Abstract
Perrault syndrome is a rare heterogeneous condition characterised by sensorineural hearing loss and premature ovarian insufficiency. Additional neuromuscular pathology is observed in some patients. There are six genes in which variants are known to cause Perrault syndrome; however, these explain only a minority of cases. We investigated the genetic cause of Perrault syndrome in seven affected individuals from five different families, successfully identifying the cause in four patients. This included previously reported and novel causative variants in known Perrault syndrome genes, CLPP and LARS2, involved in mitochondrial proteolysis and mitochondrial translation, respectively. For the first time, we show that pathogenic variants in PEX6 can present clinically as Perrault syndrome. PEX6 encodes a peroxisomal biogenesis factor, and we demonstrate evidence of peroxisomal dysfunction in patient serum. This study consolidates the clinical overlap between Perrault syndrome and peroxisomal disorders, and highlights the need to consider ovarian function in individuals with atypical/mild peroxisomal disorders. The remaining patients had variants in candidate genes such as TFAM, involved in mtDNA transcription, replication, and packaging, and GGPS1 involved in mevalonate/coenzyme Q10 biosynthesis and whose enzymatic product is required for mouse folliculogenesis. This genomic study highlights the diverse molecular landscape of this poorly understood syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perrault syndrome is a rare genetic condition characterised by sensorineural hearing loss in both sexes, as well as ovarian dysfunction in females (Newman et al. 1993). Some patients, but not all, also have neurological signs such as intellectual disability, ataxia, and peripheral neuropathy (Newman et al. 2018). The degree of hearing loss, as well as ovarian dysfunction, can vary. Hearing loss can range from a mild late-onset progressive loss to a severe loss present from birth. Similarly, ovarian dysfunction can vary from complete lack of ovarian development (streak gonads) with failure of puberty and primary amenorrhea, to ovarian dysfunction with secondary amenorrhea and premature ovarian insufficiency (POI). Perrault syndrome can be a challenge to identify given that only females present with the syndromic phenotype including ovarian dysfunction with hearing loss. As the hearing loss can be mild, the connection can be overlooked by gynaecologists treating female patients. Similarly, audiologists enquire specifically about abnormalities of hearing, but do not typically include reproductive history as part of their history-taking. The difficulty in identifying and diagnosing Perrault syndrome is further compounded by the fact that hearing loss and ovarian dysfunction may sometimes coincidently present in an individual with the two conditions having independent causes (Faridi et al. 2017).
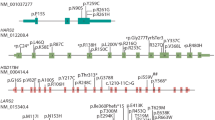
The mechanism of pathogenesis of Perrault syndrome is varied, but is usually related to mitochondrial proteostasis (Newman et al. 2018) (Fig. 1). To date, variants in six genes have been shown to cause Perrault syndrome with autosomal recessive inheritance. These include (1) CLPP, which encodes a component of a mitochondrial ATP-dependent proteolytic complex required for the unfolded protein response and for mitochondrial ribosome biogenesis; (2) ERAL1, which is required for the assembly of the small mitochondrial ribosomal subunit, genes encoding; (3) HARS2 and (4) LARS2, which are responsible for charging mitochondrial tRNAs with histidine and leucine, respectively, for mitochondrial translation; (5) HSD17B4, which is involved in fatty-acid oxidation and steroid metabolism; and (6) TWNK, which encodes an mtDNA helicase and is required for mtDNA replication and maintenance (Chatzispyrou et al. 2017; Jenkinson et al. 2013; Morino et al. 2014; Pierce et al. 2010, 2011, 2013). Current knowledge of the genetic basis of Perrault syndrome is far from saturated, with the genetic cause unknown in ~ 60% of patients, indicating that additional genes or cryptic variants await discovery (Newman et al. 2018).
Diagram depicts proteins involved in Perrault syndrome. Those indicated by the current study are highlighted by asterisks. The majority of Perrault-associated genes encode mitochondrial proteins that are involved in mtDNA replication, maintenance, or translation. For example, TWNK unwinds the two mtDNA strands making them accessible to the mitochondrial polymerases for mtDNA transcription (POLRMT) and replication (POLG). Transcription not only requires the mitochondrial polymerase, POLRMT, but also cofactor, TFAM. mtDNA is transcribed into a polycistronic molecule that is processed to form mature mitochondrial tRNAs, mRNAs, and rRNAs. ERAL1 binds the 12S mitochondrial rRNA and assembles the 28S small mitochondrial ribosomal subunit to enable protein translation. Mitochondrial tRNAs are charged with their cognate amino acids by mitochondrial tRNA synthetases such as LARS2 (leucyl-tRNA synthetase) and HARS2 (histidyl-tRNA synthetase). CLPP is a mitochondrial protein involved in the degradation of misfolded proteins. The other organelle implicated in Perrault syndrome pathogenesis is the peroxisome. The peroxisome is involved in very long-chain fatty-acid oxidation, with the enzyme HSD17B4 playing a key role in this degradation process. PEX6 is a peroxisomal biogenesis factor, tethered to the peroxisomal membrane, and involved in protein import. GGPS1 is a geranylgeranyl synthase that acts on peroxisomal products as part of the mevalonate pathway
Like Perrault syndrome, POI is clinically and genetically heterogeneous (Tucker et al. 2016). POI is defined by amenorrhea (primary or secondary) associated with elevated follicle-stimulating hormone (FSH) measured twice at greater than 1-month intervals, before the age of 40. The condition affects as many as 1 in 100 women by the age of 40, but is rarer in younger women with 1 in 1000 under the age of 30 affected (Golezar et al. 2019; Luborsky et al. 2003). POI can be an isolated condition, or can be syndromic, as is the case in Perrault syndrome where it is associated with hearing loss. There are over 50 genes in which variants cause POI. These genes affect various processes such as cell division, immunity, metabolic function, ovarian development, and ovarian function, but explain only a minority of patients, indicating that further knowledge must be sought to fully understand the aetiology of POI (Tucker et al. 2016).
Massively parallel sequencing is an ideal tool to aid the molecular diagnosis of ovarian dysfunction associated with hearing deficit. This enables the simultaneous interrogation of all known Perrault syndrome genes and genes involved in premature ovarian insufficiency. After investigation of known disease-associated genes, attention can be focused on candidate genes to explore the currently unknown genetic bases for this condition.
Here, we have performed whole-exome sequencing (WES) and/or whole-genome sequencing (WGS) on a small cohort of seven Perrault syndrome patients, as part of a larger study investigating the genetic basis of POI. We identify likely pathogenic variants in previously established Perrault syndrome genes, CLPP and LARS2, and establish a new genetic link between peroxisomal disorders and Perrault syndrome via pathogenic variants in PEX6. Three patients from two different families remain without a definitive genetic diagnosis, but homozygous variants of uncertain significance with high clinical relevance are identified in likely Perrault syndrome genes, TFAM and GGPS1. This study adds new insight into the genetic basis of Perrault syndrome and presents avenues for further investigation.
Methods
Ethics approval
Written informed consent was obtained from all participants. All procedures were in accordance with the ethical standards of the Human Research Ethics Committee of the Royal Children’s Hospital, Melbourne. WGS was performed as part of the Mitochondrial Flagship study of the Australian Genomics Health Alliance research project, which also has Human Research Ethics Committee approval (HREC/16/MH251).
Participants
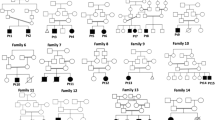
Patients were recruited after clinical consultation. Family and personal medical history were collated and are included in Table 1. Pedigrees are shown in Supplementary Figure S1. Families did not report consanguinity. All patients had POI, defined by menstrual disturbance and elevated FSH (> 20 mIU/mL) measured twice at least 1 month apart as per the European Society of Human Reproduction (ESHR) guidelines (https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-premature-ovarian-insufficiency.aspx) (ESHRE 2015). Karyotyping and/or SNP microarray were performed to confirm normal 46, XX chromosomal complement, and to exclude patients with causal chromosomal rearrangements. All included cases were negative for FMR1 premutation and negative for ovarian auto-antibodies.
Of the seven patients (from five families, including two sibling pairs), we were able to obtain parental DNA from two. Patients underwent WES as singletons or as affected sibling pairs. Parental DNA, when available, was used as confirmation of variant inheritance.
DNA extraction
Genomic DNA was extracted from EDTA-blood samples by the Victorian Clinical Genetics Service (VCGS). Concentration and integrity were assessed by Qubit dsDNA BR Assay (Thermo Fisher Scientific) and TapeStation (Agilent), respectively.
Massively parallel sequencing
DNA underwent whole-exome sequencing (WES) at the Australian Genome Research Facility (AGRF). Exome capture was performed with Agilent SureSelect Human All Exon V6 and sequencing was performed on the Illumina NovaSeq 6000.
All WES data were processed using the C-pipe pipeline (Sadedin et al. 2015) and deposited into SeqR for analysis (https://seqr.broadinstitute.org/).
We performed two phases of analysis—the first focused on gene priority and the second focused on variant priority (Supplementary Figure S2). For gene-centric analysis, we considered the potential pathogenicity of all rare (< 0.05 minor allele frequency, MAF) coding variants within candidate POI genes [571 genes, adapted from (Tucker et al. 2016), Supplementary File] or candidate genes encoding mitochondrial proteins (Calvo et al. 2016) based on the understanding that Perrault syndrome most often occurs due to mitochondrial disturbance. For variant-centric analysis, we considered the potential pathogenicity of all “high priority” variants in any gene, or “moderate priority” potentially biallelic variants in any gene. High-priority variants include variants affecting essential splice sites, introducing frameshifts or premature stop codons, whereas moderate-priority variants include missense variants and in-frame codon deletions. Variant pathogenicity was predicted using online tools including Polyphen2 (https://genetics.bwh.harvard.edu/pph2), SIFT/Provean (https://provean.jcvi.org/), CADD (Combined Annotation-Dependent Depletion) score (https://cadd.gs.washington.edu/snv), and Mutation Taster (https://www.mutationtaster.org/). MAF and the tolerance of the gene to missense and/or loss-of-function variation (ExAC and gnomAD) were also considered, as was the conservation of affected residues using the UCSC alignment of 100 vertebrates.
Whole-genome sequencing (WGS) and variant calling were performed at the Kinghorn Centre for Clinical Genomics (Garvan Institute, Sydney) as described previously (Heimer et al. 2016).
Analysis initially focused on genes associated with mitochondrial disease and then expanded to a “Mendeliome” gene list of ~ 3000 genes linked to human disease (https://panelapp.agha.umccr.org/panels/137/). We analysed the WGS data for copy-number variation (CNV) using LUMPY and CNVnator (Abyzov et al. 2011; Layer et al. 2014).
Variant phasing (de novo PEX6)
All single-nucleotide variants identified in the PEX6 gene by GATK Haplotypecaller were interrogated for linkage disequilibrium (LD) and haplotype frequencies using the LDlink tool (Machiela and Chanock 2015). The PEX6 variants were also inspected using IGV browser to confirm haplotype blocks when the SNPs were covered by a single read. Allele-specific PCR primers were designed to amplify only the allele with the de novo p.(Arg786Trp)/c.2356C>T or the allele with wild-type DNA sequence c.2356=. This enabled analysis of haplotype SNPs inherited in cis and/or trans with the de novo variant to establish its origin.
Sanger sequencing
Selected SNVs were validated by Sanger sequencing using BigDye v3.1 Terminators (Applied Biosystems) and ABI 3130X, as per the manufacturer’s protocols.
Data availability
Data were submitted to ClinVar (SUB6834830) (https://www.ncbi.nlm.nih.gov/clinvar/) (Landrum et al. 2016).
Results
Pathogenic variant in CLPP
Patient 1, of Northwest African descent, was diagnosed with POI at age 22 after experiencing secondary amenorrhea. Her FSH was elevated at 73 IU/I (normal reference: 2–8 IU/I) and oestradiol was low at 20 pg/ml (reference 20–350 pg/ml). No follicles were detected by ultrasound, nor within ovarian biopsy tissue; however, she experienced fluctuating POI with intermittent function (Bidet et al. 2011). Her medical history was notable for sensorineural hearing loss. She has a similarly affected sister, Patient 2, diagnosed with POI at age 23 (FSH 151 IU/I, oestradiol 10 pg/ml) with secondary amenorrhea and hearing loss, and a presumed diagnosis of Perrault syndrome. Both sisters underwent WES, with the only potentially biallelic variant in a known Perrault syndrome gene being a homozygous missense variant in CLPP (Table 2, Fig. 2). The homozygous NM_006012.4: c.439 T>A, NP_006003.1: p.(Cys147Ser) variant, has an MAF of zero in gnomAD, and is consistently predicted pathogenic by online algorithms (Table 3). This variant has previously been reported in two Algerian sisters with Perrault syndrome (Lerat et al. 2016), suggesting a likely founder effect. The previous association with monogenic disease and the clear phenotype match enable this variant to be curated as pathogenic and causative.
a Diagram depicts CLPP genomic structure, with functional domain beneath. Pathogenic or likely pathogenic variants reported in ClinVar (Feb, 2020) or the literature are indicated (black line = splicing; yellow line = missense). The variant carried by Patient 1 in this study is indicated in bold and underlined. Green: associated with Perrault syndrome; blue: associated with autosomal recessive hearing loss but normal menstrual cycles; grey: reported in ClinVar without condition description. b Sanger sequencing validation of the detected CLPP variant in the affected sisters, Patients 1 and 2. c Multiz alignment showing conservation of the affected residue
Novel and known pathogenic variants in LARS2
Patient 3 had hearing loss that was detected at 2 years of age due to language delay. She had a cochlear implant at 3.5 years, and as a consequence, now only has mild hearing loss. Perrault syndrome was suspected after primary amenorrhea associated with elevated FSH of 82.2 U/l. Singleton DNA was run through WES, revealing only one known Perrault syndrome gene with potential biallelic variants, LARS2 (Table 2; Fig. 3). The patient carried two different LARS2 variants: NM_015340.4: c.1237G>A, NP_056155.1: p.(Glu413Lys) and NM_015340.4: c.1987C>T, NP_056155.1: p.(Arg663Trp). The p.(Arg663Trp) has previously been reported in a boy with Perrault syndrome manifesting as intellectual disability, macrocephaly, deafness, behaviour disorder, epilepsy (atonic seizures), cerebellar syndrome, and lactate increase in blood and cerebrospinal fluid (Cherot et al. 2018; van der Knaap et al. 2019). The second variant has conflicting pathogenicity predictions (Table 3). It falls within the catalytic core, which spans amino acids 83–678. Functional investigation of a nearby pathogenic variant within this domain, p.(Ala430Val), has demonstrated aberrant editing activity (Riley et al. 2016). Parental DNA was retrospectively obtained and was used to phase the variants, which were in trans, with the c.1237G>A, p.(Glu413Lys) variant inherited maternally, and the c.1987C>T, p.(Arg663Trp) variant inherited paternally (Fig. 3). The clear phenotype match, the known pathogenicity of one variant, and the co-existence of the second variant in trans with a pathogenic variant lead to the conclusion that these LARS2 variants are likely pathogenic.
a Diagram depicts LARS2 genomic structure, with functional domains beneath. Pathogenic or likely pathogenic variants reported in ClinVar (Feb, 2020) or the literature are indicated (yellow line = missense; red line = loss of function). The variants carried by Patient 3 of this study are indicated in bold and underlined. Asterisk indicates novel variant, not reported in the literature or ClinVar. Orange: associated with mitochondrial myopathy; green: associated with Perrault syndrome; blue: associated with hydrops, lactic acidosis, and sideroblastic anaemia; red: associated with neurodevelopmental disorder; black associated with rare genetic deafness; grey: reported in ClinVar without phenotypic information. b Sanger confirmation demonstrating compound heterozygosity. c Multiz alignment of the protein sequence affected by the variants
Novel and known pathogenic variants in PEX6
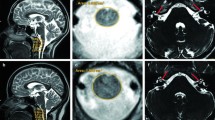
Patient 4 presented with speech delay at 2 years of age with sensorineural hearing loss diagnosed at 3 years of age. At age 12 years, it was noticed that she was not developing pre-pubertal signs, and POI was diagnosed at the age of 13 years. Pes cavus and reduced deep tendon reflexes led to the diagnosis of peripheral neuropathy at 14 years of age. Tremor and ataxia developed at the age of 15, and a brain MRI demonstrated a widespread leukodystrophy, including the cerebellar hemispheres. Despite excellent academic performance during high school years, after the age of 17 years, she gradually began to lose functional abilities. She could no longer play the violin, and had to cease university. After the age of 18 years, a pigmentary retinopathy led to a rapid loss of vision. The initial syndromic presentation of POI and associated hearing loss led to the clinical diagnosis of Perrault syndrome. WGS identified two variants in PEX6 (Tables 2, 3; Fig. 4). Peroxisomal genes are candidates for Perrault syndrome given the established link between the peroxisomal enzyme, HSD17B4, and this condition. Pathogenic variants in HSD17B4, usually cause a peroxisomal disorder characterised by infantile-onset of hypotonia, seizures, abnormal facial features, and death in infancy; however, in some cases, a milder phenotype of Perrault syndrome occurs (Pierce et al. 2010). These PEX6 variants were both missense variants [NM_000287.3: c.2356C>T, NP_000278.3: p.(Arg786Trp) and c.371 T>C, p.(Leu124Pro)], and in silico analyses predicted them to be pathogenic. The c.2356C>T, p.(Arg786Trp) variant, located within the Walker B motif of the AAA cassette domain, has previously been reported in a patient with Zellweger syndrome (Ebberink et al. 2010), and in a patient with late-onset Zellweger spectrum disorder (Tran et al. 2014). Cultured patient skin fibroblasts revealed a decreased number of peroxisomes, and elevated cytosolic catalase, providing confirmation of a peroxisome assembly defect (Tran et al. 2014).
a Diagram depicts PEX6 genomic structure, with functional domains beneath. Pathogenic or likely pathogenic variants reported in ClinVar (Feb, 2020) are indicated (yellow line = missense, red line = loss of function, and black = splicing). The variants carried by Patient 4 of this study are indicated in bold and underlined. Asterisk indicates novel variant, not reported in the literature or ClinVar. b Sanger confirmation demonstrating segregation. c Multiz protein alignment indicating the conservation of the affected residues
Sequencing of parental DNA revealed that the mother shared the c.371 T>C, p.(Leu124Pro) variant, but neither parent carried the c.2356C>T, p.(Arg786Trp). Relatedness testing confirmed paternity, indicating that the second PEX6 variant was de novo. To confirm that the de novo variant arose on the paternal allele, we used a combined population and read-based haplotype phasing approach. This identified the proband as heterozygous for a common haplotype—“Haplotype A”, which has a population database frequency of 29% (1000 Genomes Project V5). Genotyping parental tag SNPs revealed that the parents were also heterozygous carriers of haplotype A. The proband’s WGS reads, however, showed the maternal p.(Leu124Pro) variant was in trans with the haplotype A SNP, NM_000287.4:c.399G>T rs9462858, indicating that haplotype A was inherited from the father (Supplementary Figure S3). Allele-specific PCR was designed to only amplify DNA containing the de novo variant. Sanger sequencing of the resulting product confirmed that the de novo c.2356C>T; p.(Arg786Trp) variant was in cis with the paternally inherited haplotype A SNP, rs2274514 SNP NC_000006.11:g.42934500C>T c.1961+20G>A (Supplementary Figure S4), thereby confirming compound heterozygosity for the patient PEX6 variants.
The established role of PEX6 in peroxisome biogenesis prompted subsequent analysis of very-long-chain fatty acids (VLCFA) in plasma. This revealed a disturbed VLCFA profile (C26:C22 of 0.049; normal range 0–0.030, and C24:C22 of 0.993; normal range 0.550–1.050). These ratios are consistent with a defect in VLCFA oxidation, suggesting primary peroxisomal dysfunction. Further interrogation of WGS data for variants that may independently cause ovarian dysfunction revealed no other likely causative variants.
Candidate variants in TFAM
Patient 5 was of Pakistani descent and presented at 12 years of age with delayed puberty and primary amenorrhea. Maternal menopause was normal at 50 years of age. At age 20, her FSH was 96.7 IU/I, and AMH was 0.05 ng/ml (age-specific reference range 2.06–12.66 ng/ml). Her oestradiol level was 217 pg/ml, reflecting her use of hormone replacement therapy. Her ovaries appeared atrophic on ultrasound. Her medical history was significant for sensorineural hearing loss, and she had below-average IQ.
WES of patient DNA (Table 2) identified the most promising candidate variant as a homozygous missense variant in TFAM (Supplementary Figure S5). TFAM is required for mtDNA maintenance (Larsson et al. 1998; Stiles et al. 2016), as is the known Perrault syndrome gene, TWNK. The homozygous variant, NM_003201.3: c.694C>T, NP_003192.1: p.(Arg232Cys), is reported as heterozygous in three individuals in gnomAD but never in homozygosity, and is consistently predicted as pathogenic using online algorithms such as Polyphen, Mutation Taster, SIFT, and Provean (Table 3).
Candidate variants in GGPS1
Unlike the aforementioned patients who were diagnosed with POI in adolescence, Patient 6 and 7 were diagnosed at 36 and 39 years, respectively. Both sisters have bilateral hearing loss, as well as a moderate myopathy, consistent with a putative diagnosis of Perrault syndrome. Patient 6 was diagnosed with sensorineural hearing loss in childhood, which had a slow progression. Although she had a history of muscle fatigue such as difficulty climbing stairs and pain after exertion, this was attributed to venous insufficiency. Myopathy was not recognised until a formal diagnosis was achieved in her brother, who suffered clinically recognisable myopathy as well as hearing loss (DNA not available). She experienced normal puberty with menarche at age 15, followed by oligomenorrhea. She had regular withdrawal bleeding on contraception, but absence of menstruation at age 36 when contraception was ceased. Hormonal assessment revealed an FSH level of 60 IU/I, and AMH 0.01 ng/ml (age-specific reference range 0.87–9.76 ng/ml), confirming the absence of follicular activity. Her right ovary could not be visualised on ultrasound, and her left ovary had a surface area of 3.7 cm2 (normal reference range 2–6 cm2) and contained one follicle of 9 mm. Her sister, Patient 7, similarly presented with childhood-onset and slowly worsening sensorineural hearing loss and a history of unrecognised myopathy with fatigue and difficulty climbing stairs, erroneously attributed to venous insufficiency and/or smoking. She had late puberty with thelarche at 15 and menarche at 17 followed by irregular menstrual cycles. Despite unprotected sex from the age of 19, she never achieved pregnancy. At age 39 after POI was diagnosed in her sister, hormone analysis was performed, revealing an elevated FSH of 35.8 IU/I and low AMH at 0.14 ng/ml (age-specific reference range 0.56–9.49 ng/ml). Her ovaries were visualised by ultrasound with calculated surface area of 0.56 cm2 on the right and 1.408 cm2 on the left, but no detectable follicles. The family also has an unaffected 41-year-old sister who has regular cycles, no myopathy, and no hearing loss.
WES of DNA from the affected sisters was performed (Table 2). Consideration of the predicted variant pathogenicity and the literature revealed the most promising candidate was a homozygous missense variant in GGPS1 (Supplementary Figure S6). The NM_001037277.1: c.782G>A, NP_001032354.1: p.(Arg261His) variant is detected in heterozygous state in six individuals in gnomAD, but never in a homozygous state. It is predicted disease-causing/deleterious/damaging by Mutation Taster, , and SIFT, but benign by Polyphen. GGPS1 is a key enzyme required for protein prenylation, which is a post-translational modification that mediates c-terminal protein–protein interactions and lipid membrane anchoring (Xu et al. 2015). GGPS1 catalyses the synthesis of Geranylgeranyl Pyrophosphate (GGPP). Loss of GGPP in mouse oocytes leads to impaired proliferation of granulosa cells, defective primary–secondary follicle transition, and female infertility (Jiang et al. 2017). Another protein dependent on prenylation is coenzyme Q10 (ubiquinone), and its deficiency leads to hearing loss and/or myopathy (Yubero et al. 2018).
Strengthening the potential involvement of GGPS1 in Perrault syndrome pathogenesis, we identified a second family with phenotypic overlap via GeneMatcher (Sobreira et al. 2015). This family has a 7-year-old girl with a congenital muscular dystrophy as well as sensorineural hearing loss. She is able to walk short distances. Her young age precludes analysis of ovarian function. Her affected brother has a more severe muscular phenotype, with a tracheostomy for respiratory insufficiency, and a gastrostomy for feeding. At age 19, he complained of hearing loss; however, this was not confirmed by audiological testing. The affected siblings share a homozygous NM_001037277.1:c.269A>G p.(Asn90Ser) missense variant in GGPS1, affecting a residue completely conserved in mammals, birds, reptiles and fish (Supplementary Figure S6) and consistently predicted pathogenic by all tested algorithms (Table 3). These individuals share phenotypic features of Perrault syndrome, but their young age means that full clinical manifestation of their condition may yet surface, and the affected female would benefit from monitoring of ovarian function.
Discussion
Here, we have used WES and WGS to investigate the molecular cause of Perrault syndrome in seven individuals from five different families. We were able to generate conclusive diagnoses for four patients; three with variants in known Perrault syndrome genes, LARS2 and CLPP, and one with variants in PEX6, a gene not previously linked to Perrault syndrome. This latter diagnosis demonstrates the clinical overlap of peroxisomal disorders and Perrault syndrome. We present strong arguments for the potential involvement of TFAM and GGPS1 in the remaining patients, with a second affected family in the case of GGPS1. These candidate genes have not previously been associated with Perrault syndrome, but have relevant roles in biology. Cumulatively, these diagnoses have led to significant insights into the genetic basis of Perrault syndrome.
Identification of a critical CLPP codon associated with Perrault syndrome
This is the second report of the c.439 T>A, p.(Cys147Ser) variant (Lerat et al. 2016), consolidating its role in Perrault syndrome pathogenesis. Interestingly, another previously reported variant has the same protein consequence despite being a different variant at the DNA level [c.440G>C, p.(Cys147Ser)] (Jenkinson et al. 2013). This highlights the critical role of the p.Cys147 residue. This residue lies in the heart of the catalytic domain of CLPP and disruption of this domain may cause significant loss of CLPP protein function. Indeed, variation in the closely neighbouring amino acid is also pathogenic, c.433A>C [p.(Thr145Pro)] (Jenkinson et al. 2013). Eight of the nine known pathogenic variants in CLPP are missense variants within this domain, with the remaining variant being a splicing variant affecting a constitutive donor site. CLPP is moderately intolerant of LOF variants [pLI of 0.59, with two LOF variants detected compared to the expected 11, ExAC, (Samocha et al. 2014)], with no homozygous individuals with LOF variants in the gnomAD database. This, combined with the fact that all but one pathogenic variant in ClinVar is missense, indicates that homozygous null variants may not be compatible with life. In the literature, one patient is described with a homozygous “loss-of-function” frameshift variant; however, the level of CLPP expression is reduced by only 60% indicating a compensatory mechanism, such as nonsense-mediated alternative splicing or alternative start site usage (Theunissen et al. 2016). The patient does, indeed, have a more severe phenotype including white matter abnormality. This trend of pathogenic missense variants in essential proteins is seen in other genes responsible for mitochondrial disorders. For example, there is an excess of pathogenic missense variants in the core subunits of the mitochondrial complex I, in contrast to an excess of LOF variants in its supernumerary subunits (Tucker et al. 2011).
Expansion of the genotypic spectrum of variants in genes known to cause Perrault syndrome
This study has expanded the understanding of the genetic basis of Perrault syndrome (Fig. 1). We have consolidated the pathogenicity of the previously reported c.439 T>A, p.(Cys147Ser) variant in CLPP, but have also identified one known and one novel variant in another known Perrault syndrome gene, LARS2. This is the first report of a likely pathogenic c.1237G>A, p.(Glu413Lys) LARS2 variant and the second report of a c.1987C>T, p.(Arg663Trp) LARS2 variant. Interestingly, the previously reported individual harbouring the c.1987C>T, p.(Arg663Trp) variant, as one of two biallelic variants, was male, so did not demonstrate ovarian dysfunction. He was investigated for syndromic intellectual disability, presenting with macrocephaly, hearing loss, behaviour disorder, epilepsy, cerebellar syndrome, and elevated lactate in blood and cerebrospinal fluid (Cherot et al. 2018; van der Knaap et al. 2019), and the Perrault syndrome clinical diagnosis was established as a consequence of genetic diagnosis. The patient of this study, who shared one of the two variants with the previously reported patient, had a milder phenotype with ovarian dysfunction and hearing loss, but no neurological symptoms. The clinical heterogeneity of Perrault syndrome is well known and is further demonstrated here by the same variant being associated with significantly different disease severity.
Expansion of the phenotypic spectrum of PEX6 deficiency: the relationship between peroxisomal dysfunction and Perrault syndrome
We present the first case of variants in PEX6 associated with an initial diagnosis of Perrault syndrome. Patient 4 first presented with bilateral sensorineural hearing loss followed by ovarian dysfunction and POI. This gave rise to the clinical diagnosis of Perrault syndrome. Genetic analysis revealed two heterozygous likely pathogenic PEX6 variants, one de novo on the paternal allele and one inherited maternally, which prompted the analysis of VLCFA in patient plasma. Although not previously associated with Perrault syndrome, variants in PEX6 are a known cause of peroxisomal disorders such as autosomal recessive Heimler syndrome 2 (MIM 616617) and autosomal recessive Zellweger syndrome (MIM 614862). Heimler syndrome 2 is associated with sensorineural hearing loss, amelogenesis imperfecta, nail abnormalities, and occasional or late-onset retinal pigmentation. Zellweger syndrome usually has neonatal or infantile onset with hypotonia, hearing and vision loss, skeletal abnormalities, and neurological, liver, heart, and/or kidney dysfunction. Rare mild cases involve developmental delay and vision or hearing loss in adulthood. The rarity of survival to a reproductive age means that the impact of variants in this gene on ovarian function may have gone unrecognised; however, there has been one report of an individual with PEX6 variants and Zellweger spectrum disorder including “ovarian dysplasia”(Yu et al. 2019). The nature of the ovarian dysplasia is not described; however, she was assessed for Turner syndrome, which is associated with POI. Our results expand the phenotypic spectrum associated with PEX6 variants, and highlight a need to consider ovarian function in the rare individuals who survive to adulthood. The clinical course of this patient progressed into a Zellweger-like syndrome with later impairment of movement, cognition, and vision. Therefore, establishing an early genetic diagnosis for Perrault syndrome can allow for a better prediction of disease course and prognosis, potentially improving patient management.
PEX6 involvement in Perrault syndrome is reminiscent of the involvement of another peroxisomal enzyme, HSD17B4. Pathogenic variants in HSD17B4 usually cause the peroxisomal disorder, D-bifunctional protein (DBP) deficiency (MIM 261515). Almost all patients with DBP deficiency have a severe phenotype characterised by infantile onset of hypotonia, seizures, and abnormal facial features, and most die before the age of 2 years. Pierce et al (2010) identified the first cases of “mild” DBP deficiency presenting as Perrault syndrome in two sisters, and proposed the clinical overlap of peroxisomal disorders and Perrault syndrome. The involvement of HSD17B4 in Perrault syndrome pathogenesis has been consolidated by the discovery of additional patients with other causative variants (Chen et al. 2017; Demain et al. 2017). Similarly, the role of PEX6 in Perrault syndrome pathogenesis will be confirmed by the identification of additional affected individuals.
Identification of TFAM as a novel Perrault syndrome candidate gene
While genetic diagnoses were achieved in Patients 1–4, the remaining patients had no definitive cause for their Perrault syndrome phenotype. We, instead, identified candidate genes of interest, namely TFAM and GGPS1.
TFAM is a particularly strong candidate POI gene, because it has a well-established role in mtDNA transcription, replication, and packaging into nucleoids. Its disruption in bovine fibroblasts causes mtDNA depletion (de Oliveira et al. 2019). In knockout mice mtDNA depletion is also noted, however, it is also associated with embryonic lethality (Larsson et al. 1998), limiting the potential to investigate its role in mammalian ovarian biology.
Disruption of mitochondrial DNA (mtDNA) maintenance is a known cause of ovarian dysfunction and Perrault syndrome. For example, variants in POLG, the mitochondrial polymerase gamma, cause mtDNA depletion and deletions, and POI associated with progressive external ophthalmoplegia or parkinsonism (Luoma et al. 2004; Pagnamenta et al. 2006). Variants in the mitochondrial primase-helicase, TWNK, which is involved in mtDNA replication and maintenance, similarly cause POI in the context of Perrault syndrome (Morino et al. 2014).
One case of human TFAM-related mitochondrial DNA depletion syndrome has been reported in the literature (Stiles et al. 2016). The affected patient had early onset fatal liver disease. The clinical heterogeneity of patients with variants in mitochondrial disease genes is well known. For example, variants in POLG, which lead to mtDNA depletion, can cause severe fatal liver disease in some patients and a much milder presentation of POI with chronic progressive external ophthalmoplegia in other patients (Blok et al. 2009; Pagnamenta et al. 2006). Similarly, variants in TWNK can cause early onset liver failure or later-onset disease, including Perrault syndrome (Dominguez-Ruiz et al. 2019). It is, therefore, plausible that one patient with pathogenic TFAM variants may present with early onset fatal liver disease and another with Perrault syndrome, both of which can result from defects in mtDNA maintenance. The homozygous TFAM variant identified in Patient 5 affects a highly conserved residue and is consistently predicted pathogenic by online algorithms. In order for this variant to be curated as “likely pathogenic” and for TFAM to be validated as a diagnostic Perrault syndrome gene, further functional work, and/or additional families with Perrault syndrome and variants in this gene should be sought.
Identification of GGPS1 as a novel Perrault syndrome candidate gene
The remaining patients, sisters with POI, hearing loss, and muscle hypotonia, have a likely causative variant in GGPS1. This gene encodes an enzyme that catalyses the synthesis of geranylgeranyl diphosphate, GGPP, which is responsible for the C20-prenylation of downstream proteins, such as Coenzyme Q10. We identified a second family affected by sensorineural hearing loss and myopathy that also harboured a GGPS1 variant; however, the female patient was too young to assess ovarian function. The fact that two independent families with myopathy and sensorineural hearing loss carry predicted pathogenic homozygous GGPS1 variants highlights the potential for this gene to be involved. The ovarian phenotype could arise from disruption to GGPP, known to be required for folliculogenesis (Jiang et al. 2017). The sensorineural hearing loss and myopathy may be due to coenzyme Q10 deficiency (ubiquinone, an isoprenylated benzoquinone) as patients with disruption to this pathway also present with hearing loss and/or myopathy (Heeringa et al. 2011; Mollet et al. 2007; Yubero et al. 2018).
Conclusion
In summary, this study used massively parallel sequencing of seven individuals with Perrault syndrome from five families, to investigate the molecular cause of this heterogeneous condition. We identified known and novel causative variants in Perrault syndrome genes, CLPP and LARS2, as well as likely causative variants in PEX6, a gene not previously associated with Perrault syndrome. We also identified multiple affected families with GGPS1 variants, sensorineural hearing loss, and myopathy, although ovarian function could not be assessed in one family due to the affected female being pre-pubertal. Our work broadens the genotypic spectrum underpinning Perrault syndrome, and expands the phenotypic spectrum associated with variants in PEX6. This strengthens the recently proposed notion of clinical overlap between peroxisomal disorders and Perrault syndrome, and highlights a need to consider the future fertility in cases of “mild” peroxisomal disorders. We propose novel Perrault syndrome candidate genes, including the mitochondrial transcription factor, TFAM, and the geranylgeranyl pyrophosphate synthase, GGPS1. Further work is required for validation of their role in Perrault aetiology.
Data availability
Described variants are submitted to ClinVar. Further data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abyzov A, Urban AE, Snyder M, Gerstein M (2011) CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res 21:974–984. https://doi.org/10.1101/gr.114876.110
Bidet M, Bachelot A, Bissauge E, Golmard JL, Gricourt S, Dulon J, Coussieu C, Badachi Y, Touraine P (2011) Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab 96:3864–3872. https://doi.org/10.1210/jc.2011-1038
Blok MJ, van den Bosch BJ, Jongen E, Hendrickx A, de Die-Smulders CE, Hoogendijk JE, Brusse E, de Visser M, Poll-The BT, Bierau J, de Coo IF, Smeets HJ (2009) The unfolding clinical spectrum of POLG mutations. J Med Genet 46:776–785. https://doi.org/10.1136/jmg.2009.067686
Calvo SE, Clauser KR, Mootha VK (2016) MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 44:D1251–D1257. https://doi.org/10.1093/nar/gkv1003
Chatzispyrou IA, Alders M, Guerrero-Castillo S, Zapata Perez R, Haagmans MA, Mouchiroud L, Koster J, Ofman R, Baas F, Waterham HR, Spelbrink JN, Auwerx J, Mannens MM, Houtkooper RH, Plomp AS (2017) A homozygous missense mutation in ERAL1, encoding a mitochondrial rRNA chaperone, causes Perrault syndrome. Hum Mol Genet 26:2541–2550. https://doi.org/10.1093/hmg/ddx152
Chen K, Yang K, Luo SS, Chen C, Wang Y, Wang YX, Li DK, Yang YJ, Tang YL, Liu FT, Wang J, Wu JJ, Sun YM (2017) A homozygous missense variant in HSD17B4 identified in a consanguineous Chinese Han family with type II Perrault syndrome. BMC Med Genet 18:91. https://doi.org/10.1186/s12881-017-0453-0
Cherot E, Keren B, Dubourg C, Carre W, Fradin M, Lavillaureix A, Afenjar A, Burglen L, Whalen S, Charles P, Marey I, Heide S, Jacquette A, Heron D, Doummar D, Rodriguez D, Billette de Villemeur T, Moutard ML, Guet A, Xavier J, Perisse D, Cohen D, Demurger F, Quelin C, Depienne C, Odent S, Nava C, David V, Pasquier L, Mignot C (2018) Using medical exome sequencing to identify the causes of neurodevelopmental disorders: experience of 2 clinical units and 216 patients. Clin Genet 93:567–576. https://doi.org/10.1111/cge.13102
de Oliveira VC, Moreira GSA, Bressan FF, Gomes Mariano Junior C, Roballo KCS, Charpentier M, Concordet JP, Meirelles FV, Ambrosio CE (2019) Edition of TFAM gene by CRISPR/Cas9 technology in bovine model. PLoS ONE 14:e0213376. https://doi.org/10.1371/journal.pone.0213376
Demain LA, Urquhart JE, O'Sullivan J, Williams SG, Bhaskar SS, Jenkinson EM, Lourenco CM, Heiberg A, Pearce SH, Shalev SA, Yue WW, Mackinnon S, Munro KJ, Newbury-Ecob R, Becker K, Kim MJRTOK, Newman WG (2017) Expanding the genotypic spectrum of Perrault syndrome. Clin Genet 91:302–312. https://doi.org/10.1111/cge.12776
Diao F, Jiang C, Wang XX, Zhu RL, Wang Q, Yao B, Li CJ (2016) Alteration of protein prenylation promotes spermatogonial differentiation and exhausts spermatogonial stem cells in newborn mice. Sci Rep 6:28917. https://doi.org/10.1038/srep28917
Dominguez-Ruiz M, Garcia-Martinez A, Corral-Juan M, Perez-Alvarez AI, Plasencia AM, Villamar M, Moreno-Pelayo MA, Matilla-Duenas A, Menendez-Gonzalez M, Del Castillo I (2019) Perrault syndrome with neurological features in a compound heterozygote for two TWNK mutations: overlap of TWNK-related recessive disorders. J Transl Med 17:290. https://doi.org/10.1186/s12967-019-2041-x
Ebberink MS, Kofster J, Wanders RJ, Waterham HR (2010) Spectrum of PEX6 mutations in Zellweger syndrome spectrum patients. Hum Mutat 31:E1058–E1070. https://doi.org/10.1002/humu.21153
ESHRE (2015) European Society of Human Reproduction and Embryology (ESHRE) Guidelines: management of women with premature ovarian insufficiency
Faridi R, Rehman AU, Morell RJ, Friedman PL, Demain L, Zahra S, Khan AA, Tohlob D, Assir MZ, Beaman G, Khan SN, Newman WG, Riazuddin S, Friedman TB (2017) Mutations of SGO2 and CLDN14 collectively cause coincidental Perrault syndrome. Clin Genet 91:328–332. https://doi.org/10.1111/cge.12867
Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z (2019) The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. https://doi.org/10.1080/13697137.2019.1574738
Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, Xie LX, Salviati L, Hurd TW, Vega-Warner V, Killen PD, Raphael Y, Ashraf S, Ovunc B, Schoeb DS, McLaughlin HM, Airik R, Vlangos CN, Gbadegesin R, Hinkes B, Saisawat P, Trevisson E, Doimo M, Casarin A, Pertegato V, Giorgi G, Prokisch H, Rotig A, Nurnberg G, Becker C, Wang S, Ozaltin F, Topaloglu R, Bakkaloglu A, Bakkaloglu SA, Muller D, Beissert A, Mir S, Berdeli A, Varpizen S, Zenker M, Matejas V, Santos-Ocana C, Navas P, Kusakabe T, Kispert A, Akman S, Soliman NA, Krick S, Mundel P, Reiser J, Nurnberg P, Clarke CF, Wiggins RC, Faul C, Hildebrandt F (2011) COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest 121:2013–2024. https://doi.org/10.1172/JCI45693
Heimer G, Keratar JM, Riley LG, Balasubramaniam S, Eyal E, Pietikainen LP, Hiltunen JK, Marek-Yagel D, Hamada J, Gregory A, Rogers C, Hogarth P, Nance MA, Shalva N, Veber A, Tzadok M, Nissenkorn A, Tonduti D, Renaldo F, University of Washington Center for Mendelian G, Kraoua I, Panteghini C, Valletta L, Garavaglia B, Cowley MJ, Gayevskiy V, Roscioli T, Silberstein JM, Hoffmann C, Raas-Rothschild A, Tiranti V, Anikster Y, Christodoulou J, Kastaniotis AJ, Ben-Zeev B, Hayflick SJ (2016) MECR mutations cause childhood-onset dystonia and optic atrophy, a mitochondrial fatty acid synthesis disorder. Am J Hum Genet 99:1229–1244. https://doi.org/10.1016/j.ajhg.2016.09.021
Jenkinson EM, Rehman AU, Walsh T, Clayton-Smith J, Lee K, Morell RJ, Drummond MC, Khan SN, Naeem MA, Rauf B, Billington N, Schultz JM, Urquhart JE, Lee MK, Berry A, Hanley NA, Mehta S, Cilliers D, Clayton PE, Kingston H, Smith MJ, Warner TT, University of Washington Center for Mendelian G, Black GC, Trump D, Davis JR, Ahmad W, Leal SM, Riazuddin S, King MC, Friedman TB, Newman WG (2013) Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am J Hum Genet 92:605–613. https://doi.org/10.1016/j.ajhg.2013.02.013
Jiang C, Diao F, Sang YJ, Xu N, Zhu RL, Wang XX, Chen Z, Tao WW, Yao B, Sun HX, Huang XX, Xue B, Li CJ (2017) GGPP-Mediated protein geranylgeranylation in oocyte is essential for the establishment of oocyte-granulosa cell communication and primary-secondary follicle transition in mouse ovary. PLoS Genet 13:e1006535. https://doi.org/10.1371/journal.pgen.1006535
Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR (2016) ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res 44:D862–D868. https://doi.org/10.1093/nar/gkv1222
Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236. https://doi.org/10.1038/ng0398-231
Layer RM, Chiang C, Quinlan AR, Hall IM (2014) LUMPY: a probabilistic framework for structural variant discovery. Genome Biol 15:R84. https://doi.org/10.1186/gb-2014-15-6-r84
Lerat J, Jonard L, Loundon N, Christin-Maitre S, Lacombe D, Goizet C, Rouzier C, Van Maldergem L, Gherbi S, Garabedian EN, Bonnefont JP, Touraine P, Mosnier I, Munnich A, Denoyelle F, Marlin S (2016) An application of NGS for molecular investigations in Perrault syndrome: study of 14 families and review of the literature. Hum Mutat 37:1354–1362. https://doi.org/10.1002/humu.23120
Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N (2003) Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 18:199–206. https://doi.org/10.1093/humrep/deg005
Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 364:875–882. https://doi.org/10.1016/S0140-6736(04)16983-3
Machiela MJ, Chanock SJ (2015) LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31:3555–3557. https://doi.org/10.1093/bioinformatics/btv402
Mollet J, Giurgea I, Schlemmer D, Dallner G, Chretien D, Delahodde A, Bacq D, de Lonlay P, Munnich A, Rotig A (2007) Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest 117:765–772. https://doi.org/10.1172/JCI29089
Morino H, Pierce SB, Matsuda Y, Walsh T, Ohsawa R, Newby M, Hiraki-Kamon K, Kuramochi M, Lee MK, Klevit RE, Martin A, Maruyama H, King MC, Kawakami H (2014) Mutations in twinkle primase-helicase cause Perrault syndrome with neurologic features. Neurology 83:2054–2061. https://doi.org/10.1212/WNL.0000000000001036
Newman WG, Friedman TB, Conway GS, Demain LAM (1993) Perrault syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews ((R)), Seattle (WA)
Newman WG, Friedman TB, Conway GS, Demain LAM (2018) Perrault syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews((R)), Seattle (WA)
Pagnamenta AT, Taanman JW, Wilson CJ, Anderson NE, Marotta R, Duncan AJ, Bitner-Glindzicz M, Taylor RW, Laskowski A, Thorburn DR, Rahman S (2006) Dominant inheritance of premature ovarian failure associated with mutant mitochondrial DNA polymerase gamma. Hum Reprod 21:2467–2473. https://doi.org/10.1093/humrep/del076
Pierce SB, Walsh T, Chisholm KM, Lee MK, Thornton AM, Fiumara A, Opitz JM, Levy-Lahad E, Klevit RE, King MC (2010) Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome. Am J Hum Genet 87:282–288. https://doi.org/10.1016/j.ajhg.2010.07.007
Pierce SB, Chisholm KM, Lynch ED, Lee MK, Walsh T, Opitz JM, Li W, Klevit RE, King MC (2011) Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc Natl Acad Sci USA 108:6543–6548. https://doi.org/10.1073/pnas.1103471108
Pierce SB, Gersak K, Michaelson-Cohen R, Walsh T, Lee MK, Malach D, Klevit RE, King MC, Levy-Lahad E (2013) Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am J Hum Genet 92:614–620. https://doi.org/10.1016/j.ajhg.2013.03.007
Riley LG, Rudinger-Thirion J, Schmitz-Abe K, Thorburn DR, Davis RL, Teo J, Arbuckle S, Cooper ST, Campagna DR, Frugier M, Markianos K, Sue CM, Fleming MD, Christodoulou J (2016) LARS2 variants associated with hydrops, lactic acidosis, sideroblastic anemia, and multisystem failure. JIMD Rep 28:49–57. https://doi.org/10.1007/8904_2015_515
Roca-Ayats N, Balcells S, Garcia-Giralt N, Falcó-Mascaró M, Martínez-Gil N, Abril JF, Urreizti R, Dopazo J, Quesada-Gómez JM, Nogués X, Mellibovsky L, Prieto-Alhambra D, Dunford JE, Javaid MK, Russell RG, Grinberg D, Díez-Pérez A (2017) GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 376:1794–1795. https://doi.org/10.1056/NEJMc1612804
Sadedin SP, Dashnow H, James PA, Bahlo M, Bauer DC, Lonie A, Lunke S, Macciocca I, Ross JP, Siemering KR, Stark Z, White SM, Melbourne Genomics Health A, Taylor G, Gaff C, Oshlack A, Thorne NP (2015) Cpipe: a shared variant detection pipeline designed for diagnostic settings. Genome Med 7:68. https://doi.org/10.1186/s13073-015-0191-x
Samocha KE, Robinson EB, Sanders SJ, Stevens C, Sabo A, McGrath LM, Kosmicki JA, Rehnstrom K, Mallick S, Kirby A, Wall DP, MacArthur DG, Gabriel SB, DePristo M, Purcell SM, Palotie A, Boerwinkle E, Buxbaum JD, Cook EH Jr, Gibbs RA, Schellenberg GD, Sutcliffe JS, Devlin B, Roeder K, Neale BM, Daly MJ (2014) A framework for the interpretation of de novo mutation in human disease. Nat Genet 46:944–950. https://doi.org/10.1038/ng.3050
Sobreira N, Schiettecatte F, Valle D, Hamosh A (2015) GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 36:928–930. https://doi.org/10.1002/humu.22844
Stiles AR, Simon MT, Stover A, Eftekharian S, Khanlou N, Wang HL, Magaki S, Lee H, Partynski K, Dorrani N, Chang R, Martinez-Agosto JA, Abdenur JE (2016) Mutations in TFAM, encoding mitochondrial transcription factor A, cause neonatal liver failure associated with mtDNA depletion. Mol Genet Metab 119:91–99. https://doi.org/10.1016/j.ymgme.2016.07.001
Theunissen TE, Szklarczyk R, Gerards M, Hellebrekers DM, Mulder-Den Hartog EN, Vanoevelen J, Kamps R, de Koning B, Rutledge SL, Schmitt-Mechelke T, van Berkel CG, van der Knaap MS, de Coo IF, Smeets HJ (2016) Specific MRI abnormalities reveal severe Perrault syndrome due to CLPP defects. Front Neurol 7:203. https://doi.org/10.3389/fneur.2016.00203
Tran C, Hewson S, Steinberg SJ, Mercimek-Mahmutoglu S (2014) Late-onset Zellweger spectrum disorder caused by PEX6 mutations mimicking X-linked adrenoleukodystrophy. Pediatr Neurol 51:262–265. https://doi.org/10.1016/j.pediatrneurol.2014.03.020
Tucker EJ, Compton AG, Calvo SE, Thorburn DR (2011) The molecular basis of human complex I deficiency. IUBMB Life 63:669–677. https://doi.org/10.1002/iub.495
Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH (2016) Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. Endocr Rev 37:609–635. https://doi.org/10.1210/er.2016-1047
van der Knaap MS, Bugiani M, Mendes MI, Riley LG, Smith DEC, Rudinger-Thirion J, Frugier M, Breur M, Crawford J, van Gaalen J, Schouten M, Willems M, Waisfisz Q, Mau-Them FT, Rodenburg RJ, Taft RJ, Keren B, Christodoulou J, Depienne C, Simons C, Salomons GS, Mochel F (2019) Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy. Neurology 92:e1225–e1237. https://doi.org/10.1212/WNL.0000000000007098
Xu N, Shen N, Wang X, Jiang S, Xue B, Li C (2015) Protein prenylation and human diseases: a balance of protein farnesylation and geranylgeranylation. Sci China Life Sci 58:328–335. https://doi.org/10.1007/s11427-015-4836-1
Yu HL, Shen Y, Sun YM, Zhang Y (2019) Two novel mutations of PEX6 in one Chinese Zellweger spectrum disorder and their clinical characteristics. Ann Transl Med 7:368. https://doi.org/10.21037/atm.2019.06.42
Yubero D, Montero R, Santos-Ocana C, Salviati L, Navas P, Artuch R (2018) Molecular diagnosis of coenzyme Q10 deficiency: an update. Expert Rev Mol Diagn 18:491–498. https://doi.org/10.1080/14737159.2018.1478290
Acknowledgements
WGS was performed as part of the Australian Genomics Health Alliance (Australian Genomics) project, funded by a National Health and Medical Research Council (NHMRC) Targeted Call for Research Grant (1113531). This work was supported by an NHMRC program Grant (1074258, to A.H.S.), and NHMRC fellowships (1054432 to E.J.T., 1062854 to A.H.S., 1155244 to D.R.T), and a CONACYT Postgraduate Research Scholarship (R.R). The research conducted at the Murdoch Children’s Research Institute was supported by the Victorian Government's Operational Infrastructure Support Program.
Funding
WGS was performed as part of the Australian Genomics Health Alliance (Australian Genomics) project, funded by a National Health and Medical Research Council (NHMRC) Targeted Call for Research Grant (1113531). This work was supported by an NHMRC program Grant (1074258, to A.H.S.), and NHMRC fellowships (1054432 to E.J.T., 1062854 to A.H.S., 1155244 to D.R.T), and a CONACYT Postgraduate Research Scholarship (R.R). The research conducted at the Murdoch Children’s Research Institute was supported by the Victorian Government's Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Procedures were in accordance with the ethical standards of the Human Research Ethics Committee of the Royal Children’s Hospital, Melbourne (HREC/22073). WGS was performed as part of the Mitochondrial Flagship study of the Australian Genomics Health Alliance research project, which also has Human Research Ethics Committee approval (HREC/16/MH251).
Informed consent
Written informed consent was obtained from all participants.
Consent for publication
Participants consented to publication of non-identifiable data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tucker, E.J., Rius, R., Jaillard, S. et al. Genomic sequencing highlights the diverse molecular causes of Perrault syndrome: a peroxisomal disorder (PEX6), metabolic disorders (CLPP, GGPS1), and mtDNA maintenance/translation disorders (LARS2, TFAM). Hum Genet 139, 1325–1343 (2020). https://doi.org/10.1007/s00439-020-02176-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-020-02176-w