Abstract
Carotenoid dioxygenases, including 9-cis-epoxycarotenoid dioxygenases (NCEDs) and carotenoid cleavage dioxygenases (CCDs), can selectively cleave carotenoids into various apocarotenoid products that play important roles in fleshy fruit development and abiotic stress response. In this study, we identified 12 carotenoid dioxygenase genes in diploid strawberry Fragaria vesca, and explored their evolution with orthologous genes from nine other species. Phylogenetic analyses suggested that the NCED and CCDL groups moderately expanded during their evolution, whereas gene numbers of the CCD1, CCD4, CCD7, and CCD8 groups maintained conserved. We characterized the expression profiles of FveNCED and FveCCD genes during flower and fruit development, and in response to several abiotic stresses. FveNCED1 expression positively responded to osmotic, cold, and heat stresses, whereas FveNCED2 was only induced under cold stress. In contrast, FveNCED2 was the unique gene highly and continuously increasing in receptacle during fruit ripening, which co-occurred with the increase in endogenous abscisic acid (ABA) content previously reported in octoploid strawberry. The differential expression patterns suggested that FveNCED1 and FveNCED2 were key genes for ABA biosynthesis in abiotic stress responses and fruit ripening, respectively. FveCCD1 exhibited the highest expression in most stages of flower and fruit development, while the other FveCCDs were expressed in a subset of stages and tissues. Our study suggests distinct functions of FveNCED and FveCCD genes in fruit development and stress responses and lays a foundation for future study to understand the roles of these genes and their metabolites, including ABA and other apocarotenoid products, in the growth and development of strawberry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are widely distributed C40 isoprenoid pigments found in all phototrophic and many heterotrophic organisms (Kloer and Schulz 2006). In plants, carotenoids can be selectively cleaved into a wide range of apocarotenoid products that are important for plant development and abiotic stress tolerance (Winterhalter and Ebeler 2013). These cleavages have been shown to be catalyzed by different carotenoid dioxygenases, including 9-cis-epoxycarotenoid dioxygenases (NCEDs) and carotenoid cleavage dioxygenases (CCDs) (Ohmiya 2009; Walter et al. 2010). These enzymes are nonheme iron-dependent enzymes with a conserved basic structure, containing four strictly conserved histidine residues and several conserved glutamate or aspartate residues, which are necessary for their function (Sui et al. 2013; Harrison and Bugg 2014).
Carotenoid dioxygenase genes have been identified and investigated in many plant species. The maize NCED1 (Vp14) was firstly identified by analyzing viviparous (vp) mutants of maize (Schwartz et al. 1997); then 9 genes homologous to VP14 were identified in Arabidopsis thaliana, and their expression and functions were characterized (Tan et al. 2003; Lefebvre et al. 2006). The carotenoid dioxygenase gene family was previously divided into five groups in higher plants, namely NCED, CCD1, CCD4, CCD7, and CCD8 (Tan et al. 2003; Walter et al. 2010), based on analysis of phylogeny and gene function. In addition, a new group, called CCD-like (CCDL), was recently identified in tomato plants (Wei et al. 2016). NCED enzymes cleave 9-cis-violaxanthin and 9-cis-neoxanthin at the 11, 12 (11′, 12′) double bond to generate a C15 product (xanthoxin), which is the rate-limiting step for biosynthesis of the plant hormone abscisic acid (ABA) (Schwartz 2003). ABA biosynthesis can be induced by various abiotic stresses, such as drought, cold, heat, and salt, and ABA can increase plant tolerance to these stresses (Larkindale 2005; Zhang et al. 2006; Roychoudhury et al. 2013). Recently, several studies also suggested that ABA is crucial for fruit development and ripening, especially for non-climacteric fruits (Kumar et al. 2014; Leng et al. 2014; McAtee et al. 2013). For example, inhibition of endogenous ABA biosynthesis delays grape ripening (Zhang et al. 2009), whereas exogenous ABA application on grape berries can enhance their coloration (Cantin et al. 2007; Wheeler et al. 2009). Taken together, ABA plays important roles not only in abiotic stress tolerance, but also in fruit development and ripening in plants.
As they encode the key enzyme for ABA biosynthesis, the expression of NCED genes has been shown to be closely related to fruit development and ripening in several plants (Leng et al. 2014). For example, in avocado (Persea americana), PaNCED1 and PaNCED3 regulate the ripening of fruits (Chernys and Zeevaart 2000). In tomato (Solanum lycopersicum), specific suppression of SlNCED1 gene or treatment with nordihydroguaiaretic acid (NDGA; an inhibitor of NCED enzymes) in fruits delays fruit ripening and extends shelf life (Sun et al. 2012). Furthermore, down-regulation of a cultivated strawberry (Fragaria × ananassa) gene FanNCED1 using the tobacco rattle virus (TRV)-induced gene silencing (VIGS) technique in fruits results in a significant decrease in ABA levels as well as uncolored fruits (Jia et al. 2011). NCED genes also play an important role in the abiotic stress response. It has been reported that the SgNCED1 gene from Stylosanthes guianensis can induce ABA accumulation under stress caused by cold, drought, dehydration, and salt (Yang and Guo 2007). Moreover, dehydration and high salinity can significantly induce AhNCED1 gene transcription in Arachis hypogasa (Wan and Li 2006). Studies have revealed that Arabidopsis NCED3 mutants were unable to accumulate ABA following hyperosmotic stress and overexpression of NCED genes led to an increase in ABA accumulation and drought tolerance (Ruggiero 2004). Together, these studies show that NCED genes can influence fruit development and plant stress responses by controlling ABA biosynthesis.
CCD1, CCD4, CCD7, and CCD8 enzymes can cleave a group of carotenoids at specific double-bond positions to produce a large variety of apocarotenoids with various functions (Kloer and Schulz 2006; Ohmiya 2009). CCD1 cleaves multiple carotenoids to produce volatile apocarotenoids, which play important roles in the synthesis of aroma and flavor in flowers and fruits (Ohmiya 2009). Inactivation of the CCD4 gene results in yellow fruit flesh in peach plants (Ma et al. 2014; Falchi et al. 2013) and yellow flowers in Brassica species (Zhang et al. 2015), suggesting that CCD4 is involved in the cleavage of carotenoids affecting the colors of flowers and fruits. CCD7 can cleave 9-cis-β-carotene to produce 9-cis-β-apo-10′-carotenal, which is then cleaved by CCD8 to produce carlactone, the precursor of strigolactone (Walter et al. 2010; Harrison and Bugg 2014), a plant hormone involved in shoot branching, seed germination, hypocotyl elongation, and reproductive development (Walter et al. 2010). It was recently reported that SlCCD7 is highly expressed in immature tomato fruits (Vogel et al. 2010), as are AcCCD7 and AcCCD8 in young Actinidia chinensis fruits and seeds (Ledger et al. 2010). SlCCD8 knockdown lines exhibited smaller fruits, and fewer and smaller seeds than wild-type lines (Kohlen et al. 2012). These findings suggest that strigolactone may play a role in fruit development. Accordingly, all four classes of CCD genes may also be associated with fruit development and ripening.
The fleshy fruit of strawberry refers to the enlarged receptacle, and the true fruits are the achenes embedded on the receptacle. The strawberry receptacle is used as a model for studying non-climacteric fruit ripening (Li et al. 2011). The importance of ABA for plant/fruit development and stress response makes NCED and CCD genes well worthy of study in strawberry. To date, few studies have been conducted on NCED and CCD genes in strawberry, and all were confined to the cultivated strawberry, Fragaria × ananassa. F. × ananassa is an octoploid strawberry (2n = 8x = 56) with a complex genomic background that makes laboratory studies more difficult. In comparison, the diploid strawberry, F. vesca, has many advantages, including a small and sequenced genome (2n = 14, 240 Mb genome), small stature, easy cultivation, short life cycle, and facile transformation, which make it highly suitable for genetic and molecular analyses (Shulaev et al. 2011; Hollender et al. 2012). Over the last few years, F. vesca has become a useful model for genomics in octoploid strawberry, as well as for other members of the Rosaceae family including apples, peaches, raspberries, and roses, all of which are important agricultural crops. In this paper, we first identified and investigated the entire carotenoid dioxygenase gene family in F. vesca and 9 other angiosperm species. Then, we characterized the expression profiles of FveNCED and FveCCD genes during flower and fruit development and in response to various abiotic stresses. We demonstrated that the 3 FveNCED genes are differentially expressed in fruit development and abiotic stress responses. Our results will provide useful information for further investigation of NCED and CCD gene functions and the effects of ABA and other carotenoid metabolites on the growth, development, and stress tolerance of strawberries.
Materials and methods
Data retrieve
Complete proteome and corresponding annotation information of F. vesca were obtained from Phytozome (version 11.0; http://phytozome.jgi.doe.gov/pz/portal.html). Additionally, nine other plants were selected for the evolutionary analysis of the carotenoid dioxygenase gene family, including one basal angiosperm (Amborella trichopoda), six eudicots (Arabidopsis thaliana, Brassica rapa, Pyrus bretschneideri, Prunus persica, Solanum lycopersicum and Vitis vinifera), and two monocots (Oryza sativa and Zea mays) (Details were listed in Supplemental Table S1). The longest protein was used for further analysis, if more than one protein were annotated for a same gene due to alternative splicing. The transcriptome data of CCD and NCED genes in F. vesca flowers and early development stages of fruits were downloaded from http://bioinformatics.towson.edu/strawberry/ (Kang et al. 2013; Darwish et al. 2013; Hollender et al. 2014).
Identification of carotenoid dioxygenase genes
The RPE65 domain has been reported to be the characteristic domain of all known carotenoid dioxygenases (Kloer and Schulz 2006; Rodriguez-Avila et al. 2011). Thus, the full-alignment sequences of RPE65 domain (Retinal pigment epithelial membrane protein, Pfam: PF03055) were downloaded from the Pfam database v30.0 (Finn et al. 2010). The sequences were then used as query in HMMER search against the proteomes of the ten species with an E-value cutoff of 1e-4 to identify all candidate carotenoid dioxygenase proteins. The presence of RPE65 domain in the matching proteins was confirmed using the Pfam (http://pfam.xfam.org/search) and the Simple Modular Architecture Research Tool database (SMART; http://smart.embl-heidelberg.de/).
Phylogenetic analyses and gene nomenclature
MUSCLE v3.8.31 with default parameters (Edgar 2004) was used to align the RPE65 domain sequences of all the carotenoid dioxygenase proteins we obtained. The best model LG + G+I was selected by Model Generator software (Keane et al. 2006). Then the maximum likelihood phylogenetic tree was constructed by PhyML with 1000 bootstrapping replicates (Guindon and Gascuel 2003). The genes that have been named in previous papers were named accordingly (Schwartz et al. 1997; Tan et al. 2003; Vallabhaneni et al. 2010; Young et al. 2012; Ma et al. 2014; Wei et al. 2016), and others were named on the basis of their homologies to the Arabidopsis NCED and CCD genes following the species abbreviation. Three-letter species abbreviations were used for genes in Rosaceae family plants according to the nomenclature system proposed by the Rosaceae research community (Jung et al. 2015). The accession number for each gene is listed in Table 1 and Supplemental Table S2.
Conserved motif and gene structure analysis
To investigate the motif features among the carotenoid dioxygenase members, the full-length protein sequences were subjected to the MEME online system (Version 4.10.2, http://meme-suite.org/- tools/meme) (Bailey and Elkan 1994) with the following parameters: the maximum number of motifs was 20; the minimum and the maximum motif length were 5 and 50, respectively; default settings were used for other parameters. The exon–intron structures of NCED and CCD genes in each species were obtained from their genomic sequences and annotation information.
Plant growth conditions, material collection and stress treatments
All strawberry materials we used came from a seventh generation inbred line of F. vesca, Ruegen F7-4 (gift from Janet Slovin). The plants were grown in 10 cm × 10 cm pots in a controlled-environment growth chamber with a 16-h light (22 °C)/8 h dark (20 °C) cycle and 65% relative humidity. Light was supplied by sodium lamps at about 160 µmol m−2 s−1 irradiance. Five developmental stages of Ruegen receptacles were collected for quantitative PCR (qPCR) analysis: big green (BG, 13–15 days after anthesis, DPA), little white (LW, white flesh with green achenes, 19–20 DPA), pre-turning (PT, white flesh with red achenes, 24–25DPA), pink (light pink flesh with red achenes, 26–27 DPA), and red (the flesh is all red, 28–29 DPA) stages.
For abiotic stress treatments, strawberry achenes were surface-sterilized for 5 min with 75% ethanol, and 8 min with 2% (w/v) sodium hypochlorite solution, followed by eight times of washing with sterilized-distilled water. Sterile seeds were germinated on wet sterile filter papers in dark at 28 °C. Germinated seeds were transferred to the media containing basal salts and vitamins as described by Murashige and Skoog (1962), supplemented with 3% sucrose (w/v) and 7.5 g L−1Agar. The pH value of the media was adjusted to 5.8 before autoclaving. The aseptic seedlings were then grown in a growth chamber with 16 h light (22 °C)/8 h dark (20 °C) cycles for 4 weeks. Light was supplied by white fluorescent lamps at about 55 µmol m−2 s−1 irradiance. Heat, cold, salinity, and osmotic stress treatments were carried out as described previously (Gu et al. 2016). All samples after collection were immediately frozen in liquid nitrogen and then stored at −80 °C for RNA extraction.
RNA extraction and qPCR analysis of FveNCED and FveCCD genes
Total RNA was extracted from strawberry receptacles with achenes removed at different ripening stages and from abiotic stress-treated seedlings with a modified CTAB method (Yu et al. 2012). The cDNA was generated by Primerscript RT reagent Kit with gDNA Erase (Takara) according to the manufacturer’s protocol. qPCRs were carried out with SYBR Premix Ex Taq II (Takara) on a Bio-Rad CFX96 using the cDNA as the template. The relative expression levels were analyzed with the ΔΔCT method using GAPDH as the reference gene (Amil-Ruiz et al. 2013). Three biological and three technical replicates were performed for each experiment. All of the specific primers we used are listed in Supplemental Table S3.
Results
Identification of carotenoid dioxygenase genes in F. vesca and other plants
To identify carotenoid dioxygenase genes, a full alignment of the RPE65 domain downloaded from the Pfam database was used to search the F. vesca proteome using the HMMER toolset. Twelve genes were identified containing this domain. Using SMART with Pfam, we verified that all the 12 protein contained only the RPE65 domain. Multiple protein sequence alignments revealed that 11 of the 12 proteins contained 4 conserved histidine residues and glutamate or aspartate residues, while 1, mrna08831, was truncated and lacked most conserved active sites (Fig. S1). However, further structural and phylogenetic analyses indicated that mrna08831 should also be a member of the carotenoid dioxygenase gene family in F. vesca. Therefore, we included mrna08831 as FveCCDL5 in our following analysis (Table 1).
To trace the evolutionary history of carotenoid dioxygenase genes in F. vesca, members of this family were also identified in nine other species, including Amborella, six eudicots, and two monocots (Materials and methods). Amborella is a basal angiosperm that originated prior to the split of eudicots and monocots (Albert et al. 2013), which can help us to better investigate gene number variation of the family along angiosperms. A total of 119 genes were detected, and almost all of them contained a single RPE65 domain. Two proteins, GRMZM2G328612 in maize and LOC_Os01g54270 in rice, also contained Rif1_N (PF12231) and RVT_1 (PF00078) domains, respectively. We included these two genes in our study in accordance with previously published methods (Vallabhaneni et al. 2010). Across all species, 131 carotenoid dioxygenase genes were identified. The number of genes in each species ranged from 9 (in Arabidopsis and Amborella) to 19 (in pear and maize) (Table 2).
Phylogenetic analysis of carotenoid dioxygenase genes
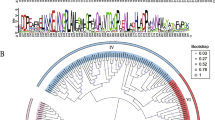
To better understand the relationships among carotenoid dioxygenase genes in F. vesca and other plants, a phylogenetic tree was constructed using the best-fit model in PhyML (LG + G + I) based on the conserved RPE65 domain sequences of the 131 proteins. According to the tree topology and the nine known carotenoid dioxygenase genes in Arabidopsis (Tan et al. 2003), all genes could be classified into six groups, named NCED, CCD1, CCD4, CCD7, CCD8, and CCDL (Fig. 1). The bootstrap values at the nodes of each group were all greater than 90%, suggesting that the grouping of the phylogenetic tree was reliable. In addition, each of the six groups contained clusters of genes exclusively from monocot or dicot species, which is consistent with previous studies (Priya and Siva 2014, 2015). Based on this grouping, the carotenoid dioxygenase gene family in F. vesca included five FveCCDL genes, three FveNCED genes, and one gene each from FveCCD1, FveCCD4, FveCCD7, and FveCCD8.
Maximum likelihood phylogeny and exon–intron composition of predicted carotenoid dioxygenase genes identified from the 10 species, including F. vesca (Fve), Arabidopsis (At), B. rapa (Br), pear (Pbr), peach (Ppe), tomato (Sl), grape (Vv), rice (Os), maize (Zm) and Amborella (Am). The phylogenetic tree, shown as broken up into the NCEDs (a) and CCDs (b) groups, was constructed based on the RPE65 domain sequences with 1000 bootstrapping replicates. The schematic diagram for exon–intron composition was made on the Evolview Web site (http://www.evolgenius. info/evolview/#login) and shown on the right of the tree. The lines for introns of the genes longer than 7000 bp were compressed and marked with two parallel lines (||). The protein/gene IDs in F. vesca and the other nine species have been listed in Table 1 and Supplemental Table S2, respectively
The NCED group can be further divided into two subgroups: NCED-I and NCED-II (Fig. 1). FveNCED1 and FveNCED2 genes belonged to the NCED-I subgroup, whereas FveNCED6 fell into the NCED-II subgroup. In the NCED-I subgroup, the basal angiosperm Amborella had only 1 NCED gene, but the number of NCED genes in other species ranged from 2 to 11 (Table 2), implying that moderate expansion of genes in this subgroup occurred during angiosperm evolution. In the NCED-II subgroup, each species had a unique NCED gene orthologous to AtNCED6 in Arabidopsis, indicating that this subgroup of NCED genes is highly conserved among angiosperm species.
The gene phylogeny of the CCD1, CCD4, CCD7, and CCD8 groups was congruent with the species phylogeny (Table 2; Fig. 1). Every species has only one or two orthologous genes in each of these four groups, except B. rapa CCD1s and pear and rice CCD4s, suggesting that the number of CCD1, CCD4, CCD7, and CCD8 genes in angiosperms is evolutionarily stable. Additionally, there is a group recently proposed in tomato (Wei et al. 2016) that we called CCDL; all species, including Amborella and the two monocots, had at least one such gene, except Arabidopsis and B. rapa. This suggests that CCDL genes may have been lost in the common ancestor of Arabidopsis and B. rapa after its divergence from other angiosperms. There was only 1 CCDL gene in Amborella, whereas 5, 3, and 3 CCDL genes were found in F. vesca, pear, and peach, respectively, suggesting that CCDL genes probably expanded moderately within the Rosaceae.
Gene structures and motif analysis of carotenoid dioxygenase proteins
To further elucidate the functional conservation and divergence among carotenoid dioxygenase proteins, we carried out motif analysis using MEME with the total number of motifs set to 20. As a result, motif 1, 3, 5, 7 and 18 contained conserved histidine residues, and motif 4, 6, 14 and 16 contained conserved glutamate or aspartate residues (Table S4 or Fig S2). Both NCED and all groups of CCD proteins contained motif 1, 4, 5, 8, 14, and 15 (Table S5; Fig. S2), suggesting that these motifs may be responsible for their common functions. Furthermore, most NCED proteins contained all 20 motifs with motif 19 and 20 specific to them, whereas CCD proteins contained fewer motifs. CCD4 proteins lacked motifs 19 and 20, CCD1 proteins lacked motif 10, 19 and 20, and CCDL, CCD7, and CCD8 proteins lacked multiple motifs (Table S5; Fig. S2). In summary, using motif analysis, we demonstrated that each group of carotenoid dioxygenase proteins had a different motif composition, which further supports the topology of our phylogenetic tree, showing that CCD4 proteins are most closely related to the NCED proteins, whereas CCD7s and CCD8s had the most distant relationship with NCEDs.
We also analyzed the exon–intron structures of all 131 genes and found that the number of introns varied greatly among different NCED and CCD genes, but was similar within each group (Fig. 1). Most NCED and CCD4 genes had no more than one intron, whereas in other groups, all genes contained more than three introns, particularly in the CCD1 and CCDL groups. For instance, FveCCD7, FveCCD8, FveCCD1, FveCCDL1, FveCCDL2, FveCCDL3, and FveCCDL4 contained 4, 4, 12, 7, 12, 13, and 11 introns, respectively, while the 3 FveNCED genes and FveCCD4 had 0 introns (Table 1). Based on our phylogeny, NCED and CCD4 genes are likely derived from other groups of CCDs, most likely CCD1 genes. Therefore, the observed loss of introns may have occurred during the gene duplication event that gave rise to the ancestor of NCED and CCD4 genes, which then maintained their intron-free structure.
Transcriptomic analysis of FveNCED and FveCCD genes in flowers and early-stage fruits
To investigate the roles of FveNCED and FveCCD genes in reproductive tissues and organs, we downloaded and analyzed their transcriptomic data in F. vesca flowers and early-stage fruits (from anthesis to 13 DPA) (Darwish et al. 2013; Kang et al. 2013; Hollender et al. 2014). The results showed that, in F. vesca flowers, FveNCED1 exhibited the highest expression, whereas FveNCED2 and FveNCED6 appeared to be expressed at lower levels (Fig. 2). The exceptions are found in anthers, where FveNCED2 displayed similarly high mRNA levels with FveNCED1 and in mature pollen, where all 3 FveNCED genes appeared to be scarcely expressed. In particular, it appeared that the expression levels of FveNCED1 in styles increased, FveNCED2 decreased slightly, and FveNCED6 declined significantly after pollination, suggesting differential roles for these 3 FveNCED genes in styles after the pollination.
Expression profiles of carotenoid dioxygenase genes in the flowers and early-stage fruits of F. vesca. Data were retrieved from http://bioinformatics.towson.edu/strawberry/ (Kang et al. 2013; Darwish et al. 2013; Hollender et al. 2014). The heat map showed log2 “relative RPKM values” of individual carotenoid dioxygenase genes. Gray boxes indicate undetectable expression of the genes. Numbers after flower tissues (including perianth, carpel, anther, microspore, pollen, and style) and fruit tissues (including receptacle, embryo, ovule, ghost, wall, cortex, and pith) indicate the developmental stages of flowers and fruits, respectively. For detailed description of the stages and tissues, please see http://bioinformatics.towson.edu/strawberry/newpage/Tissue_Description.aspx
In the early-stage achene, FveNCED1 and FveNCED2 exhibited similar expression patterns during development (Fig. 2). Their expression in the developing embryos was highest during Fruit Stage 3 (the heart stage for embryos), followed by a gradual reduction, and reached a minimum level at Fruit Stage 5 (10–13 DPA), when embryos were considered to be mature (Hollender et al. 2012). In the early-stage receptacle, FveNCED2 appeared to be more highly expressed than FveNCED1 in both the cortex and pith tissues. It appeared that the expression level of FveNCED1 gradually declines as the receptacle developed, whereas the expression of FveNCED2 first decreased and then increased after Fruit Stage 4 (8–10 DPA). The expression of FveNCED6 was near the detection limit in both the early-stage achene and receptacle, suggesting that it may not be important in early fruit development.
CCD genes also display diversified expression patterns in the flowers of F. vesca (Fig. 2). Our analyses showed that FveCCD1 and FveCCD4 were expressed in all flower tissues, where it appeared that the expression level of FveCCD1 was the highest and FveCCD4 was the second highest. The expression of the other CCD genes was much lower and confined to a subset of tissues. Moreover, all were expressed in flowers from Flower Stages 1–4, in perianths at Flower Stage 5/6, and in microspores at Flower Stage 10. Specifically, the level of FveCCD7 mRNA appeared to remain stable in the styles after flower opening, while FveCCDL3 and FveCCDL4 expression appeared to increase during the 3 days of the pollination period. The other FveCCD genes, including FveCCD8, FveCCDL1, FveCCDL2, and FveCCDL5, appeared to have almost no detectable transcripts in the developing anthers, carpels, and styles.
In early fruits, FveCCD1 and FveCCD4 maintained high and stable expression levels in both developing achenes and receptacles, and their expression appeared to gradually increase when the fruits developed (Fig. 2). In receptacle tissues, FveCCD7 and FveCCD8 exhibited similar patterns of expression; in achene tissues, it appeared that FveCCD7 was moderately expressed while FveCCD8 transcripts were undetectable. FveCCDL3 appeared to show similar expression levels to FveCCD4 in most tissues aside from the achene wall, suggesting that this gene is important for early-stage fruit development. The expression of all other FveCCD genes was very low, suggesting minor roles in these tissues and stages.
Expression profiles of FveNCED and FveCCD genes during fruit ripening
Analysis of the expression patterns of NCED and CCD genes during fruit ripening can help us to better understand the role of these genes. In our study, qPCR was performed to analyze the expression of these genes at five stages of fruit ripening (from 13 to 29 DPA, Fig. 3c), namely BG, LW, PT, Pink, and Red, with achenes entirely removed. Our results demonstrated that among the 3 FveNCED genes, the abundance of FveNCED2 increased rapidly when fruits developed and ripened, and reached its highest level during the red stage (Fig. 3a). FveNCED1 maintained a much lower expression level, with maximum expression observed during the pre-turning stage (Fig. 3a); the expression of FveNCED6 could not be detected at any stage. Therefore, FveNCED2 most likely enables ABA biosynthesis during fruit ripening of the diploid strawberry. For CCD genes, FveCCD1 exhibited relatively high expression throughout all five stages, with a peak at the pre-turning stage followed by a continuous decline (Fig. 3b). Expression levels of FveCCD4, FveCCD7, FveCCD8, and FveCCDL3 were much lower and mostly declined during ripening (Fig. 3b). The expression of the remaining four FveCCD genes, including FveCCDL1, FveCCDL2, FveCCDL4, and FveCCDL5, was too low to be detected. In the transcriptomic data, as a whole, these four genes were scarcely expressed in receptacles, suggesting that they may not be important in receptacle development.
Expression profiles of carotenoid dioxygenase genes during fruit ripening in F. vesca. a b Expression levels of FveNCED (a) and FveCCD (b) genes. The transcript values were measured using qPCR with GAPDH as reference gene and displayed in the log2 values. Each data point was obtained by three biological replicates and three technical replicates. c The five stages of fruit ripening investigated in a and b: BG big green, 13–15 days post-anthesis (DPA); LW little white, 19–20 DPA; PT pre-turning, 24–25 DPA; Pink, 26–27 DPA; Red, 28–29 DPA
Expression profiles of FveNCED genes in F. vesca during abiotic stresses
To study how FveNCED genes respond to osmotic, heat, cold, and salinity stresses, we treated F. vesca seedlings with these stresses and then characterized the expression profiles of the 3 FveNCED genes. Our qPCR results showed that FveNCED genes had diversified responses to different environmental stresses (Fig. 4). FveNCED1 exhibited continuously enhanced expression during cold, PEG-osmotic, and heat stress treatments; this enhancement was most prominent under cold stress. During the recovery stage after the heat stress treatment, while the temperature returned to 22 °C, FveNCED1 expression gradually decreased. With salt treatment, FveNCED1 expression exhibited a minor increase at 1 h, but decreased when the treatment lasted more than 1 h, which differed from the patterns observed under other stress conditions. On the other hand, the expression of FveNCED2 was significantly enhanced only under cold stress (Fig. 4). Its expression slightly increased after 3-h PEG-osmotic stress treatment or at the recovery stage after the heat stress treatment, but continuously decreased under salt and heat stresses. FveNCED6 was undetectable in both control and all four abiotic stress treatments. The expression patterns of the 3 FveNCED genes during abiotic stress suggest that FveNCED1 plays a more important role in stress-induced ABA biosynthesis in F. vesca.
Expression patterns of FveNCED1 and FveNCED2 genes in response to abiotic stresses including heat, cold, PEG-osmotic and salinity. The expression values were performed using qPCR with GAPDH as reference gene and displayed in the log2 values. Each data point was obtained by three biological replicates and three technical replicates. T3 h + R1 h/R5 h means 3-h heat shock treatment followed by 1-h/5-h recovery, respectively. PEG polyethylene glycol, T treatment, R recovery
Discussion
In this study, we identified 131 carotenoid dioxygenase genes in ten angiosperm species, which could be divided into six groups based on phylogenic and motif analyses. Most previous phylogenetic analyses have demonstrated that the carotenoid dioxygenase gene family can be divided into five groups in higher plants, namely NCED, CCD1, CCD4, CCD7, and CCD8 (Walter et al. 2010; Vallabhaneni et al. 2010; Tan et al. 2003). Recently, Wei et al. (2016) identified the SlCCDL gene in tomato and classified it into a new group called CCDL, because it lacked an ortholog in Arabidopsis. In our study, we also found CCD genes belonging to the CCDL group in eight species, including F. vesca and tomato but excluding Arabidopsis and B. rapa, which supports the findings of Wei et al. (2016). Our results demonstrate that, compared with nine in the basal angiosperm Amborella, the number of carotenoid dioxygenase genes in higher angiosperms (9–19) did not increase greatly, revealing that only a moderate expansion occurred during angiosperm evolution. The numbers of CCD1, CCD4, CCD7, and CCD8 genes were stable, whereas NCED and CCDL gene numbers varied from 2 to 11 and 0 to 5, respectively, suggesting that the expansion of NCED and CCDL genes was primarily responsible for the expansion of the family. Four of the five FveCCDL genes were most likely derived from tandem duplication (Fig. S3), providing a possible mechanism for the expansion of the carotenoid dioxygenase genes in F. vesca.
Gene structure analysis revealed that most NCED and CCD4 genes in F. vesca and other species had no more than one intron. Previous reports have found that NCED genes in Arabidopsis and other species had no introns (Tan et al. 2003; Vallabhaneni et al. 2010). The absence of introns may be due to intron loss during the gene duplication event that produced the NCED and CCD4 ancestors and was maintained during subsequent plant evolution. It has been demonstrated that the fidelity of alternative splicing could be perturbed under cold stress (Bournay et al. 1996). Splicing errors and alternative splicing giving rise to truncated proteins are energy costly for the cell (Kalyna et al. 2012), and affect gene expression as well. Therefore, the intron-free structure of NCED genes may be a result of maintaining highly accurate posttranscriptional processing under stress, which is essential to rapid ABA biosynthesis and ABA-mediated stress responses in plants.
Based on our transcriptomic data analyses and qPCR results, FveNCED1 is probably responsible for ABA synthesis during carpel and achene development. Its expression significantly increased in the styles after pollination while the other two FveNCEDs’ expression decreased, suggesting that FveNCED1 is a good candidate for further study regarding the role of ABA in the pollinated styles, which is probably related to drying of the styles as they are senescing. On the other hand, FveNCED2 expression most likely accounts for ABA biosynthesis in the receptacle during fruit development and ripening in F. vesca. Strawberry fruit ripening is closely related to endogenous ABA content (Symons et al. 2012; Ayub et al. 2016). FveNCED2 had the highest expression level among the 3 NCED genes in the two receptacle tissues, the cortex and pith, during all stages of fruit development. Its expression continuously and highly increased during the ripening of the receptacle, which was in accordance with the increase in endogenous ABA content after the onset of fruit ripening previously reported in F. × ananassa (Symons et al. 2012). Expression of the other two genes was either undetectable or decreased while the ABA content increased. Therefore, FveNCED2 appears to be the dominant contributor to ABA biosynthesis in strawberry receptacles.
NCED expression is related to fruit ripening in non-climacteric fruits, such as grape (Zhang et al. 2009), sweet cherry (Luo et al. 2014), and octoploid strawberry (Jia et al. 2011; Ji et al. 2012). Ji et al. (2012) demonstrated that, in the receptacle of octoploid strawberry, FanNCED2 (HQ008770) was highly expressed, whereas the expression of FanNCED1 (HQ290318) was much lower. Moreover, enhanced FanNCED2 expression co-occurred with the increase in ABA content during fruit ripening. Our protein sequence alignments showed that FanNCED2 and FanNCED1 displayed the highest amino acid identity to FveNCED2 and FveNCED1, respectively, suggesting that orthologous gene expression patterns during receptacle development of diploid and octoploid strawberries are congruent. On the other hand, Jia et al. (2011) showed that the expression pattern of FanNCED1 was also consistent with ABA content variation during fruit ripening and FanNCED1 knockdown resulted in reduced ABA content and uncolored fruits. Hence, they proposed that FanNCED1 contributed to ABA biosynthesis during fruit ripening. Further experiments are needed to determine whether both FveNCED1 and FveNCED2 are responsible for the elevation of ABA levels.
It is well known that ABA can be induced under abiotic stress conditions, such as cold (Lang et al. 1994; Chen et al. 1983), heat (Larkindale 2005), salt (Zhu 2002), and PEG-osmotic stresses (Zhang et al. 2006), consistent with the upregulation of NCED gene expression (Iuchi et al. 2001). Our qPCR profiling of the FveNCED genes in F. vesca seedlings revealed that FveNCED1 was most likely the only enzyme for the induced ABA biosynthesis under PEG-osmotic and heat stresses, and the major one under cold stress, as the abundance of FveNCED2 also increased during cold treatment. This suggests that manipulating the gene expression of FveNCED1 would provide an effective method for studying the role of ABA in diploid strawberry under most abiotic stresses. During salt stress with 150 mM NaCl treatment, none of the 3 FveNCED genes were significantly induced. The expression of FveNCED1 was slightly elevated at 1-h treatment, but declined as the stress duration increased. Therefore, whether FveNCED genes and ABA are involved in the response of F. vesca to salt stress requires further verification.
CCD1 expression plays an important role in the production of apocarotenoid volatiles in Petunia corollas (Simkin et al. 2004b) and in grape (Mathieu et al. 2005) and tomato fruits (Simkin et al. 2004a). Consistent with the previous report in F. × ananassa (García-Limones et al. 2008), our results showed that FveCCD1 maintained strong expression during the processes of achene and receptacle development and ripening, suggesting that FveCCD1 and its products play an important role in F. vesca fruits. However, it reached its maximum expression at the pre-turning stage, not the red stage, as observed in octoploid strawberry. The expression of FanCCD1 was shown to continuously increase during receptacle ripening of octoploid strawberry, with the content of lutein, a main substrate of FanCCD1, drastically reduced (García-Limones et al. 2008). In general, diploid strawberry receptacles will be red and ripe within 3 or 4 days after the pre-turning stage (Fig. 3c). The differential expression patterns of FveCCD1 and FanCCD1 genes suggest that there may be some differences between carotenoid metabolisms of diploid and octoploid strawberries during receptacle ripening.
CCD7 and CCD8 are key enzymes in the biosynthesis of strigolactone, which is related to the size of tomato fruits and seeds (Vogel et al. 2010; Kohlen et al. 2012). During the early development stages of F. vesca fruits, FveCCD7 and FveCCD8 showed similarly high levels of expression in receptacle tissues, but quite low levels in achene tissues. Thus, strigolactone may be involved in the early-stage development of receptacles but not achenes. In strigolactone biosynthesis, the product of the CCD7 enzyme is the substrate for CCD8 (Harrison and Bugg 2014). However, our transcriptomic analysis demonstrated high expression of FveCCD7 and almost no expression of FveCCD8 in strawberry styles both before and after pollination, suggesting that the product of FveCCD7 may be involved in a pathway other than strigolactone biosynthesis.
SlCCDL gene expression has been reported in flowers, pistils, and mature green fruits (Wei et al. 2016). In this study, we identified 5 CCDL genes in F. vesca, more than that can be found in other species. Among these 5 FveCCDL genes, FveCCDL3 always had the highest expression levels while FveCCDL1, 2, and 5 were scarcely expressed. A large increase in FveCCDL3 and FveCCDL4 expression was observed in the styles during the 3 days of the pollination period, suggesting that these two genes might be involved in the changes in the pollinated styles, such as pollen tube growth. In addition, FveCCDL3 exhibited high expression in the early stages of both cortex and pith development, indicating that FveCCDL3 is also involved in the development of receptacles. Taken together, our results provide useful information for further studies on the roles of FveCCDL3 and other carotenoid dioxygenase genes in flower and fruit development in F. vesca, as well as the role of FveNCED genes in response to abiotic stress.
References
Albert VA, Barbazuk WB, DePamphilis CW, Der JP, Leebens-Mack J, Ma H, Palmer JD, Rounsley S, Sankoff D, Schuster SC, Soltis DE, Soltis PS, Wessler SR, Wing RA, Albert VA, Ammiraju JSS, Barbazuk WB, Chamala S, Chanderbali AS, DePamphilis CW, Der JP, Determann R, Leebens-Mack J, Ma H, Ralph P, Rounsley S, Schuster SC, Soltis DE, Soltis PS, Talag J, Tomsho L, Walts B, Wanke S, Wing RA, Albert VA, Barbazuk WB, Chamala S, Chanderbali AS, Chang T, Determann R, Lan T, Soltis DE, Soltis PS, Arikit S, Axtell MJ, Ayyampalayam S, Barbazuk WB, Burnette JMI, Chamala S, De Paoli E, DePamphilis CW, Der JP, Estill JC, Farrell NP, Harkess A, Jiao Y, Leebens-Mack J, Liu K, Mei W, Meyers BC, Shahid S, Wafula E, Walts B, Wessler SR, Zhai J, Zhang X, Albert VA, Carretero-Paulet L, DePamphilis CW, Der JP, Jiao Y, Leebens-Mack J, Lyons E, Sankoff D, Tang H, Wafula E, Zheng C, Albert VA, Altman NS, Barbazuk WB, Carretero-Paulet L, DePamphilis CW, Der JP, Estill JC, Jiao Y, Leebens-Mack J, Liu K, Mei W, Wafula E, Altman NS, Arikit S, Axtell MJ, Chamala S, Chanderbali AS, Chen F, Chen J, Chiang V, De Paoli E, DePamphilis CW, Der JP, Determann R, Fogliani B, Guo C, Harholt J, Harkess A, Job C, Job D, Kim S, Kong H, Leebens-Mack J, Li G, Li L, Liu J, Ma H, Meyers BC, Park J, Qi X, Rajjou L, Burtet-Sarramegna V, Sederoff R, Shahid S, Soltis DE, Soltis PS, Sun Y, Ulvskov P, Villegente M, Xue J, Yeh T, Yu X, Zhai J, Acosta JJ, Albert VA, Barbazuk WB, Bruenn RA, Chamala S, de Kochko A, DePamphilis CW, Der JP, Herrera-Estrella LR, Ibarra-Laclette E, Kirst M, Leebens-Mack J, Pissis SP, Poncet V, Schuster SC, Soltis DE, Soltis PS, Tomsho L (2013) The Amborella genome and the evolution of flowering plants. Science 342:1467
Amil-Ruiz F, Garrido-Gala J, Blanco-Portales R, Folta KM, Muñoz-Blanco J, Caballero JL (2013) Identification and validation of reference genes for transcript normalization in strawberry (Fragaria × ananassa) defense responses. PLoS One 8:e70603
Ayub RA, Bosetto L, Galvao CW, Etto RM, Inaba J, Lopes PZ (2016) Abscisic acid involvement on expression of related gene and phytochemicals during ripening in strawberry fruit Fragaria × ananassa cv. Camino Real. Sci Hortic 203:178–184
Bailey TL, Elkan C (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2:28–36
Bournay AS, Hedley PE, Maddison A, Waugh R, Machray GC (1996) Exon skipping induced by cold stress in a potato invertase gene transcript. Nucleic Acids Res 24:2347–2351
Cantin CM, Fidelibus MW, Crisostoc CH (2007) Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biol Technol 46:237–241
Chen HH, Li PH, Brenner ML (1983) Involvement of abscisic Acid in potato cold acclimation. Plant Physiol 71:362–365
Chernys JT, Zeevaart J (2000) Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol 124:343–353
Darwish O, Slovin JP, Kang C, Hollender CA, Geretz A, Houston S, Liu Z, Alkharouf NW (2013) SGR: an online genomic resource for the woodland strawberry. BMC Plant Biol 13:223
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Falchi R, Vendramin E, Zanon L, Scalabrin S, Cipriani G, Verde I, Vizzotto G, Morgante M (2013) Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J 76:175–187
Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer ELL, Eddy SR, Bateman A (2010) The Pfam protein families database. Nucleic Acids Res 381:D211–D222
García-Limones C, Schnäbele K, Blanco-Portales R, Luz Bellido M, Caballero JL, Schwab W, Muñoz-Blanco J (2008) Functional characterization of FaCCD1: a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening. J Agric Food Chem 56:9277–9285
Gu T, Ren S, Wang Y, Han Y, Li Y (2016) Characterization of DNA methyltransferase and demethylase genes in Fragaria vesca. Mol Genet Genom 291:1333–1345
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Harrison PJ, Bugg TDH (2014) Enzymology of the carotenoid cleavage dioxygenases: reaction mechanisms, inhibition and biochemical roles. Arch Biochem Biophys 544:105–111
Hollender CA, Geretz AC, Slovin JP, Liu Z (2012) Flower and early fruit development in a diploid strawberry, Fragaria vesca. Planta 235:1123–1139
Hollender CA, Kang C, Darwish O, Geretz A, Matthews BF, Slovin J, Alkharouf N, Liu Z (2014) Floral transcriptomes in woodland strawberry uncover developing receptacle and anther gene networks. Plant Physiol 165:1062–1075
Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333
Ji K, Chen P, Sun L, Wang Y, Dai S, Li Q, Li P, Sun Y, Wu Y, Duan C, Leng P (2012) Non-climacteric ripening in strawberry fruit is linked to ABA, FaNCED2 and FaCYP707A1. Funct Plant Biol 39:351–357
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157:188–199
Jung S, Bassett C, Bielenberg DG, Cheng C, Dardick C, Main D, Meisel L, Slovin J, Troggio M, Schaffer RJ (2015) A standard nomenclature for gene designation in the Rosaceae. Tree Genet Genomes 11:108
Kalyna M, Simpson CG, Syed NH, Lewandowska D, Marquez Y, Kusenda B, Marshall J, Fuller J, Cardle L, McNicol J, Dinh HQ, Barta A, Brown JWS (2012) Alternative splicing and nonsense-mediated decay modulate expression of important regulatory genes in Arabidopsis. Nucleic Acids Res 40:2454–2469
Kang C, Darwish O, Geretz A, Shahan R, Alkharouf N, Liu Z (2013) Genome-scale transcriptomic insights into early-stage fruit development in woodland strawberry Fragaria vesca. Plant Cell 25:1960–1978
Keane TM, Creevey CJ, Pentony MM, Naughton TJ, Mclnerney JO (2006) Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol 6:1–17
Kloer DP, Schulz GE (2006) Structural and biological aspects of carotenoid cleavage. Cell Mol Life Sci 63:2291–2303
Kohlen W, Charnikhova T, Lammers M, Pollina T, Toth P, Haider I, Pozo MJ, de Maagd RA, Ruyter-Spira C, Bouwmeester HJ, Lopez-Raez JA (2012) The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol 196:535–547
Kumar R, Khurana A, Sharma AK (2014) Role of plant hormones and their interplay in development and ripening of fleshy fruits. J Exp Bot 65:4561–4575
Lang V, Mantyla E, Welin B, Sundberg B, Palva ET (1994) Alterations in water status, endogenous abscisic acid content, and expression of rab18 Gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol 104:1341–1349
Larkindale J (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138:882–897
Ledger SE, Janssen BJ, Karunairetnam S, Wang T, Snowden KC (2010) Modified CAROTENOID CLEAVAGE DIOXYGENASE8 expression correlates with altered branching in kiwifruit (Actinidia chinensis). New Phytol 188:803–813
Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion-Poll A (2006) Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45:309–319
Leng P, Yuan B, Guo Y (2014) The role of abscisic acid in fruit ripening and responses to abiotic stress. J Exp Bot 65:4577–4588
Li C, Jia H, Chai Y, Shen Y (2011) Abscisic acid perception and signaling transduction in strawberry: a model for non-climacteric fruit ripening. Plant Signal Behav 6:1950–1953
Luo H, Dai S, Ren J, Zhang C, Ding Y, Li Z, Sun Y, Ji K, Wang Y, Li Q, Chen P, Duan C, Wang Y, Leng P (2014) The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit. J Plant Growth Regul 33:373–383
Ma J, Li J, Zhao J, Zhou H, Ren F, Wang L, Gu C, Liao L, Han Y (2014) Inactivation of a gene encoding carotenoid cleavage dioxygenase (CCD4) leads to carotenoid-based yellow coloration of fruit flesh and leaf midvein in peach. Plant Mol Biol Rep 32:246–257
Mathieu S, Terrier N, Procureur J, Bigey F, Gunata Z (2005) A carotenoid cleavage dioxygenase from Vitis vinifera L.: functional characterization and expression during grape berry development in relation to C-13-norisoprenoid accumulation. J Exp Bot 56:2721–2731
McAtee P, Karim S, Schaffer R, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci 4:79–85
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ohmiya A (2009) Carotenoid cleavage dioxygenases and their apocarotenoid products in plants. Plant Biotechnol-Nar 26:351–358
Priya R, Siva R (2014) Phylogenetic analysis and evolutionary studies of plant carotenoid cleavage dioxygenase gene. Gene 548:223–233
Priya R, Siva R (2015) Analysis of phylogenetic and functional diverge in plant nine-cis epoxycarotenoid dioxygenase gene family. J Plant Res 128:519–534
Rodriguez-Avila NL, Narvaez-Zapata JA, Ramirez-Benitez JE, Aguilar-Espinosa ML, Rivera-Madrid R (2011) Identification and expression pattern of a new carotenoid cleavage dioxygenase gene member from Bixa orellana. J Exp Bot 62:5385–5395
Roychoudhury A, Paul S, Basu S (2013) Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep 32:985–1006
Ruggiero B (2004) Uncoupling the effects of abscisic acid on plant growth and water relations. Analysis of sto1/nced3, an abscisic acid-deficient but salt stress-tolerant mutant in Arabidopsis. Plant Physiol 136:3134–3147
Schwartz SH (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131:1591–1601
Schwartz SH, Tan BC, Gage DA, Zeevaart J, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874
Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, Burns P, Davis TM, Slovin JP, Bassil N, Hellens RP, Evans C, Harkins T, Kodira C, Desany B, Crasta OR, Jensen RV, Allan AC, Michael TP, Setubal JC, Celton J, Rees DJG, Williams KP, Holt SH, Rojas JJR, Chatterjee M, Liu B, Silva H, Meisel L, Adato A, Filichkin SA, Troggio M, Viola R, Ashman T, Wang H, Dharmawardhana P, Elser J, Raja R, Priest HD Jr, Bryant DW, Fox SE, Givan SA, Wilhelm LJ, Naithani S, Christoffels A, Salama DY, Carter J, Girona EL, Zdepski A, Wang W, Kerstetter RA, Schwab W, Korban SS, Davik J, Monfort A, Denoyes-Rothan B, Arus P, Mittler R, Flinn B, Aharoni A, Bennetzen JL, Salzberg SL, Dickerman AW, Velasco R, Borodovsky M, Veilleux RE, Folta KM (2011) The genome of woodland strawberry (Fragaria vesca). Nat Genet 43:109–116
Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ (2004a) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J 40:882–892
Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ (2004b) Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant Physiol 136:3504–3514
Sui X, Kiser PD, Lintig JV, Palczewski K (2013) Structural basis of carotenoid cleavage: from bacteria to mammals. Arch Biochem Biophys 539:203–213
Sun L, Sun Y, Zhang M, Wang L, Ren J, Cui M, Wang Y, Ji K, Li P, Li Q, Chen P, Dai S, Duan C, Wu Y, Leng P (2012) Suppression of 9-cis-Epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol 158:283–298
Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB (2012) Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot 63:4741–4750
Tan B, Joseph LM, Deng W, Liu L, Li Q, Cline K, McCarty DR (2003) Molecular characterization of theArabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Vallabhaneni R, Bradbury LMT, Wurtzel ET (2010) The carotenoid dioxygenase gene family in maize, sorghum, and rice. Arch Biochem Biophys 504:104–111
Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T, Simkin AJ, Goulet C, Strack D, Bouwmeester HJ, Fernie AR, Klee HJ (2010) SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant Journal 61:300–311
Walter MH, Floss DS, Strack D (2010) Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232:1–17
Wan X, Li L (2006) Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochem Biophys Res Commun 347:1030–1038
Wei Y, Wan H, Wu Z, Wang R, Ruan M, Ye Q, Li Z, Zhou G, Yao Z, Yang Y (2016) A comprehensive analysis of carotenoid cleavage dioxygenases genes in Solanum lycopersicum. Plant Mol Biol Rep 34:512–523
Wheeler S, Loveys B, Ford C, Davies C (2009) The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust J Grape Wine Res 15:195–204
Winterhalter P, Ebeler SE (2013) Carotenoid cleavage products: an introduction. In: Winterhalter P, Ebeler SE (eds) Carotenoid cleavage products. American Chemical Society, Washington, DC, pp 3–9
Yang J, Guo Z (2007) Cloning of a 9-cis-epoxycarotenoid dioxygenase gene (SgNCED1) from Stylosanthes guianensis and its expression in response to abiotic stresses. Plant Cell Rep 26:1383–1390
Young PR, Lashbrooke JG, Alexandersson E, Jacobson D, Moser C, Velasco R, Vivier MA (2012) The genes and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genom 13:1–17
Yu D, Tang H, Zhang Y, Du Z, Yu H, Chen Q (2012) Comparison and improvement of different methods of RNA isolation from strawberry (Fragria × ananassa). J Agric Sci 4:51–56
Zhang JH, Jia WS, Yang JC, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res 97:111–119
Zhang M, Leng P, Zhang G, Li X (2009) Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol 166:1241–1252
Zhang B, Liu C, Wang Y, Yao X, Wang F, Wu J, King GJ, Liu K (2015) Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytol 206:1513–1526
Zhu J (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC 31471860, 31672123) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
Author contribution
JD and YL conceived and designed the project; YW and GD performed the experiments and carried out the analysis; YW and JD wrote the manuscript; and TG and YL edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that no competing interests deserved. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/9P2sze.
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Ding, G., Gu, T. et al. Bioinformatic and expression analyses on carotenoid dioxygenase genes in fruit development and abiotic stress responses in Fragaria vesca . Mol Genet Genomics 292, 895–907 (2017). https://doi.org/10.1007/s00438-017-1321-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-017-1321-5