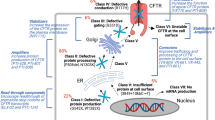
Abstract
Cystic fibrosis (CF) is a chronic and progressive autosomal recessive disorder of secretory epithelial cells, which causes obstructions in the lung airways and pancreatic ducts of 70,000 people worldwide (for recent review see Cutting Nat Rev Genet 16(1):45–56, 2015). The finding that mutations in the CFTR gene cause CF (Kerem et al. Science 245(4922):1073–1080, 1989; Riordan et al. Science 245(4922):1066–1073, 1989; Rommens et al. Science 245(4922):1059–1065, 1989), was hailed as the very happy middle of a story whose end is a cure for a fatal disease (Koshland Science 245(4922):1029, 1989). However, despite two licensed drugs (Ramsey et al. N Engl J Med 365(18):1663–1672, 2011; Wainwright et al. N Engl J Med 373(3):220–231, 2015), and a formal demonstration that repeated administration of CFTR cDNA to patients is safe and effects a modest but significant stabilisation of disease (Alton et al. Lancet Respir Med 3(9):684–691, 2015), we are still a long way from a cure, with many patients taking over 100 tablets per day, and a mean age at death of 28 years. The aim of this review is to discuss the impact on the study of CF of gene-editing techniques as they have developed over the last 30 years, up to and including the possibility of editing as a therapeutic approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Precise editing of the mammalian genome, the ability to manipulate the DNA sequence at the level of single base-pair resolution in cells, requires homologous recombination (HR) between the target region of the genome and a donor template molecule with the desired sequence, but typically occurs in less than 1:1000 treated cells (Smithies et al. 1985; Thomas et al. 1986). Nonetheless, the development of powerful selection strategies to isolate edited cells, and techniques to generate mice from embryonic stem (ES) cells, enabled successful germline editing to create gene-targeted mice (Thompson et al. 1989) which opened up a new field of in vivo modelling of genetic disorders. The first impact of gene editing on the study of CF was the development of knockout mice in which a whole exon was removed (Snouwaert et al. 1992; Dorin et al. 1992; Ratcliff et al. 1993), or precisely-edited with a 3-bp deletion equivalent to the most common CF-causing mutation, ∆F508 (Colledge et al. 1995; van Doorninck et al. 1995; Zeiher et al. 1995). As discussed in detail below, these models recapitulated many features of this human disease; a notable exception was that these mice have a very mild lung phenotype, a stark contrast to the progressive deterioration of lung function which is the cause of death in the vast majority of CF individuals. The exact cause of this difference was not known, and an inability to derive ES cell lines from other mammalian species effectively blocked the development of other models to study lung function for over a decade.
One small step—shorter templates for editing
A considerable limitation to widespread use of gene-editing techniques at the time was the need to create very large (>10 kb) and complicated template molecules or targeting constructs (Koller et al. 1989; Zijlstra et al. 1989). A significant breakthrough in editing for CF was the demonstration that short (≪1 kb) DNA fragments (SDFs) could precisely modify the human CFTR gene in both transformed and non-transformed primary normal airway epithelial cells (Kunzelmann et al. 1996; Goncz et al. 1998), proving the principle that isogenic human cells could be created. The small fragment homologous replacement (SFHR) approach was also used to stably introduce a 3-bp deletion (equivalent to ∆F508) in the Cftr gene of mouse embryonic stem (ES) cells (Sangiuolo et al. 2008). However, it was the fusion of two different techniques exploiting the recombinogenic properties of adeno-associated virus (AAV) template molecules (Cathomen 2004) and somatic cell nuclear transfer (Campbell et al. 1996), that facilitated the development of CF-null ferrets (Sun et al. 2008) and CF-null and ∆F508 pigs (Rogers et al. 2008).
A key feature of CF pigs and ferrets was the development of lung disease similar to that seen in CF patients, a pathological feature that had not been observed in any of the CF mouse models. The availability of three gene-edited species placed CF researchers in a unique position for a genetic disease to undertake comparative pathophysiological studies which have revealed a number of less well-characterised features of the disease, such as abnormalities in alveolar macrophages, bone, and cartilage (reviewed by Wilke et al. 2011; Keiser and Engelhardt 2011). These models will also be critical to optimization of delivery of editing machinery (Cao et al. 2013, 2016) and/or edited cells (Butler et al. 2016).
A giant leap in editing efficiency—targeted double-stranded breaks by programmable and RNA-guided nucleases
For nearly 20 years, gene editing relied solely upon the donor to mediate changes to the genome, even though early plasmid recombination studies indicated that a targeted double-stranded break (DSB) in the genomic DNA close to the site to be edited would boost repair efficiency by at least one order of magnitude (Kucherlapati et al. 1984). Proof of principle for this idea was established with the intron-encoded meganuclease I-SceI (Rouet et al. 1994), but to make DSBs at custom sites in the genome, an endonuclease with a programmable recognition site of at least 16 bp would be required (see Table 1). The first step towards this was the creation of zinc finger nucleases (ZFNs), obligate dimers of concatemerised zinc finger transcription factor domains fused to the nuclease domain of FokI which together recognised and cut a unique 18-bp sequence (Kim et al. 1996). The second step was a decade-long search to determine the rules to program ZFNs to bind any desired target site in the genome, culminating in the use of ZFNs to boost the efficiency of template-mediated editing to as many as 1 in 5 transfected mammalian cells (Urnov et al. 2005). With a relatively simple set of rules in place, the ability to make targeted DSBs at any point in the genome marked the start of a revolution in gene editing with the demonstration that ZFNs could be used to edit fertilised eggs and, thus, create models of disease in a broad range of species other than mice, and with the speed of transgenic techniques (Geurts et al. 2009). The therapeutic potential for in vivo editing was elegantly demonstrated by delivering ZFNs and donor using virus vectors to effectively cure haemophilia A in a mouse model (Li et al. 2011).
The widespread uptake of ZFN editing was relatively slow, partly due to the finding that robust programming of ZFNs was harder than originally anticipated, with the first CFTR-specific designer nucleases only capable of DSB formation in 1.2 % of transfected cells (Maeder et al. 2008). Using different design rules (Dreier et al. 2001; Liu et al. 2002), we created a ZFN pair based on the original nuclease scaffold (Kim et al. 1996) to successfully target a different site in the human CFTR gene and edit and repair the ∆F508 mutation (Lee et al. 2012) in patient-derived tracheal epithelial cells (Kunzelmann et al. 1993). Around this time, a second programmable nuclease system, TAL-effector nucleases (TALENs), was described, with human CFTR-specific TALENs reported to show good activity in a yeast-based assay, but not tested in human cells (Cermak et al. 2011). However, the breakthrough that brought gene editing to the public attention and to thousands of labs around the world was CRISPR Cas9/gRNA (Jinek et al. 2012). This RNA-guided DNA-specific nuclease system was quickly shown to be capable of doing essentially everything that ZFNs and TALENs could do, but via much simpler, quicker and cheaper protocols (Cong et al. 2013; Mali et al. 2013).
Isogenic models to study CF
The first published report of Cas9/gRNA to study CF was conducted in the gut stem cell organoid model (Sato et al. 2009); gut stem cells from a patient with the ∆F508 mutation were repaired using Cas9/gRNA and a donor plasmid containing a selectable marker. Correctly edited cells were selected and subsequently expanded in culture to form organoids; when exposed to cyclic AMP, a CFTR agonist, the gene-edited organoids rapidly swelled confirming that genetic correction results in a concomitant functional correction of the CFTR ion channel (Schwank et al. 2013). A host of other strategies to generate isogenic human cell models quickly followed, the first of which used ZFNs to integrate a CFTR transcription unit into a potential safe-harbour locus within inducible pluripotent stem (iPS) cells (Ramalingam et al. 2013). Direct repair of the ∆F508 mutation in iPS cells using ZFNs (Crane et al. 2014), followed by differentiation of corrected cells into epithelia, resulted in the full restoration of Cl− channel function, though the excision of the selection marker to enrich edited cells left a single 34-bp loxP site in the genome. Truly scarless gene editing of CFTR in iPS cells was achieved using Cas9/gRNA and a selection marker flanked by PiggyBac transposon sequences (Firth et al. 2015), whereas a cyclic enrichment strategy involving allele-specific PCR enabled the isolation of edited cells generated using SDFs with TALENs with significantly fewer genetic manipulations (Suzuki et al. 2016). Upon differentiation, these cells also showed fully restored CFTR function as assessed by several different assays.
Study of CF with isogenic models
These isogenic models provide the opportunity for a systematic evaluation of the 2000 mutations in the CFTR2.org database, only 127 of which have been definitively shown to cause CF (Castellani and CFTR2 team 2013); the disease-causing status is of particular relevance where pregnant women and their partners are offered testing for mutations in CFTR and one or both shown to carry a mutation of unknown status (see Pearson 2009). These models may also uncover novel targets for drug development (Farinha and Matos 2016), and could also be adapted for high throughput screening (Verkman et al. 2015) to identify lead compounds for clinical evaluation, analogous to the strategy (van Goor et al. 2009) which gave rise to Ivacaftor (Kalydeco), a drug that has radically improved lung function and quality of life for CF patients with the G551D mutation (Ramsey et al. 2011; Harrison et al. 2013). The iPSc models may also be useful in identifying other mutations which may respond to existing medications, an approach which has already been successful with the stem cell organoid model (Mini guts for Cystic Fibrosis). The therapeutic potential of corrected iPS cells is explored below.
Third generation CF animal models—rats, rabbits and more mice
As mentioned above, the demonstration that the programmable nucleases or RNA-guided nucleases can be used to edit the nuclei of a fertilised egg, radically altered the way in which germline editing can be used to make gene-modified animal models. One of the first examples was the development of CF knockout rats using ZFNs (Tuggle et al. 2014). These animals showed many of the features seen in CF patients including abnormalities in the ileum and airway surface liquid height. A robust system to generate knockout rabbits from Cas9/gRNA-edited pronuclear-stage rabbit embryos has also been described, and the generation of Cftr-null embryos (Yang et al. 2014) should provide a rabbit model of CF disease.
Whilst existing mouse models continue to give mechanistic insight into the various aspects of CF disease such as mucoviscidosis, the production of viscous mucus in the glands and ducts of affected organs (Liu et al. 2015), the next generation of models, a suite of humanised CF mice in development by this group where the endogenous Cftr gene is replaced with the complete human CFTR gene, will be particularly useful for the evaluation of small molecule drugs and gene-editing therapies that are being developed in human isogenic cell models.
Editing as a therapeutic approach
Within a year of cloning the CFTR gene, a number of studies demonstrated that the CFTR cDNA could functionally complement the genetic defect (Drumm et al. 1990; Gregory et al. 1990; Rich et al. 1990) and served as proof of concept for cDNA addition as a therapeutic approach to treat CF. After more than 20 clinical trials, the safety of DNA delivery to the CF lung is now well established, but the best clinical outcome so far is a small but significant stabilisation of lung function during a multiple-dose protocol (Alton et al. 2015).
So, given that the editing machinery can be delivered by similar size DNA molecules to those used in the complementation trials, is it now appropriate to try editing as a therapeutic approach? The feasibility of site-specific lung editing was originally demonstrated using SFHR (Goncz et al. 2001), and whilst programmable and/or RNA-guided nucleases in combination with template molecules or SFHR have not yet been reported in vivo for CF, two alternative approaches have demonstrated editing in the CF lung cells in situ.
Using triplex-forming peptide nucleic acids and donor DNA delivered by nanoparticles, efficient correction of the ∆F508 mutation with concomitant restoration of Cl− efflux was observed in up to 25 % of human cells in vitro (McNeer et al. 2015). When the same triplex-forming peptide nucleic acids were used with a different donor (due to sequence differences between human CFTR and mouse Cftr genes), deep sequencing analysis revealed correct editing of the ∆F508 in ~1 % of mouse lung cells in vivo (McNeer et al. 2015).
The development of strategies to correct the ∆F508 CFTR mutation at the RNA level has also been reported. Proof of principle for mRNA editing was established using a complex of two modified RNA molecules to insert the three missing bases, possibly via an RNaseH-dependent process, resulting in restored ion channel activity in a CF cell line (Zamecnik et al. 2004). Following on from this work, two clinical trials are now in progress (ProQR clinical trials), evaluating the ability of a single antisense oligonucleotide to correct the ∆F508 mRNA. The mechanism of repair is not clear, although the use of a single oligonucleotide appears to rule out a role for RNaseH in the repair process (De Boer and Ritsema, 2014). Identity across a 35-bp region centred almost exactly on the ∆F508 mutation in the human and mouse genes means that the same editing tools can be used provided they bind within this region. However, until humanised animal models of CF become available, the correction of mutations outside this region will require the creation of species-specific reagents.
Alternatives to editing—characterisation and alteration of CFTR gene expression
The regulation of the CFTR gene is critical to understanding the pathophysiology of CF, and studies of gene expression using Cas9/gRNA to remove control elements have already revealed much about the role of cis-acting elements in terms of CFTR expression, its chromatin landscape, and higher order organisation (Yang et al. 2016). Further studies should also reveal the identity of proteins involved in tissue-specific levels of CFTR expression and post-transcriptional regulation by microRNAs (Gosalia and Harris 2015). Such studies could lead to the use of editing to increase expression levels sufficient to address the pathophysiological effects of a small subset of specific CF-causing mutations, for example, the premature stop codon (PTC) class of CF-causing mutants which generally show reduced mRNA levels due to nonsense-mediated decay (NMD), so a strategy to increase the mRNA levels in combination with PTC suppression strategies may provide a critical breakthrough for this group of mutations (see Cutting 2015). The option also exists to use non-editing CRISPRa (activation) strategies to regulate levels of gene expression (Dominguez et al. 2016). In the case discussed here, gRNA could be used to guide dCas9 variants fused to transcriptional activators such as VP64 to boost gene expression. Alternatively, one could evaluate the CRISPRi (interference) approach which used gRNA to guide dCas9 variants fused to transcriptional repressors such as KRAB to block expression of, for example, ATP12A, in an attempt to ameliorate lung disease. For cell models, inducible regulation of gene regulation via the doxycycline-inducible system is also possible (Mandegar et al. 2016).
Outstanding questions on the road to a cure
Are there still better ways to edit?
A concern about off-target effects of gene editing, that is the creation of DSBs at other regions of the genome which may have deleterious effects, has been assuaged by a number of developments that have gradually increased nuclease specificity. Shortly after the first mammalian use of ZFNs for gene correction, two new scaffolds with substantial reductions in off-target effects were described (Miller et al. 2007; Szczepek et al. 2007), and TALENs have an inherently high level of specificity as elegantly demonstrated by their ability to discern between two closely related human genes, CCR2 and CCR5, at a site which differs by a single base pair (Mussolino et al. 2011). There have been numerous advances to increase the specificity of Cas9/gRNA, most recently the rational design of enhanced-specificity Cas9 (Slaymaker et al. 2016) and Cas9-high fidelity (Kleinstiver et al. 2016) which show almost undetectable levels of off-target DSB formation, even with exquisitely sensitive detection assays. Small-scale clinical studies using cells edited by ZFNs (Tebas et al. 2014) and TALENs (Poirot et al. 2015) have shown no serious adverse reactions, and it is likely that Cas9/gRNA will be evaluated in humans in the near future. The use of asymmetric donors (Richardson et al. 2016) and careful choice of target locus, nuclease, and cell type (Miyaoka et al. 2016) can substantially improve the efficiency of on-target editing by increasing the ratio of precision repair events by template-directed HR relative to imperfect non-homologous end joining (NHEJ) events. One other challenge is to conquer the low efficiency of HR-dependent editing in cells that are terminally differentiated or divide slowly, with obligate ligation-gated recombination (ObLiGaRe) currently offering the most promise (Maresca et al. 2013). Though feasible with ZFNs and TALENs, the blunt end DSB generated by Cas9/gRNA has meant this strategy has not found widespread use. However, the discovery that CRISPR Cpf1/gRNA generates a DSB with a 5′ overhang suggests that Obligare may be feasible using this newly identified RNA-guided endonuclease (Zetsche et al. 2015). The use of microhomology-mediated end-joining (MMEJ) techniques such as PITCh may also be feasible with TALENs and Cas9/gRNA (Sakuma et al. 2016).
Why don’t mice get lung disease?
As mentioned above, a significant obstacle in CF research for many years was the lack of an animal model for lung disease, solved only with the availability of pig and ferret models. Possibly the most exciting finding from comparative studies is the recent discovery that humans and pigs express a H+/K+-ATPase (ATP12A) in the lung, such that when CFTR is absent, the ensuing loss of bicarbonate secretions [CFTR is a bicarbonate and Cl− channel (Gray et al. 1993; Poulsen et al. 1994; Quinton 2001)] leads to an unchecked H+ secretion resulting in acidified airway surface liquid which subsequently impairs airway defence mechanisms. In stark contrast, mouse airways express very low levels of ATP12A, and so, there is minimal H+ secretion even when CFTR was absent, thus, the airway surface liquid in CF and wild-type mice has similar pH (Shah et al. 2016). When CF mice were analysed 3 days after infection with an adenovirus vector expressing ATP12A, their airways had become acidified which impaired defences and increased bacterial load in the lungs. These findings suggest that CF mice engineered to upregulate or overexpress ATP12A would most likely have a significant lung phenotype. If combined with the humanisation strategy described above, this could create a very powerful in vivo model to study CF.
How many cells need to be edited to correct disease?
Two key studies from the last century suggested that only a relatively small number of cells would need to be corrected to restore, at least in part, lung function. The first study established that the overall Cl− transport properties of an epithelial layer comprising mainly homozygous ∆F508 cells with just 6–10 % functionally corrected cells were essentially indistinguishable from an epithelial layer where all the homozygous ∆F508 cells had been functionally corrected (Johnson et al. 1992). The second, a study of healthy non-CF volunteers, showed that as little as 8 % of the normal level of bronchial CFTR transcripts are needed to maintain normal airway function (Chu et al. 1992). With the increase in editing technology, it should be possible to revisit this problem in a number of novel ways, particularly if a mouse model with significant lung disease becomes available (see above). One option could be to mirror the recent approach used to study Duchenne muscular dystrophy (DMD), an X-linked progressive muscle disorder caused by mutations in the gene encoding dystrophin, by simply using Cas9/gRNA to edit single cell mouse embryos. The F1 generation of animals produced were genetically mosaic containing 2–100 % correction of the DMD gene, allowing a correlation between genetic correction and disease severity to be robustly established (Long et al. 2014). A similar approach could shed new light on the number of cells required to cause, or prevent CF, depending on whether the starting cells are wild-type or CF, respectively.
Functional studies of modifier genes
A long-standing and open question is which genes contribute to the variability of disease symptoms and drug responsiveness amongst different CF individuals. A recent genome-wide association analysis of over 6000 CF patients identified five loci that display significant association with variation in lung disease (Corvol et al. 2015). Given that Cas9/gRNA tools are now available to simultaneously edit multiple genes in cells or in vivo (Wang et al. 2013), it is now feasible to generate biological models to test these predictions.
Delivery challenges for therapeutic application
For therapeutic use, essentially two options exist: direct editing of lung cells in vivo, or adminstration of gene-modified cells. The repeated delivery of plasmids expressing the CFTR cDNA to stablise lung function in patients (Alton et al. 2015) suggests that plasmids of a similar size could be used to deliver gene-editing nucleases, and clinical trials to assess the safety and efficacy of direct RNA-editing delivered by non-viral vectors are underway (ProQR clinical trials). Nanocomplex formulation of lipids and peptides to efficiently deliver minicircle DNA-encoding reporter genes to mouse lung also provides proof of principle for delivery of the cDNAs encoding gene-editing nucleases (Munye et al. 2016). Adeno-associated virus (AAV) has been used to deliver cDNA encoding ZFNs (Li et al. 2011) and Cas9 derived from S. aureus (Ran et al. 2015), and adenovirus has been used to deliver cDNA encoding TALENs (Holkers et al. 2014). Lentivirus vectors have even been used to deliver the ZFN or TALEN proteins and edit cells (Cai et al. 2014), and more recently, direct delivery of Cas9/gRNA ribonucleoprotein complexes has been described (Choi et al. 2016). Of particular interest for CF, the combination of non-viral and viral delivery, specifically the use of chemically modified mRNA encoding site-specific nucleases delivered with chitosan nanoparticles, and AAV to deliver the donor, resulted in precise editing in the lungs, within a notable phenotypic change in a well-established transgenic mouse model of surfactant protein B deficiency (Mahiny et al. 2015). The availability of large animal CF models has also established the feasibility of delivery of aerosolised virus vectors intratracheally into pigs under bronchoscopic guidance (Cao et al. 2013; Yan et al. 2015). With regard to cell-based therapies for CF, there are less data at present, but a recent proof-of-concept study in mice showed successful lung reconstitution by canicular-stage lung cells (Rosen et al. 2016) raising the possibility of testing some of the recently described gene-edited iPSc models in conjunction with CF animal models as an alternative to direct editing of the lung.
In conclusion
Gene editing has had a long-standing impact on the study of CF, and as new techniques have been developed, many by CF researchers, they have been fully exploited to increase our understanding of the disease and develop new treatments. The CF field now has a surfeit of human and animal models at its disposal which will only add to the growing number of mutation-specific therapeutic approaches already available. CF is already a shining example of personalised medicine (Corvol et al. 2016), and the transition from classifying DNA variants by disease-causing properties to theratypes, a classification according to the molecular-based treatment to which they respond (Cutting 2015) is now well under way.
References
Alton EW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, Boyd AC, Brand J, Buchan R, Calcedo R, Carvelli P, Chan M, Cheng SH, Collie DD, Cunningham S, Davidson HE, Davies G, Davies JC, Davies LA, Dewar MH, Doherty A, Donovan J, Dwyer NS, Elgmati HI, Featherstone RF, Gavino J, Gea-Sorli S, Geddes DM, Gibson JS, Gill DR, Greening AP, Griesenbach U, Hansell DM, Harman K, Higgins TE, Hodges SL, Hyde SC, Hyndman L, Innes JA, Jacob J, Jones N, Keogh BF, Limberis MP, Lloyd-Evans P, Maclean AW, Manvell MC, McCormick D, McGovern M, McLachlan G, Meng C, Montero MA, Milligan H, Moyce LJ, Murray GD, Nicholson AG, Osadolor T, Parra-Leiton J, Porteous DJ, Pringle IA, Punch EK, Pytel KM, Quittner AL, Rivellini G, Saunders CJ, Scheule RK, Sheard S, Simmonds NJ, Smith K, Smith SN, Soussi N, Soussi S, Spearing EJ, Stevenson BJ, Sumner-Jones SG, Turkkila M, Ureta RP, Waller MD, Wasowicz MY, Wilson JM, Wolstenholme-Hogg P; UK Cystic Fibrosis Gene Therapy Consortium (2015) Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 3(9):684–691
Butler CR, Hynds RE, Gowers KH, Lee DD, Brown JM, Crowley C, Teixeira VH, Smith CM, Urbani L, Hamilton NJ, Thakrar RM, Booth HL, Birchall MA, De Coppi P, Giangreco A, O’Callaghan C, Janes SM (2016) Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med. doi:10.1164/rccm.201507-1414OC
Cai Y, Bak RO, Mikkelsen JG (2014) Targeted genome editing by lentiviral protein transduction of zinc-finger and TAL-effector nucleases. Elife 3:e01911
Campbell KH, McWhir J, Ritchie WA, Wilmut I (1996) Sheep cloned by nuclear transfer from a cultured cell line. Nature 380(6569):64–66
Cao H, Machuca TN, Yeung JC, Wu J, Du K, Duan C, Hashimoto K, Linacre V, Coates AL, Leung K, Wang J, Yeger H, Cutz E, Liu M, Keshavjee S, Hu J (2013) Efficient gene delivery to pig airway epithelia and submucosal glands using helper-dependent adenoviral vectors. Mol Ther Nucleic Acids 2:e127. doi:10.1038/mtna.2013.55
Cao H, Wu J, Duan C, Du K, Lee CM, Yeger H, Hu J (2016) Long-term expression of the human cftr gene in mouse airway via helper-dependent adenoviral vector delivery and transient immunosuppression. Hum Gene Ther 27(1):83–91. doi:10.1089/hum.2015.108
Castellani C, CFTR2 team (2013) CFTR2: how will it help care? Paediatr Respir Rev 14(Suppl 1):2–5
Cathomen T (2004) AAV vectors for gene correction. Curr Opin Mol Ther 6(4):360–366
Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39(12):e82. doi:10.1093/nar/gkr218 (Epub 2011 Apr 14. Erratum in: Nucleic Acids Res. 39(17):7879)
Choi JG, Dang Y, Abraham S, Ma H, Zhang J, Guo H, Cai Y, Mikkelsen JG, Wu H, Shankar P, Manjunath N (2016) Lentivirus pre-packed with Cas9 protein for safer gene editing. Gene Ther. doi:10.1038/gt.2016.27
Chu CS, Trapnell BC, Curristin SM, Cutting GR, Crystal RG (1992) Extensive posttranscriptional deletion of the coding sequences for part of nucleotide-binding fold 1 in respiratory epithelial mRNA transcripts of the cystic fibrosis transmembrane conductance regulator gene is not associated with the clinical manifestations of cystic fibrosis. J Clin Invest 90(3):785–790
Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, MacVinish LJ, Anderson JR, Cuthbert AW, Evans MJ (1995) Generation and characterization of a delta F508 cystic fibrosis mouse model. Nat Genet 10(4):445–452
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339(6121):819–823
Corvol H, Blackman SM, Boëlle PY, Gallins PJ, Pace RG, Stonebraker JR, Accurso FJ, Clement A, Collaco JM, Dang H, Dang AT, Franca A, Gong J, Guillot L, Keenan K, Li W, Lin F, Patrone MV, Raraigh KS, Sun L, Zhou YH, O’Neal WK, Sontag MK, Levy H, Durie PR, Rommens JM, Drumm ML, Wright FA, Strug LJ, Cutting GR, Knowles MR (2015) Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun 6:8382
Corvol H, Thompson KE, Tabary O, le Rouzic P, Guillot L (2016) Translating the genetics of cystic fibrosis to personalized medicine. Transl Res 168:40–49
Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, Wang J, Sun HC, Paschon DE, Guschin DY, Gregory PD, Kotton DN, Holmes MC, Sorscher EJ, Davis BR (2014) Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Rep 4(4):569–577
Cutting GR (2015) Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 16(1):45–56
De Boer DA, Ritsema T (2014) Oligonucleotides for making a change in the sequence of a target RNA molecule present in a living cell. Patent WO 2014011053 A1
Dominguez AA, Lim WA, Qi LS (2016) Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol 17(1):5–15
Dorin JR, Dickinson P, Alton EW, Smith SN, Geddes DM, Stevenson BJ, Kimber WL, Fleming S, Clarke AR, Hooper ML, Anderson L, Beddington RSP, Porteous DJ (1992) Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature 359(6392):211–215
Dreier B, Beerli RR, Segal DJ, Flippin JD, Barbas CF 3rd (2001) Development of zinc finger domains for recognition of the 5′-ANN-3′ family of DNA sequences and their use in the construction of artificial transcription factors. J Biol Chem 276(31):29466–29478
Drumm ML, Pope HA, Cliff WH, Rommens JM, Marvin SA, Tsui LC, Collins FS, Frizzell RA, Wilson JM (1990) Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell 62(6):1227–1233
Farinha CM, Matos P (2016) Repairing the basic defect in cystic fibrosis—one approach is not enough. FEBS J 283(2):246–264
Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH, Verma IM (2015) Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12(9):1385–1390
Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R (2009) Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325(5939):433
Goncz KK, Kunzelmann K, Xu Z, Gruenert DC (1998) Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum Mol Genet 7(12):1913–1919
Goncz KK, Colosimo A, Dallapiccola B, Gagné L, Hong K, Novelli G, Papahadjopoulos D, Sawa T, Schreier H, Wiener-Kronish J, Xu Z, Gruenert DC (2001) Expression of DeltaF508 CFTR in normal mouse lung after site-specific modification of CFTR sequences by SFHR. Gene Ther 8(12):961–965
Gosalia N, Harris A (2015) Chromatin dynamics in the regulation of CFTR expression. Genes (Basel) 6(3):543–558
Gray MA, Plant S, Argent BE (1993) cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol 264(3 Pt 1):C591–C602
Gregory RJ, Cheng SH, Rich DP, Marshall J, Paul S, Hehir K, Ostedgaard L, Klinger KW, Welsh MJ, Smith AE (1990) Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature 347(6291):382–386
Harrison MJ, Murphy DM, Plant BJ (2013) Ivacaftor in a G551D homozygote with cystic fibrosis. N Engl J Med 369(13):1280–1282
Holkers M, Maggio I, Henriques SF, Janssen JM, Cathomen T, Gonçalves MA (2014) Adenoviral vector DNA for accurate genome editing with engineered nucleases. Nat Methods 11(10):1051–1057
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821
Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC (1992) Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet 2(1):21–25
Keiser NW, Engelhardt JF (2011) New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med 17(6):478–483
Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245(4922):1073–1080
Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA 93(3):1156–1160
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK (2016) High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529(7587):490–495
Koller BH, Hagemann LJ, Doetschman T, Hagaman JR, Huang S, Williams PJ, First NL, Maeda N, Smithies O (1989) Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci USA 86(22):8927–8931
Koshland DE Jr (1989) The cystic fibrosis gene story. Science 245(4922):1029
Kucherlapati RS, Eves EM, Song KY, Morse BS, Smithies O (1984) Homologous recombination between plasmids in mammalian cells can be enhanced by treatment of input DNA. Proc Natl Acad Sci USA 81(10):3153–3157
Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo WL, Stanton BA, Gruenert DC (1993) An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the delta F508 CFTR mutation. Am J Respir Cell Mol Biol 8(5):522–529
Kunzelmann K, Legendre JY, Knoell DL, Escobar LC, Xu Z, Gruenert DC (1996) Gene targeting of CFTR DNA in CF epithelial cells. Gene Ther 3(10):859–867
Lee CM, Flynn R, Hollywood JA, Scallan MF, Harrison PT (2012) Correction of the ΔF508 mutation in the cystic fibrosis transmembrane conductance regulator gene by zinc-finger nuclease homology-directed repair. Biores Open Access 1(3):99–108
Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, Malani N, Anguela XM, Sharma R, Ivanciu L, Murphy SL, Finn JD, Khazi FR, Zhou S, Paschon DE, Rebar EJ, Bushman FD, Gregory PD, Holmes MC, High KA (2011) In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 475(7355):217–221
Liu Q, Xia Z, Zhong X, Case CC (2002) Validated zinc finger protein designs for all 16 GNN DNA triplet targets. J Biol Chem 277(6):3850–3856
Liu J, Walker NM, Ootani A, Strubberg AM, Clarke LL (2015) Defective goblet cell exocytosis contributes to murine cystic fibrosis-associated intestinal disease. J Clin Invest 125(3):1056–1068
Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN (2014) Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345(6201):1184–1188
Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, Unger-Wallace E, Sander JD, Müller-Lerch F, Fu F, Pearlberg J, Göbel C, Dassie JP, Pruett-Miller SM, Porteus MH, Sgroi DC, Iafrate AJ, Dobbs D, McCray PB Jr, Cathomen T, Voytas DF, Joung JK (2008) Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell 31(2):294–301
Mahiny AJ, Dewerth A, Mays LE, Alkhaled M, Mothes B, Malaeksefat E, Loretz B, Rottenberger J, Brosch DM, Reautschnig P, Surapolchai P, Zeyer F, Schams A, Carevic M, Bakele M, Griese M, Schwab M, Nürnberg B, Beer-Hammer S, Handgretinger R, Hartl D, Lehr CM, Kormann MS (2015) In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat Biotechnol 33(6):584–586
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided human genome engineering via Cas9. Science 339(6121):823–826
Mandegar MA, Huebsch N, Frolov EB, Shin E, Truong A, Olvera MP, Chan AH, Miyaoka Y, Holmes K, Spencer CI, Judge LM, Gordon DE, Eskildsen TV, Villalta JE, Horlbeck MA, Gilbert LA, Krogan NJ, Sheikh SP, Weissman JS, Qi LS, So PL, Conklin BR (2016) CRISPR interference efficiently induces specific and reversible gene silencing in human iPSCs. Cell Stem Cell 18(4):541–553
Maresca M, Lin VG, Guo N, Yang Y (2013) Obligate ligation-gated recombination (ObLiGaRe): custom-designed nuclease-mediated targeted integration through nonhomologous end joining. Genome Res 23(3):539–546
McNeer NA, Anandalingam K, Fields RJ, Caputo C, Kopic S, Gupta A, Quijano E, Polikoff L, Kong Y, Bahal R, Geibel JP, Glazer PM, Saltzman WM, Egan ME (2015) Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat Commun 6:6952
Miller JC, Holmes MC, Wang J, Guschin DY, Lee YL, Rupniewski I, Beausejour CM, Waite AJ, Wang NS, Kim KA, Gregory PD, Pabo CO, Rebar EJ (2007) An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol 25(7):778–785
Mini guts for Cystic Fibrosis. http://www.ncfs.nl/organoids. Accessed 17 May 2016
Miyaoka Y, Berman JR, Cooper SB, Mayerl SJ, Chan AH, Zhang B, Karlin-Neumann GA, Conklin BR (2016) Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing. Sci Rep 6:23549
Munye MM, Tagalakis AD, Barnes JL, Brown RE, McAnulty RJ, Howe SJ, Hart SL (2016) Minicircle DNA provides enhanced and prolonged transgene expression following airway gene transfer. Sci Rep 6:23125
Mussolino C, Morbitzer R, Lütge F, Dannemann N, Lahaye T, Cathomen T (2011) A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res 39(21):9283–9293
Pearson H (2009) Human genetics: one gene, twenty years. Nature 460(7252):164–169
Poirot L, Philip B, Schiffer-Mannioui C, Le Clerre D, Chion-Sotinel I, Derniame S, Potrel P, Bas C, Lemaire L, Galetto R, Lebuhotel C, Eyquem J, Cheung GW, Duclert A, Gouble A, Arnould S, Peggs K, Pule M, Scharenberg AM, Smith J (2015) Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res 75(18):3853–3864
Poulsen JH, Fischer H, Illek B, Machen TE (1994) Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 91(12):5340–5344
ProQR clinical trials. https://clinicaltrials.gov/ct2/show/NCT02532764 and https://clinicaltrials.gov/ct2/show/NCT02564354. Accessed 17 May 2016
Quinton PM (2001) The neglected ion: HCO3 −. Nat Med 7(3):292–293
Ramalingam S, London V, Kandavelou K, Cebotaru L, Guggino W, Civin C, Chandrasegaran S (2013) Generation and genetic engineering of human induced pluripotent stem cells using designed zinc finger nucleases. mStem Cells Dev 22(4):595–610
Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordoñez C, Elborn JS, VX08-770-102 Study Group (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365(18):1663–1672
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F (2015) In vivo genome editing using Staphylococcus aureus Cas9. Nature 520(7546):186–191
Ratcliff R, Evans MJ, Cuthbert AW, MacVinish LJ, Foster D, Anderson JR, Colledge WH (1993) Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet 4(1):35–41
Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, McCann JD, Klinger KW, Smith AE, Welsh MJ (1990) Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 347(6291):358–363
Richardson CD, Ray Graham J, DeWitt Mark A, Curie Gemma L, Corn Jacob E (2016) Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat Biotechnol 34:339–344
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245(4922):1066–1073
Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z, Spate L, Wax D, Murphy CN, Rieke A, Whitworth K, Linville ML, Korte SW, Engelhardt JF, Welsh MJ, Prather RS (2008) Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest 118(4):1571–1577
Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N et al (1989) Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245(4922):1059–1065
Rosen C, Shezen E, Aronovich A, Klionsky YZ, Yaakov Y, Assayag M, Biton IE, Tal O, Shakhar G, Ben-Hur H, Shneider D, Vaknin Z, Sadan O, Evron S, Freud E, Shoseyov D, Wilschanski M, Berkman N, Fibbe WE, Hagin D, Hillel-Karniel C, Krentsis IM, Bachar-Lustig E, Reisner Y (2016) Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med 21(8):869–879. doi:10.1038/nm.3889 (Epub 2015 Jul 13)
Rouet P, Smih F, Jasin M (1994) Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci USA 91(13):6064–6068
Sakuma T, Nakade S, Sakane Y, Suzuki KT, Yamamoto T (2016) MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat Protoc 11(1):118–133. doi: 10.1038/nprot.2015.140
Sangiuolo F, Scaldaferri ML, Filareto A, Spitalieri P, Guerra L, Favia M, Caroppo R, Mango R, Bruscia E, Gruenert DC, Casavola V, De Felici M, Novelli G (2008) Cftr gene targeting in mouse embryonic stem cells mediated by Small Fragment Homologous Replacement (SFHR). Front Biosci 13:2989–2999
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13(6):653–658
Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PB Jr, Ostedgaard LS, Stoltz DA, Randak CO, Welsh MJ (2016) Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351(6272):503–507
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F (2016) Rationally engineered Cas9 nucleases with improved specificity. Science 351(6268):84–88
Smithies O, Gregg RG, Boggs SS, Doralewski MA, Kucherlapati RS (1985) Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature 317:230–234
Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH (1992) An animal model for cystic fibrosis made by gene targeting. Science 257(5073):1083–1088
Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, Chen J, Zhang Y, Welsh MJ, Leno GH, Engelhardt JF (2008) Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest 118(4):1578–1583. doi:10.1172/JCI34599
Suzuki S, Sargent RG, Illek B, Fischer H, Esmaeili-Shandiz A, Yezzi MJ, Lee A, Yang Y, Kim S, Renz P, Qi Z, Yu J, Muench MO, Beyer AI, Guimarães AO, Ye L, Chang J, Fine EJ, Cradick TJ, Bao G, Rahdar M, Porteus MH, Shuto T, Kai H, Kan YW, Gruenert DC (2016) TALENs facilitate single-step seamless SDF correction of F508del CFTR in airway epithelial submucosal gland cell-derived CF-iPSCs. Mol Ther Nucleic Acids 5:e273. doi:10.1038/mtna.2015.43
Szczepek M, Brondani V, Büchel J, Serrano L, Segal DJ, Cathomen T (2007) Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat Biotechnol 25(7):786–793
Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang WT, Levine BL, June CH (2014) Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370(10):901–910
Thomas KR, Folger KR, Capecchi MR (1986) High frequency targeting of genes to specific sites in the mammalian genome. Cell 44(3):419–428
Thompson S, Clarke AR, Pow AM, Hooper ML, Melton DW (1989) Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell 56(2):313–321
Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, Chambers A, Ji D, Gamber K, Chu KK, Tearney G, Tang LP, Fortenberry JA, Du M, Cadillac JM, Bedwell DM, Rowe SM, Sorscher EJ, Fanucchi MV (2014) Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One 9(3):e91253
Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC (2005) Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435(7042):646–651
van Doorninck JH, French PJ, Verbeek E, Peters RH, Morreau H, Bijman J, Scholte BJ (1995) A mouse model for the cystic fibrosis delta F508 mutation. EMBO J 14(18):4403–4411
Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 106(44):18825–18830
Verkman AS, Edelman A, Amaral M, Mall MA, Beekman JM, Meiners T, Galietta LJ, Bear CE (2015) Finding new drugs to enhance anion secretion in cystic fibrosis: toward suitable systems for better drug screening. Report on the pre-conference meeting to the 12th ECFS Basic Science Conference, Albufeira, 25–28 March 2015. J Cyst Fibros 14(6):700–705
Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP, TRAFFIC Study Group, TRANSPORT Study Group (2015) Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 373(3):220–231
Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153(4):910–918
Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ (2011) Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros 10(Suppl 2):S152–S171
Yan Z, Stewart ZA, Sinn PL, Olsen JC, Hu J, McCray PB Jr, Engelhardt JF (2015) Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Hum Gene Ther Clin Dev 26(1):38–49
Yang D, Xu J, Zhu T, Fan J, Lai L, Zhang J, Chen YE (2014) Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J Mol Cell Biol 6(1):97–99
Yang R, Kerschner JL, Gosalia N, Neems D, Gorsic LK, Safi A, Crawford GE, Kosak ST, Leir SH, Harris A (2016) Differential contribution of cis-regulatory elements to higher order chromatin structure and expression of the CFTR locus. Nucleic Acids Res 44(7):3082–3094. doi:10.1093/nar/gkv1358
Zamecnik PC, Raychowdhury MK, Tabatadze DR, Cantiello HF (2004) Reversal of cystic fibrosis phenotype in a cultured Delta508 cystic fibrosis transmembrane conductance regulator cell line by oligonucleotide insertion. Proc Natl Acad Sci USA 101(21):8150–8155
Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB Jr, Capecchi MR, Welsh MJ, Thomas KR (1995). A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995 Oct;96(4):2051-64
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F (2015) Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163(3):759–771
Zijlstra M, Li E, Sajjadi F, Subramani S, Jaenisch R (1989) Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature 342(6248):435–438
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrison, P.T., Sanz, D.J. & Hollywood, J.A. Impact of gene editing on the study of cystic fibrosis. Hum Genet 135, 983–992 (2016). https://doi.org/10.1007/s00439-016-1693-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-016-1693-3