression across all of these cell types (Fig. 3b) compared to spiral ganglion neurons (SGN) (Fig. 3c). Perrault syndrome-associated genes are ubiquitously and prominently expressed across all type 1 SGNs (1A, 1B, 1C) (Shrestha et al. 2018). With the exception of Eral1, similar expression of these genes was noted in type 2 SGNs. However, there are only seven type 2 SGN single cell transcriptomes included in the dataset making it difficult to draw conclusions about the expression of these genes in type 2 SGNs. These data are consistent with Perrault syndrome gene expression in regions of the adult mouse cochlea (organ of Corti, lateral wall, and spiral ganglion region) from laser-capture micro-dissected adult mouse tissues (Nishio et al. 2017). Twnk was not found to be expressed amongst type 1 SGNs but is expressed in the bulk RNA-Seq of P8 glial cells reported by Li and colleagues (Li et al. 2020) visualized in the “Neuron” datasets in gEAR (https://umgear.org/p?l=fee360e8).
Cochlear localization of Perrault syndrome genes using single-cell and single-nucleus RNA-Seq datasets to pinpoint the possible cell types associated with deafness in this genetically heterogenous disorder. Single-cell RNA-Seq datasets from the P7 mouse organ of Corti (Kolla et al. 2020) and P25-27 mouse spiral ganglion neurons (Shrestha et al. 2018) along with a published P30 adult mouse stria vascularis single-nucleus RNA-seq dataset (Gu et al. 2020) were utilized to identify Perrault gene expression levels in the cell types of the inner ear. Heatmaps display Perrault syndrome-associated genes along the vertical axis and cell types are grouped along the horizontal axis. Expression is in normalized counts scaled from 0 to 1. Higher expression of a gene of interest is indicated by an increasing darker purple color. a Heatmap of Perrault syndrome gene expression among P30 stria vascularis cell types including marginal, intermediate, basal, spindle, root, and Reissner’s membrane cells, as well as macrophages. Note the low level of expression of these genes across the stria vascularis. b Heatmap of Perrault syndrome gene expression among cell types in the P7 organ of Corti including inner hair cells (IHC), outer hair cells (OHC), pillar cells, and Deiters cells. Note the low level of expression of Clpp in these cell types in the organ of Corti. c Heatmap of Perrault syndrome gene expression among type 1 (1A, 1B, and 1C) and type 2 spiral ganglion neurons (SGN). Note that the prominent expression of these Perrault syndrome-associated genes across all SGN types suggests the hypothesis that the hearing loss for variants of the majority of Perrault syndrome genes is related to dysfunctional SGNs
From the combined datasets in these reported RNAseq studies (Fig. 3), we hypothesize a common cellular pathophysiology for Perrault syndrome. We propose that the pathogenic variants of the Perrault syndrome-associated genes discussed in this review damage essential processes in spiral ganglion neurons resulting in deafness. This hypothesis rests on the assumption, perhaps simplistic, that a distinct high level of expression in a cell type of a complex organ, such as the inner ear, points towards the cell type where a gene serves an essential function. These RNA-Seq data also suggest that rehabilitation with a hearing aid or cochlear implant (CI) might be problematic in some Perrault syndrome individuals if spiral ganglion neurons are not functioning properly. Spiral ganglion neurons are necessary to transmit a CI-initiated electrical stimulation to the brainstem. Nevertheless, two recent reviews on this subject point to individuals with auditory neuropathy spectrum disorders (ANSD) who do benefit from cochlear implants (Eshraghi et al. 2020; Shearer and Hansen 2019). Benefit from a CI depends on the site and magnitude of the physiological lesion. Importantly, although these two reviews discussed results of cochlear implantation in ANSD, which is thought to be genetically heterogeneous, neither discussed the results of cochlear implantation in patients with genetic mutations in genes associated with Perrault syndrome. While data on CI outcomes in patients with Perrault syndrome is limited and mixed, some studies of patients with LARS2 variants indicated that these patients achieved expected hearing levels from cochlear implantation (Carminho-Rodrigues et al. 2020; Solda et al. 2016; van der Knaap et al. 2019). However, a patient was reported who failed to develop speech after receiving a cochlear implant at 1.5 years of age (van der Knaap et al. 2019). Thus, more detailed reporting of clinical outcomes with cochlear implantation are needed to identify whether specific genetic variants associated with Perrault syndrome will impact the ability of these patients to benefit from cochlear implantation. Examining the spiral ganglion neurons in the temporal bones from humans with Perrault syndrome may also help to resolve this question by identifying structures in the cochlea that have undergone degeneration, possibly revealing if and to what extent SGNs degenerate in the setting of Perrault syndrome.
Pleiotropic manifestations of variants in genes associated with Perrault syndrome
It is important to emphasize that more deleterious variants in some of the genes associated with Perrault syndrome can result in a more severe childhood onset clinical phenotype (Theunissen et al. 2016). Riley and coauthors reported biallelic variants of LARS2 in a neonate who died in the first few days of life with hydrops, lactic acidosis, sideroblastic anemia (HLASA), and multisystem failure (Riley et al. 2016). One variant in the child was shown to have reduced aminoacylation activity (Riley et al. 2016). These investigators have recently expanded this observation of severe phenotypes associated with variants of LARS2, with the description now of three patients with a HLASA‐like phenotype, an individual with Perrault syndrome whose affected siblings also had leukodystrophy, and an individual with a reversible mitochondrial myopathy, lactic acidosis, and developmental delay (Riley et al. 2020). Leukodystrophy appears to be an emerging clinical phenotype caused by a number of variants in genes associated with Perrault syndrome. Many individuals with variants of LARS2 and CLPP have been described with white matter changes and neurological features including ataxia, intellectual disability and epilepsy (Theunissen et al. 2016; van der Knaap et al. 2019). Importantly, complete loss of function of the genes associated with Perrault syndrome is likely to be incompatible with life and so interpretation of hypomorphic variants of uncertain significance is challenging.
Genotype–phenotype correlations in Perrault syndrome
When the clinical phenotypes of the published individuals with Perrault syndrome are collated some interesting genotype–phenotype correlations emerge (Table 1 and Supplementary Table 2). Variable degrees of SNHL are found in affected individuals, ranging from mild-moderate to profound and associated with variants in all of the genes, except for CLPP where the majority of individuals have severe to profound hearing loss. Variants of the Perrault syndrome genes, except for HARS2 and ERAL1, have been associated with evidence of neurological sequelae and have all resulted in more severe childhood onset phenotypes. This is most striking for TWNK, HSD17B4, CLPP and GGPS1. It is difficult to draw conclusions about ERAL1 due to the report of only a single disease associated variant, but biallelic variants in HARS2 do not appear to result in neurological problems. Therefore, based on the classification into Type 1 and Type 2, it appears that variants in all of the Perrault syndrome associated genes can result in either form, except for HARS2 and possibly ERAL1. Consistent with ascertainment biases in novel disease gene discovery, variants in the more recently described Perrault syndrome-associated genes, TWNK, ERAL1 and GGPS1 have been described predominantly in females with ovarian dysfunction as well as SNHL. It is likely that more males with hearing loss will be identified with variants in these genes.
Coincidental Perrault syndrome
There are many non-genetic causes of permanent deafness including ototoxic drugs and viral infections (Kros and Steyger 2019). Nongenetic etiologies such as chemotherapy and microbial infections also cause infertility (Cohen et al. 2014; Grosse et al. 2008). Some sporadic cases of Perrault syndrome may be caused in part or entirely to nongenetic etiologies. Additionally, there are hundreds of genes with variants associated with just deafness (Griffith and Friedman 2016) and similarly there are many genetic variants associated with infertility (Imtiaz et al. 2018; Kosova et al. 2012; Zorrilla and Yatsenko 2013).
John M. Opitz suggested there may be coincidental syndromes caused by the co-occurrence of more than one etiology for a multisystem disorder with features taken together that define a distinct syndrome (Opitz et al. 1979), for example, the combination of POI and deafness. We reported such a case of coincidental Perrault syndrome in a consanguineous family (Faridi et al. 2017). The 24-year-old proband was deaf, presented with oligomenorrhea and had an endocrine profile in the reference range for post-menopausal women consistent with hypergonadotropic hypogonadism. After exome sequencing, the POI was associated with a homozygous frameshift variant of SGO2 (previous gene symbol, SGOL2). So far, there is only one homozygous loss of function variant of SGO2 (c.1453_1454delGA, p.E485Kfs*5) reported in the Human Gene Mutation Database (HGMD). In mouse, shugoshin 2a encoded by Sgol2a, the orthologue of human SGO2, is essential for meiosis and its absence causes infertility in males and females (Llano et al. 2008; Salic et al. 2004).
While the proband’s infertility was likely due to homozygous null alleles of SGO2, the profound deafness was associated with homozygosity for pathogenic variants of CLDN14 encoding claudin 14, a member of the claudin family of tight junctions (Wilcox et al. 2001). In mouse, a homozygous knockout mutation of Cldn14 also causes deafness by partial loss of the cation-restrictive paracellular barrier in the organ of Corti and the subsequent post-natal death of hair cells (Ben-Yosef et al. 2003). Perhaps there are other unexplained cases of deafness and infertility resulting from coincidental Perrault syndrome. Importantly, individuals classified as Perrault syndrome who subsequently turn out to have two independent genetic disorders, one causing deafness and the other POI, would benefit from counseling that their clinical phenotype is the result of more than one monogenic disorder.
Features of Perrault syndrome can be part of a multisystem disorder
RMND1 is a nuclear encoded mitochondrial protein involved in the translation of mitochondrial encoded respiratory chain proteins (Broenen et al. 2019). Biallelic variants in RMND1 were initially described to result in a severe childhood disorder of encephalopathy associated with defective mitochondrial translation (Janer et al. 2012). Subsequent reports expanded the phenotype to include hearing loss and renal defects as key clinical features (Ng et al. 2016). We described a family segregating a known pathogenic variant in RMND1: c.713A > G, p.N238S with clinical features of Perrault syndrome and distal renal tubular acidosis (Demain et al. 2018) (Fig. 1). This association between RMND1 and features of Perrault syndrome with renal impairment has been replicated recently (Ozieblo et al. 2020).
Biallelic loss of function variants in the gene PEX1 and PEX6 are associated with the fatal peroxisomal disorder Zellweger syndrome. Hypomorphic variants in these genes result in a milder phenotype characterized by amelogenesis imperfecta, retinal dysfunction and SNHL (Ratbi et al. 2015). Recently an individual with SNHL, POI, retinitis pigmentosa and leukodystophy was reported to be compound heterozygous for c.2356C > T, p.R786W and c.371 T > C, p.L124P in PEX6 (Tucker et al. 2020). This further shows the clinical overlap between peroxisomal dysfunction including HSD17B4 and dysfunction of mitochondrial translation evident in other individuals with Perrault syndrome. In light of this finding, it will be important to assess the ovarian reserve in woman identified to have biallelic hypomorphic variants in PEX1, PEX6 and other peroxisomal genes.
Therapeutic options
Treatment for individuals with Perrault syndrome is symptomatic. Presently, there are no specific therapeutic approaches based on genotype. Dependent on the degree of impairment, restoring hearing capacity is either through hearing aids or cochlear implantation. Anecdotal evidence suggests that cochlear implantation is effective. Eventually otolaryngologists will be able to provide personalized gene therapies to correct either one or both copies of the recessive variant allele or provide a wild-type transgene to compensate for the difunctional endogenous gene. A pediatric endocrinologist may treat the primary amenorrhea with estrogen replacement to induce secondary sexual characteristics. The degree of residual ovarian function in women with POI is variable and despite reduced ovarian reserve, some women with Perrault syndrome may experience spontaneous ovulation, resulting in conception and live birth (Jenkinson et al. 2013). In vitro fertilization (IVF) with autologous oocytes is an effective treatment for women with POI when the residual ovarian reserve is sufficient for ovarian stimulation (Chae-Kim and Gavrilova-Jordan 2018). Oocyte cryopreservation should be considered in young women with Perrault syndrome with ovarian reserve who wish to defer starting a family. In contrast, women with follicular depletion, diminished ovarian reserve and clinical hypoestrogenism can benefit from IVF with donor oocytes.
Conclusions
Perrault syndrome is a complex genetically and clinically heterogeneous condition. Recent work has expanded the clinical phenotype to encompass more severe childhood onset presentations with multisystem metabolic and neurological features. The dysfunction of mitochondrial protein translation underlying the pathogenesis of most cases of Perrault syndrome provides important insight into the role of this pathway in the normal development of hearing function and offers opportunities for therapeutic intervention.
References
Bae JW et al (2019) Ras-related proteins (Rab) are key proteins related to male fertility following a unique activation mechanism. Reprod Biol 19:356–362. https://doi.org/10.1016/j.repbio.2019.10.001
Baes M, Huyghe S, Carmeliet P, Declercq PE, Collen D, Mannaerts GP, Van Veldhoven PP (2000) Inactivation of the peroxisomal multifunctional protein-2 in mice impedes the degradation of not only 2-methyl-branched fatty acids and bile acid intermediates but also of very long chain fatty acids. J Biol Chem 275:16329–16336. https://doi.org/10.1074/jbc.M001994200
Ben-Yosef T et al (2003) Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet 12:2049–2061. https://doi.org/10.1093/hmg/ddg210
Bonner ER, Waszak SM, Grotzer MA, Mueller S, Nazarian J (2020) Mechanisms of imipridones in targeting mitochondrial metabolism in cancer cells. Neuro Oncol. https://doi.org/10.1093/neuonc/noaa283
Brodie EJ, Zhan H, Saiyed T, Truscott KN, Dougan DA (2018) Perrault syndrome type 3 caused by diverse molecular defects in CLPP. Sci Rep 8:12862. https://doi.org/10.1038/s41598-018-30311-1
Broenen E et al (2019) RMND1 mutations in two siblings: severe renal hypoplasia but different levels of extrarenal abnormality severity: the ethics of decision making. Arch Pediatr 26:377–380. https://doi.org/10.1016/j.arcped.2019.08.004
Carminho-Rodrigues MT et al (2020) LARS2-Perrault syndrome: a new case report and literature review. BMC Med Genet 21:109. https://doi.org/10.1186/s12881-020-01028-8
Chae-Kim JJ, Gavrilova-Jordan L (2018) Premature ovarian insufficiency: procreative management and preventive strategies. Biomedicines. https://doi.org/10.3390/biomedicines7010002
Chatzispyrou IA et al (2017) A homozygous missense mutation in ERAL1, encoding a mitochondrial rRNA chaperone, causes Perrault syndrome. Hum Mol Genet 26:2541–2550. https://doi.org/10.1093/hmg/ddx152
Chen K et al (2017) A homozygous missense variant in HSD17B4 identified in a consanguineous Chinese Han family with type II Perrault syndrome. BMC Med Genet 18:91. https://doi.org/10.1186/s12881-017-0453-0
Cherot E et al (2018) Using medical exome sequencing to identify the causes of neurodevelopmental disorders: Experience of 2 clinical units and 216 patients. Clin Genet 93:567–576. https://doi.org/10.1111/cge.13102
Cohen BE, Durstenfeld A, Roehm PC (2014) Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. https://doi.org/10.1177/2331216514541361
de Launoit Y, Adamski J (1999) Unique multifunctional HSD17B4 gene product: 17beta-hydroxysteroid dehydrogenase 4 and D-3-hydroxyacyl-coenzyme A dehydrogenase/hydratase involved in Zellweger syndrome. J Mol Endocrinol 22:227–240. https://doi.org/10.1677/jme.0.0220227
Demain LA et al (2017) Expanding the genotypic spectrum of Perrault syndrome. Clin Genet 91:302–312. https://doi.org/10.1111/cge.12776
Demain LAM, Antunes D, O’Sullivan J, Bhaskhar SS, O’Keefe RT, Newman WG (2018) A known pathogenic variant in the essential mitochondrial translation gene RMND1 causes a Perrault-like syndrome with renal defects. Clin Genet 94:276–277. https://doi.org/10.1111/cge.13255
Eshraghi AA et al (2020) Genotype-phenotype correlation for predicting cochlear implant outcome: current challenges and opportunities. Front Genet 11:678. https://doi.org/10.3389/fgene.2020.00678
Faridi R et al (2017) Mutations of SGO2 and CLDN14 collectively cause coincidental Perrault syndrome. Clin Genet 91:328–332. https://doi.org/10.1111/cge.12867
Faridi R et al (2019) Mutational and phenotypic spectra of KCNE1 deficiency in Jervell and Lange-Nielsen Syndrome and Romano-Ward Syndrome. Hum Mutat 40:162–176. https://doi.org/10.1002/humu.23689
Fiumara A, Sorge G, Toscano A, Parano E, Pavone L, Opitz JM (2004) Perrault syndrome: evidence for progressive nervous system involvement. Am J Med Genet A 128A:246–249. https://doi.org/10.1002/ajmg.a.20616
Flanagan JM, Wall JS, Capel MS, Schneider DK, Shanklin J (1995) Scanning transmission electron microscopy and small-angle scattering provide evidence that native Escherichia coli ClpP is a tetradecamer with an axial pore. Biochemistry 34:10910–10917. https://doi.org/10.1021/bi00034a025
Foley AR et al (2020) GGPS1 Mutations Cause Muscular Dystrophy/Hearing Loss/Ovarian Insufficiency Syndrome. Ann Neurol 88:332–347. https://doi.org/10.1002/ana.25772
Fujiki Y et al (2020) Recent insights into peroxisome biogenesis and associated diseases. J Cell Sci. https://doi.org/10.1242/jcs.236943
Gispert S et al (2013) Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum Mol Genet 22:4871–4887. https://doi.org/10.1093/hmg/ddt338
Gotta F et al (2020) A novel mutation of Twinkle in Perrault syndrome: a not rare diagnosis? Ann Hum Genet 84:417–422. https://doi.org/10.1111/ahg.12384
Gottesman S (1996) Proteases and their targets in Escherichia coli. Annu Rev Genet 30:465–506. https://doi.org/10.1146/annurev.genet.30.1.465
Gottschalk ME, Coker SB, Fox LA (1996) Neurologic anomalies of Perrault syndrome. Am J Med Genet 65:274–276. https://doi.org/10.1002/(SICI)1096-8628(19961111)65:4%3c274::AID-AJMG5%3e3.0.CO;2-P
Griffith AJ, Friedman TB (2016) Hereditary hearing loss. In: Snow JB (ed) Ballenger’s otorhinolaryngology head and neck surgery. People’s medical publishing house
Grosse SD, Ross DS, Dollard SC (2008) Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol 41:57–62. https://doi.org/10.1016/j.jcv.2007.09.004
Gu S, Olszewski R, Taukulis I, Wei Z, Martin D, Morell RJ, Hoa M (2020) Characterization of rare spindle and root cell transcriptional profiles in the stria vascularis of the adult mouse cochlea. Sci Rep 10:18100. https://doi.org/10.1038/s41598-020-75238-8
Gupta A et al (2016) Compound heterozygous RMND1 gene variants associated with chronic kidney disease, dilated cardiomyopathy and neurological involvement: a case report. BMC Res Notes 9:325. https://doi.org/10.1186/s13104-016-2131-2
Imtiaz A et al (2018) CDC14A phosphatase is essential for hearing and male fertility in mouse and human. Hum Mol Genet 27:780–798. https://doi.org/10.1093/hmg/ddx440
Ishizawa J et al (2019) Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell 35(721–737):e729. https://doi.org/10.1016/j.ccell.2019.03.014
Janer A et al (2012) An RMND1 mutation causes encephalopathy associated with multiple oxidative phosphorylation complex deficiencies and a mitochondrial translation defect. Am J Hum Genet 91:737–743. https://doi.org/10.1016/j.ajhg.2012.08.020
Janer A et al (2015) RMND1 deficiency associated with neonatal lactic acidosis, infantile onset renal failure, deafness, and multiorgan involvement. Eur J Hum Genet 23:1301–1307. https://doi.org/10.1038/ejhg.2014.293
Jenkinson EM et al (2013) Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am J Hum Genet 92:605–613. https://doi.org/10.1016/j.ajhg.2013.02.013
Josso N, De Grouchy J, Frezal J, Lamy M (1963) Familial turner’s syndrome. Study of 2 families with Xo and Xx Karyotypes. Ann Pediatr (paris) 10:163–167
Kang SG, Maurizi MR, Thompson M, Mueser T, Ahvazi B (2004) Crystallography and mutagenesis point to an essential role for the N-terminus of human mitochondrial ClpP. J Struct Biol 148:338–352. https://doi.org/10.1016/j.jsb.2004.07.004
Kim MJ, Kim SJ, Kim J, Chae H, Kim M, Kim Y (2013) Genotype and phenotype heterogeneity in perrault syndrome. J Pediatr Adolesc Gynecol 26:e25-27. https://doi.org/10.1016/j.jpag.2012.10.008
Kolla L et al (2020) Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat Commun 11:2389. https://doi.org/10.1038/s41467-020-16113-y
Konovalova S, Tyynismaa H (2013) Mitochondrial aminoacyl-tRNA synthetases in human disease. Mol Genet Metab 108:206–211. https://doi.org/10.1016/j.ymgme.2013.01.010
Kosova G, Scott NM, Niederberger C, Prins GS, Ober C (2012) Genome-wide association study identifies candidate genes for male fertility traits in humans. Am J Hum Genet 90:950–961. https://doi.org/10.1016/j.ajhg.2012.04.016
Kros CJ, Steyger PS (2019) Aminoglycoside- and cisplatin-induced ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a033548
Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G (2003) A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet 33:40–48. https://doi.org/10.1038/ng1056
Leenders F, Prescher G, Dolez V, Begue A, de Launoit Y, Adamski J (1996) Assignment of human 17 beta-hydroxysteroid dehydrogenase IV to chromosome 5q2 by fluorescence in situ hybridization. Genomics 37:403–404. https://doi.org/10.1006/geno.1996.0578
Li C, Li X, Bi Z, Sugino K, Wang G, Zhu T, Liu Z (2020) Comprehensive transcriptome analysis of cochlear spiral ganglion neurons at multiple ages. Elife. https://doi.org/10.7554/eLife.50491
Llano E et al (2008) Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev 22:2400–2413. https://doi.org/10.1101/gad.475308
Morino H et al (2014) Mutations in twinkle primase-helicase cause perrault syndrome with neurologic features. Neurology 83:2054–2061. https://doi.org/10.1212/WNL.0000000000001036
Newman WG, Friedman TB, Conway GS, Demain LAM (2018) Perrault syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A (eds) GeneReviews((R)). Seattle (WA),
Ng YS et al (2016) The clinical, biochemical and genetic features associated with RMND1-related mitochondrial disease. J Med Genet 53:768–775. https://doi.org/10.1136/jmedgenet-2016-103910
Nishio SY, Takumi Y, Usami SI (2017) Laser-capture micro dissection combined with next-generation sequencing analysis of cell type-specific deafness gene expression in the mouse cochlea. Hear Res 348:87–97. https://doi.org/10.1016/j.heares.2017.02.017
Oldak M et al (2017) Novel neuro-audiological findings and further evidence for TWNK involvement in Perrault syndrome. J Transl Med 15:25. https://doi.org/10.1186/s12967-017-1129-4
Opitz JM, Herrmann J, Pettersen JC, Bersu ET, Colacino SC (1979) Terminological, diagnostic, nosological, and anatomical-developmental aspects of developmental defects in man. Adv Hum Genet 9:71–164. https://doi.org/10.1007/978-1-4615-8276-2_2
Ozieblo D, Pazik J, Stepniak I, Skarzynski H, Oldak M (2020) Two novel pathogenic variants confirm RMND1 causative role in perrault syndrome with renal involvement. Genes (basel). https://doi.org/10.3390/genes11091060
Pallister PD, Opitz JM (1979) The Perrault syndrome: autosomal recessive ovarian dysgenesis with facultative, non-sex-limited sensorineural deafness. Am J Med Genet 4:239–246. https://doi.org/10.1002/ajmg.1320040306
Perrault M, Klotz B, Housset E (1951) Deux cas de syndrome de Turner avec surdi-mutite dans une meme fratrie. Bull Mem Soc Med Hop Paris 67:79–84
Pierce SB et al (2010) Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome. Am J Hum Genet 87:282–288. https://doi.org/10.1016/j.ajhg.2010.07.007
Pierce SB et al (2011) Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc Natl Acad Sci U S A 108:6543–6548. https://doi.org/10.1073/pnas.1103471108
Pierce SB et al (2013) Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am J Hum Genet 92:614–620. https://doi.org/10.1016/j.ajhg.2013.03.007
Ratbi I et al (2015) Heimler syndrome is caused by hypomorphic mutations in the peroxisome-biogenesis genes PEX1 and PEX6. Am J Hum Genet 97:535–545. https://doi.org/10.1016/j.ajhg.2015.08.011
Ravn K, Neland M, Wibrand F, Duno M, Ostergaard E (2016) Hearing impairment and renal failure associated with RMND1 mutations. Am J Med Genet A 170A:142–147. https://doi.org/10.1002/ajmg.a.37399
Riley LG et al (2016) LARS2 variants associated with hydrops lactic acidosis, sideroblastic anemia, and multisystem failure. JIMD Rep 28:49–57. https://doi.org/10.1007/8904_2015_515
Riley LG et al (2020) The expanding LARS2 phenotypic spectrum: HLASA, Perrault syndrome with leukodystrophy, and mitochondrial myopathy. Hum Mutat 41:1425–1434. https://doi.org/10.1002/humu.24050
Ripstein ZA, Vahidi S, Houry WA, Rubinstein JL, Kay LE (2020) A processive rotary mechanism couples substrate unfolding and proteolysis in the ClpXP degradation machinery. Elife. https://doi.org/10.7554/eLife.52158
Salic A, Waters JC, Mitchison TJ (2004) Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118:567–578. https://doi.org/10.1016/j.cell.2004.08.016
Shayota BJ et al (2019) Characterization of the renal phenotype in RMND1-related mitochondrial disease. Mol Genet Genomic Med 7:e973. https://doi.org/10.1002/mgg3.973
Shearer AE, Hansen MR (2019) Auditory synaptopathy, auditory neuropathy, and cochlear implantation. Laryngoscope Investig Otolaryngol 4:429–440. https://doi.org/10.1002/lio2.288
Shrestha BR, Chia C, Wu L, Kujawa SG, Liberman MC, Goodrich LV (2018) Sensory neuron diversity in the inner ear is shaped by activity. Cell 174(1229–1246):e1217. https://doi.org/10.1016/j.cell.2018.07.007
Solda G et al (2016) First independent replication of the involvement of LARS2 in Perrault syndrome by whole-exome sequencing of an Italian family. J Hum Genet 61:295–300. https://doi.org/10.1038/jhg.2015.149
Suzuki Y et al (1997) D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein deficiency: a newly identified peroxisomal disorder. Am J Hum Genet 61:1153–1162. https://doi.org/10.1086/301599
Szczepanowska K et al (2016) CLPP coordinates mitoribosomal assembly through the regulation of ERAL1 levels. EMBO J 35:2566–2583. https://doi.org/10.15252/embj.201694253
Szyk A, Maurizi MR (2006) Crystal structure at 1.9A of E. coli ClpP with a peptide covalently bound at the active site. J Struct Biol 156:165–174. https://doi.org/10.1016/j.jsb.2006.03.013
Theunissen TE et al (2016) Specific MRI abnormalities reveal severe perrault syndrome due to CLPP defects. Front Neurol 7:203. https://doi.org/10.3389/fneur.2016.00203
Tucker EJ et al (2020) Genomic sequencing highlights the diverse molecular causes of Perrault syndrome: a peroxisomal disorder (PEX6), metabolic disorders (CLPP, GGPS1), and mtDNA maintenance/translation disorders (LARS2, TFAM). Hum Genet 139:1325–1343. https://doi.org/10.1007/s00439-020-02176-w
Tyynismaa H et al (2005) Mutant mitochondrial helicase Twinkle causes multiple mtDNA deletions and a late-onset mitochondrial disease in mice. Proc Natl Acad Sci U S A 102:17687–17692. https://doi.org/10.1073/pnas.0505551102
van der Knaap MS et al (2019) Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy. Neurology 92:e1225–e1237. https://doi.org/10.1212/WNL.0000000000007098
Violante S et al (2019) Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J 33:4355–4364. https://doi.org/10.1096/fj.201801498R
Wang J, Hartling JA, Flanagan JM (1997) The structure of ClpP at 2.3 A resolution suggests a model for ATP-dependent proteolysis. Cell 91:447–456. https://doi.org/10.1016/s0092-8674(00)80431-6
Wang XX et al (2013) Altered protein prenylation in Sertoli cells is associated with adult infertility resulting from childhood mumps infection. J Exp Med 210:1559–1574. https://doi.org/10.1084/jem.20121806
Weaver SP, Schweitzer L (1994) Development of gerbil outer hair cells after the onset of cochlear function: an ultrastructural study. Hear Res 72:44–52. https://doi.org/10.1016/0378-5955(94)90204-6
Wilcox ER et al (2001) Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104:165–172. https://doi.org/10.1016/s0092-8674(01)00200-8
Zorrilla M, Yatsenko AN (2013) The genetics of infertility: current status of the field. Curr Genet Med Rep. https://doi.org/10.1007/s40142-013-0027-1
Acknowledgements
We thank participants in our studies, Dr. Hafiz Muhammad Waqas Munir assistance with recruitment and Drs. Isabelle Roux and Mhamed Grati for critiques of our manuscript.
Funding
WGN was supported by Action Medical Research (GN2494) and the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007) while SN was supported by the Higher Education Commission, Islamabad, Pakistan (HEC 3288). This research was also supported (in part) by the Intramural Research Programs of the NIH, NIDCD, DC000088 to M.H. and DC000039 to T.B.F.
Author information
Authors and Affiliations
Contributions
RF, AR, TBF, WGN, MH, CFF, SN, RTO’K wrote the manuscript, all authors revised drafts of the manuscript. Figures and tables were prepared by RF, TBF, CFF, SG, MH and AR. ZM ascertained family HLZM05 and arranged clinical testing.
Corresponding authors
Ethics declarations
Conflict of interest
None to declare.
Ethical approval
This study was approved by the Institutional Review Boards of the School of Biological Sciences, University of the Punjab, Lahore Pakistan (IRB No. 00005281 to SN), The National Centre of Excellence in Molecular Biology at the University of the Punjab (to SR) and Combined Neurosciences Blue Panel at the National Institutes of Health (OH-93-N-016 to TBF).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Faridi, R., Rea, A., Fenollar-Ferrer, C. et al. New insights into Perrault syndrome, a clinically and genetically heterogeneous disorder. Hum Genet 141, 805–819 (2022). https://doi.org/10.1007/s00439-021-02319-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-021-02319-7