Abstract
Yellow fruit flesh color, resulting from the accumulation of carotenoids, is one of the most important commercial traits of peach. Yellow flesh is controlled by a single locus (Y), with white flesh dominant over yellow flesh. In this study, the Y locus was narrowed to a 2.6-cM interval flanked by two markers, SSRy and W2691. SSRy, which is located on the first exon of a gene encoding carotenoid cleavage dioxygenase (CCD4), was cosegregated with the Y locus in two peach F1 populations. RNA-Seq and qRT-PCR analysis revealed transcript level of CCD4 was consistent with carotenoid degradation in peach fruits. All these results suggest that CCD4 is responsible for white and yellow coloration of peach fruit flesh. In fruits of white-fleshed peach, carotenoids are synthesized but subsequently degraded into colorless compounds, leading to the formation of white color. CCD4 is likely to utilize β-carotene as the substrate in peach. Interestingly, CCD4 also controls white and yellow coloration of leaf midveins of peach. Moreover, LCYE was highly expressed in peach leaves, whereas its transcript was not detectable in fruits. This suggests the difference of carotenoid biosynthesis between peach fruits and leaves. Our study not only shows for the first time the pleiotropic effects of CCD4 gene in peach but also provides a morphological marker for easy selection of new peach cultivars with desirable white or yellow flesh colors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are tetraterpenoid organic pigments that are naturally synthesized in chloroplasts and chromoplasts of plants. In chloroplasts, carotenoids play indispensable roles in photosynthesis, whereas in chromoplasts, they are recognized as secondary metabolites (Hirschberg 2001). Carotenoids are essential to both plants and humans. First, carotenoids provide flowers and fruits with distinct colors, ranging from yellow to orange or red, to attract insects and animals for pollination as well as seed dispersal. Second, carotenoids have multiple benefits to human health. For example, carotenoids act as antioxidants by oxidizing the superoxide radical anion, and may thus reduce the risk of certain cancers (Fraser and Bramley 2004). Some carotenoids can also serve as precursors to vitamin A, which is required for healthy skin and mucus membranes, and for night vision. Hence, carotenoid biosynthesis is becoming a hot topic worldwide in recent years.
The carotenoid metabolism pathway has been well established in higher plants (Isaacson 2002; Park et al. 2002; Schwartz et al. 2003; Li et al. 2007; Maass et al. 2009; Chen et al. 2010). Briefly, the biosynthesis pathway of carotenoids begins with the condensation of geranylgeranyl diphosphate, leading to the formation of phytoene (Fig. 1), and this reaction is catalyzed by the enzyme phytoene synthase (PSY). Phytoene is desaturated by phytoene desaturase (PDS) and zeta-carotene desaturase (ZDS), and then isomerized by carotenoid isomerase (CRTISO) and zeta-carotene isomerase (Z-ISO) to form the all-trans-lycopene. Subsequently, lycopene is cyclized at each end by lycopene β-cyclase (LCYB) or lycopene ε-cyclase (LCYE) to yield β- or α-carotene, respectively. Hydroxylation of β- and α-carotenes at C3 and C3′ by β-ring hydroxylase (HYB) and ε-ring hydroxylase (HYE) results in lutein and zeaxanthin. Zeaxanthin epoxidase (ZEP) further converts zeaxanthin into violaxanthin through two-step epoxidation. Finally, violaxanthin is converted to neoxanthin by neoxanthin synthase (NSY). Carotenoid cleavage dioxygenases (CCDs) cleave carotenoids into apocarotenoids at different double-bond positions, while 9-cis-epoxycarotenoid dioxygenases (NCEDs) catalyzes the cleavage of 9-cis-violaxanthin or 9′-cis-neoxanthin to produce C25 epoxy-apocarotenal and an ABA precursor xanthoxin.
Carotenoid biosynthetic pathway in higher plants. The dash line indicates the branch is blocked in peach fruit, but activated in peach leaf. PSY phytoene synthase, PDS phytoene desaturase, ZDS zeta-carotene desaturase, CRTISO carotenoid isomerase, Z-ISO zeta-carotene isomerase, LCYE lycopene ε-cyclase, LCYB lycopene β-cyclase, HYB β-ring hydroxylase, HYE ε-ring hydroxylase, NSY neoxanthin synthase, ZEP zeaxanthin epoxidase, NCED 9-cis-epoxycarotenoid dioxygenase, CCD carotenoid cleavage dioxygenase
Structural genes of the carotenoid biosynthesis pathway have been isolated and characterized in such plant species as tomato and citrus. In tomato, lycopene is responsible for red coloration, and its accumulation coincides with both expression of genes encoding PSY and PDS and suppression of genes encoding LCYB and LCYE (Bramley 2002). In citrus, the accumulation of xanthophylls is associated with the expression balance between the upstream carotenogenic genes encoding PSY, PDS, ZDS, and LCYB and the downstream genes encoding HYB and ZEP (Kato et al. 2004). Recently, transcription factors (TFs) involved in regulation of carotenoid biosynthesis have been identified. For example, ERF/RAP2.2 TFs mediate the expression of genes encoding PSY and PDS (Welsch et al. 2007). Phytochrome-interacting factors (PIFs) repress the expression of genes encoding PSY to downregulate the accumulation of carotenoids (Toledo-Ortiz et al. 2010). Besides transcriptional regulation, post-translational regulation has also been reported to be involved in the regulation of carotenoid biosynthesis (Egea et al. 2010). For example, inactive form of ZDS and differential membrane-binding capacity of NCEDs are two potential mechanisms regulating carotenoid accumulation in peach and Arabidopsis, respectively (Tan et al. 2003; Marty et al. 2005). In addition, carotenoid accumulation can be regulated by controlling the formation of chromoplasts in plants (Lu et al. 2006).
Peach (Prunus persica), a member of the Rosaceae family, is one of the most important fruit crops grown in the temperate zone of the world. Peach is a diploid with a base chromosome number of 8 and has a small genome size of ~230 Mb/haploid (The International Peach Genome Initiative 2013). Thus, peach is an important model plant for the study of carotenoid biosynthesis in fruit trees. Yellow flesh is controlled by a single locus (Y) on linkage group 1, with white flesh dominant over yellow flesh (Bliss et al. 2002; Dirlewanger et al. 2004; Williamson et al. 2006). However, there are few studies on mechanisms underlying carotenoid biosynthesis in Prunus (Kita et al. 2007; Brandi et al. 2011).
To investigate metabolic and genetic differences among yellow- and white-fleshed peaches, genetic mapping of the Y locus and RNA-Seq analysis were conducted in this study. A gene encoding CCD4, designated PpCCD4, was identified to control white or yellow colors of both peach fruit and leaf midvein. This result will be very helpful for understanding and manipulation of carotenoid accumulation in peach fruits. The yellow color of leaf vein can also serve as a user-friendly marker for selection of yellow-fleshed varieties.
Materials and Methods
Plant Material
Two segregating F1 populations, “NJ250” × “96-7-41” and “98-5-21” × “96-7-41,” were collected from the Institute of Forestry and Pomology of the Beijing Academy of Agriculture and Forestry Sciences, Beijing, China. Yellow- and white-fleshed cultivars used in this study are all maintained at Wuhan Botanical Garden of the Chinese Academy of Sciences (Wuhan, Hubei Province, People's Republic of China). Leaves were collected during juvenile stage in spring season. Fruit samples were collected at different stages after pollination and cut into small pieces. All samples were immediately frozen in liquid nitrogen, and then stored at −75 °C until use.
RNA Isolation and cDNA Synthesis
Total RNA was isolated using Total RNA Kit (Bioteke, China) according to the manufacturer's instructions and then adjusted to 500 ng/μL using ND-1000 UV–Vis spectrophotometer (NanoDrop, USA). The RNA samples were treated with RNase-free DNase I (Invitrogen, USA) to avoid any genomic DNA contamination. Approximate 2 μg of total RNA was used for first-strand cDNA synthesis with oligodT (Promega, Madison, WI, USA) and SuperScriptIII reverse transcriptase (Invitrogen).
Quantitative Real-Time PCR (qRT-PCR)
qRT-PCR was conducted using a total volume of 20 μL reaction containing 100 ng of template cDNA, 0.2 μM of each primer, and 10 μL of 2× SYBR Green I Master Mix (Takara, Dalian, China). Amplifications were performed using Applied Biosystems® 7500 Real-Time PCR Systems (Applied Biosystems, USA). The amplification program consisted of one cycle of 30 s at 95 °C, followed by 40 cycles of 5 s at 95 °C and 34 s at 58 °C. Melting curve analysis was performed at the end of 40 cycles to ensure proper amplification of target fragments. A peach EF2 gene (GDR accession no. ppa001368m) was used as a constitutive control. Transcripts were quantified using the standard curve method. All analyses were repeated three times using three biological replicates. Primer sequences used for real-time PCR are listed in Table S1.
Carotenoid Extraction and HPLC Analysis
Two grams of peach fruits was ground into fine powder in liquid nitrogen and added to 15 mL extraction buffer of hexane:ethanol:acetone (v/v/v 2:1:1) containing 0.01 % of butylated hydroxytoluene (BHT). The mixture was vortexed briefly for 30 min and then centrifuged for 10 min at 6,000 × g. The supernatant was collected, and the residual was resuspended using extraction buffer. The extraction was repeated twice until the residual was colorless. The extracts were combined and washed three times with saturated NaCl. The supernatant was transferred to a centrifuge tube and diluted with hexane to 25 mL. A total of 5 mL dilution was subjected to measurement of absorbance at 470 nm using ultraviolet spectrophotometer. The rest of 20 mL dilution was dried and then resuspended in 2 mL of MTBE with 0.01 % BHT. The solution was saponified by adding 2 mL KOH in methanol and incubated overnight. After the saponification, water-soluble extracts were collected by adding NaCl-saturated water. Subsequently, the residue was dried and redissolved in 0.3 mL of MTBE solution.
Carotenoids were identified by their characteristic absorption spectra and distinctive retention times. Quantification was performed by integrating the peak areas of the HPLC results using Millennium chromatography software (Waters, Milford, MA, USA).
Phylogenetic Analysis
Protein sequences of CCD genes in plants were used for phylogenetic analysis. Sequences were aligned using the CLUSTAL X and adjusted manually. The resulting data were analyzed using equally weighted neighbor joining (NJ). NJ trees were constructed using the heuristic search strategies of MEGA version 5. Bootstrap values were calculated by 1,000 replicates of analysis.
Development of SSRs for Mapping of the Y Locus
The Y locus of peach was previously mapped to an interval flanked by two SSR markers, C-PP04A01 and UPD96-005 (Ogundiwin et al. 2009). The genomic DNA sequences between the two SSRs were extracted from the draft genome of cv. Lovell (Peach v1.0, http://www.rosaceae.org/species/prunus_persica/genome_v1.0) and subjected to identify SSR loci using online SSR finding program SSRIT (http://www.gramene.org/db/markers/ssrtool). A total of 43 SSR primer pairs were then designed to amplify genomic DNA of two parents of the mapping population 98-5-21 × 96-7-41. Amplification program consisted of one cycle of 3 min at 95 °C, followed by 35 cycles of 45 s at 94 °C, 45 s at 55 °C, 45 s at 72 °C, and a final extension step at 72 °C for 10 min. PCR products were separated on 6 % polyacrylamide gel and visualized after silver staining according to our previously reported protocol (Zhang et al. 2012).
Polymorphic SSRs between the two parents were further subjected to screen progenies of the mapping population using polyacrylamide gel electrophoresis as mentioned above. The linkage analysis was carried out using JoinMap version 4.0 (Van Ooijen 2006). The consensus genetic linkage map was constructed using the Kosambi mapping function with a LOD score threshold of 4.0.
Results
Characterization of Carotenoid Accumulation in White- and Yellow-Fleshed Peach Fruits
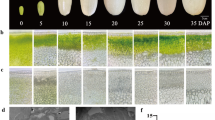
A yellow-fleshed cv. Jinxiang (JX) and a white-fleshed cv. Baifeng (BF) were selected to investigate accumulation patterns of carotenoid in peach fruits. Fruits of cv. JX and BF ripened 89 and 105 days after pollination (DAP), respectively. The flesh of cv. JX and BF was green in color during the early stages of fruit development and changed color to yellow ~3 weeks before ripening (Fig. 2a). Overall, the concentration of total carotenoids was the highest in young fruitlet and then decreased significantly during pit hardening phase (Fig. 2b). Later, accumulation of carotenoids increased in yellow-fleshed fruit until the fruit ripening. However, the accumulation of carotenoids decreased rapidly in white-fleshed fruit during late stage of fruit development and was almost undetectable in ripening.
The flesh color and total carotenoid content of peach fruits. a Fruit developmental stages of yellow-fleshed cv. Jinxing (JX) and white-fleshed cv. Baifeng (BF). b The total carotenoid content of fruits at different developmental stages. Fruit developmental stages are as follows: (I) 30 days after pollination (DAP), (II) 45 DAP, (III) 60 DAP, (IV) 77 DAP, (V) 89 DAP, and (VI) 105 DAP
HPLC analysis further revealed the major components of carotenoids were β-carotene, antheraxanthin, and violaxanthin (Table 1). The accumulation of antheraxanthin and violaxanthin in yellow-fleshed fruit was significantly higher than that in white-fleshed fruit. Several minor components of carotenoids were also identified in peach fruits, including lutein, α-carotene, and cryptoxanthin. Lutein and cryptoxanthin were present in young fruitlet, but not detectable in fruits at middle and late stages of development.
Expression Profiling of Carotenoid Biosynthetic Pathway Genes in Developing Fruits
A total of 13 genes in carotenoid biosynthetic pathway were identified in the reference genome of peach “Lovell,” and their expression profiles in developing fruit were examined using qRT-PCR (Fig. 3). Two PSY genes, PSY1 and PSY2, were identified in the peach genome. PSY1 was expressed in both yellow- and white-fleshed fruits in late stage of development, while PSY2 was predominately expressed in white-fleshed fruit. Similarly, two copies of LCYB were present in the peach genome. LCYB1 was expressed in yellow- and white-fleshed fruits throughout fruit development, while LCYB2 was expressed in fruitlets of yellow-fleshed cultivar. The transcript of LCYE was not detected in yellow- and white-fleshed fruits. Several genes such as PSY2, ZEP, and NSY showed difference in expression level between yellow-fleshed cv. JX and white-fleshed cv. BF. However, the expression profile of carotenoid biosynthetic pathway genes was not consistent with the pattern of carotenoid accumulation in peach fruit.
Expression Profiling of Genes Involved in Carotenoid Cleavage in Developing Fruits
Four CCD genes (CCD1, CCD4, CCD7, and CCD8) and three NCED genes (NCED3A, NCED3B, and NCED6) were identified in the reference genome of cv. Lovell (Fig. 4). The expression profiling of these genes involved in carotenoid oxidation was examined in developing fruits of cvs. BF and JX using qRT-PCR. The results indicated the transcript of CCD1 was detected in both yellow- and white-fleshed fruits throughout fruit development, with slightly high levels in yellow-fleshed fruit than in white-fleshed fruit. NCED3A and NCED3B were predominately expressed in yellow-fleshed fruit at ripening stage. CCD8 and NCED6 were expressed in both yellow- and white-fleshed fruits during the whole development stage. Interestingly, CCD4 was highly expressed in white-fleshed fruit in late stage of development, but its transcript was almost undetectable in yellow-fleshed fruit (Fig. 5). This result was consistent with the dramatic degradation of carotenoids in white-fleshed fruits in late stage of development. In addition, expression of CCD4 in fruit was also assessed in yellow-fleshed cvs. Jinxiu, Jinyuan, and Huyou018 and white-fleshed cvs. Gailiangbaifeng, Hujingmilu, and Zhihebaitao. All white-fleshed cultivars tested showed high levels of CCD4 transcripts in fruits during early stages of ripening, whereas yellow-fleshed cultivars showed very low levels. In summary, CCD4 was closely related to white/yellow fruit flesh color of peach.
A phylogenetic tree of CCD genes in plants. Genes encoding CCDs in peach are highlighted in green color. The values near branch represent bootstrap value, and the scale bar indicates 0.1 nucleotide substitutions per site. The GenBank accession numbers of the published CCD genes are as follows: AtCCD1 (NP_191911), AtNCED2 (NP_193569), AtNCED3 (NP_188062), AtCCD4 (NP_193652), AtNCED5 (NP_174302), AtNCED6 (NP_189064), AtCCD7 (NP_182026.1), AtCCD8 (NP_195007.2), and AtNCED9 (NP_177960) in Arabidopsis thaliana; CsCCD4a (ACD62476.1), CsCCD4b (ACD62477.1), and CsZCD (AJ489276) in Crocus sativus; MdCCD4 (ABY47995) in Malus × domestica; RdCCD4 (ABY60886) in Rosa × damascene; CmCCD4a (ABY60885) and CmCCD4b (BAF36656) in Chrysanthemum × morifolium; Vb14(NP_001147527.1) in Zea mays; and OfCCD4 (ABY60887) in Osmanthus fragans. The GDR accession numbers of CCD genes in peach are as follows: CCD1 (ppa003814m), CCD8 (ppa006042m), CCD7 (ppa017865m), CCD4 (ppa006109m), NCED3A (ppa002804m), NCED3B (ppa002314m), and NCED6 (ppa014647m)
Fine Mapping of the Y Locus in Peach
Genetic mapping was conducted using a segregating F1 population 98-5-21 × 96-7-41. The two parents both bear white-fleshed fruits. The population consisted of 50 white- and 13 yellow-fleshed individuals, with a 3.8:1 ratio of white to yellow. This result suggested both parents are heterozygous at the Y locus. Thus, the genotypes for the Y locus of yellow- and white-fleshed individuals were marked as kk and h- (hh or hk), respectively.
Seven SSR markers were polymorphic between the two parents, 98-5-21 and 96-7-41, and their primer sequences are listed in Table 2. These SSRs were successfully used to narrow the Y locus to a 2.6-cM interval flanked by two markers, SSRy and W2691 (Fig. 6). The 2.6-cM interval was 1.3 Mb in size and contained 158 predicted genes. SSRy was tightly linked to the Y locus, and three genotypes for SSRy locus, TC8/TC8, TC7/TC7, and TC8/TC7, were identified in progeny (Table 3). TC8/TC8 was present in yellow-fleshed individuals, while both TC7/TC7 and TC8/TC7 were related to white-fleshed individuals. SSRy was further used to screen another segregating F1 population NJ250 × 96-7-41. Similarly, yellow-fleshed individuals had the same genotype TC8/TC8, while white-fleshed individuals had TC7/TC7 or TC8/TC7 genotypes (Table 3). In short, SSRy was cosegregated with the Y locus in peach. The SSRy was developed from a microsatellite locus located in the first exon of CCD4 gene. Thus, the fine-mapping result further suggested CCD4 gene was likely the candidate gene of the Y locus.
Comparison of RNA-Seq-Based Transcriptome Analysis between White- and Yellow-Fleshed Fruits of Peach
Three RNA-Seq libraries were prepared from flesh tissues of cv. BF at 89 DAP and cv. JX at 60 and 77 DAP, respectively. Each library was deep sequenced using Illumina platform. Approximate 9.7 million of pair-end reads were generated for the flesh sample of cv. BF, and 9.1 and 10.2 million of pair-end reads for flesh samples of cv. JX at 60 and 77 DAP, respectively. Identification of differentially expressed genes between cv. BF and JX samples was conducted according to FPKM (fragments per kilo bases per million reads) values as we previously reported (Wang et al. 2013). As a result, 768 and 560 transcripts were significantly up- or downregulated in white flesh (Fig. 7). Of the 1,328 differently expressed transcripts, three (CCD4 and two functional unknown genes with GDR accession no. PPa017003m and PPa025705m, respectively) were located in the 2.6-cM interval as mentioned above. The expression levels of PPa017003m or PPa025705m were less than two-fold higher in yellow flesh than those in white flesh.
Moreover, the expression profiles of carotenoid pathway genes were estimated using the RNA-Seq analysis, and the result was shown in Fig. 8. Only CCD4 gene showed significant difference in expression level between white and yellow flesh samples. This was consistent with the result of expression profiling of carotenoid pathway genes using qRT-PCR method. In summary, transcriptome analysis further confirmed that CCD4 was the candidate gene of the Y locus.
Expression Profiling of Carotenoid-Related Genes in White and Yellow Midveins of Peach Leaves
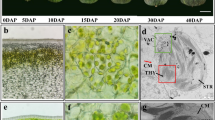
Leaves of white-fleshed cv. BF develop white-colored midveins, whereas leaves of yellow-fleshed cv. JX have yellow-colored midveins (Fig. 9a). Overall, levels of total carotenoids were ~2-fold higher in yellow midveins than in white midveins (Fig. 9b). Real-time PCR analysis further revealed that transcripts of CCD4 were abundant in white midveins, but not detectable in yellow midveins (Fig. 9c). Moreover, progeny of two F1 populations were evaluated for segregation of leaf midvein color, and it was found that all white-fleshed seedlings developed white-colored midveins, while all yellow-fleshed seedlings developed yellow-colored midveins. All these findings suggested the CCD4 gene was also responsible for white or yellow coloration in leaf midveins of peach.
Carotenoid accumulation in leaf vein of peach. a Leaves from white-fleshed cv. BF and yellow-fleshed cv. JX, respectively. b Concentration of total carotenoid in leaf veins with yellow or white colors. c Comparison of expression profile of carotenoid-related genes in leaf and vein between Jinxiang and Baifeng
Discussion
Inactivation of CCD4 Leads to Carotenoid Accumulation in Fruit Flesh and Leaf Midvein of Peach
Carotenoid cleavage dioxygenases (CCDs) catalyze the oxidative cleavage of carotenoids, resulting in various apocarotenoids (Auldridge et al. 2006). In plants, the CCD family consist of nine members: CCD1, NCED2, NCED3, CCD4, NCED5, NCED6, CCD7, CCD8, and NCED9 (Tan et al. 2003). Among these CCDs, the NCEDs catalyze neoxanthin and violaxanthin to yield xanthoxin, and the rest four CCDs have different substrate specificity (Tan et al. 2003; Auldridge et al. 2006). To date, however, only CCD4 has been so far reported to be involved in the regulation of carotenoid-based coloration in plants (Ohmiya et al. 2006). This scenario is also observed in this study. Seven copies of CCD genes are identified in the peach genome. Among the seven genes, only CCD4 is demonstrated to be responsible for white and yellow coloration of peach fruit flesh and leaf midvein through transcription analysis and genetic mapping.
It has been reported that transcription inactivation of CmCCD4a causes yellow coloration of flowers in chrysanthemum (Ohmiya et al. 2006). All white-flowered cultivars have high levels of CmCCD4a transcript in petals, whereas most of yellow-flowered cultivars show extremely low levels. Here, we show that all the white-fleshed varieties tested have high levels of CCD4 transcript in fruits at early ripening stage, but extremely low levels for all the tested yellow-fleshed varieties. Likewise, CCD4 is highly expressed in white-colored leaf midvein, but its transcript is extremely low in yellow-colored leaf midvein. Thus, it seems that the accumulation of carotenoids in fruit flesh and leaf midvein is due to inactivation of CCD4 in peach. However, it is important to note that the mechanisms underlying carotenoid-based yellow coloration are different between peach and chrysanthemum. In yellow-flowered chrysanthemum cultivars, the accumulation of carotenoids is mainly due to the loss of CmCCD4a in the genome (Ohmiya et al. 2006). In this study, a (TC)n microsatellite (SSRy) is located 47 bp downstream of the CCD4 start codon. Two alleles, TC8 and TC7, are identified in the two segregating F1 populations. The TC7 allele encodes a 597-amino acid protein, whereas the TC8 allele contains a premature stop codon due to the insertion of a CT repeat unit, resulting in a truncated and nonfunctional protein. White fruit flesh is dominant over yellow fruit flesh in peach (Williamson et al. 2006). Theoretically, three combinations of alleles of CCD4 gene, TC8/TC8, TC8/TC7, and TC7/TC7, will produce yellow-, white-, and white-fleshed fruits, respectively. This hypothesis can be well validated in the two segregating F1 populations tested in this study. Besides length change in the SSR locus, nucleotide substitution and retrotransposon insertion are also found to cause loss-of-function mutation of CCD4 gene in peach (Adami et al. 2013; Falchi et al. 2013). In summary, CCD4 can be inactivated by various mutational events, and its inactivation is responsible for carotenoid accumulation in fruit flesh and leaf midvein of peach. The white/yellow color of leaf vein can serve as a morphological trait to select new varieties with desirable white or yellow flesh colors.
It is worth noting that the transcript level of CCD4 is very low in fruits of white-fleshed cv. BF during the early stage of fruit development. PIFs have been shown to directly repress the expression of phytoene synthase to downregulate the accumulation of carotenoids (Toledo-Ortiz et al. 2010). Moreover, ethylene has also been reported to be involved in the regulation of carotenoid accumulation and carotenogenic gene expression in apricot (Marty et al. 2005). We have analyzed the promoter sequences of CCD4 genes from both cv. BF and JX using PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Several cis-regulatory elements for light or hormone responses are identified. Thus, further studies are needed to address whether or not the temporal expression pattern of CCD4 is regulated by TFs or hormones in peach.
CCD4 Is Likely to Utilize β-Carotene as a Substrate in Peach
Several studies have been reported on the enzyme activity of CCD4 in plants. For example, two CCD4 genes, designated CsCCD4 and BoLCD, have been isolated in Crocus and Bixa orellana, respectively. CsCCD4 catalyzes the conversion of β-carotene to β-ionone (Rubio et al. 2008), whereas BoLCD catalyzes the oxidative cleavage of lycopene to generate bixin (Bouvier et al. 2003). Here, we show that the concentration of β-carotene in fruits of white-fleshed cv. BF decreases throughout fruit development, and is very low in late stage of fruit development. In contrast, the transcript level of CCD4 is very high in fruits of white-fleshed cv. BF in late stage of fruit development, while extremely low in fruits during the early stage of fruit development. The transcript level of CCD4 shows an inverse correlation with the concentration of β-carotene in fruits of white-fleshed cv. BF. Moreover, the transcript levels of genes responsible for the synthesis of β-carotene, including PSY1, PSY2, PDS, ZPS, Z-ISO, and LCYB1, are high in fruits of white-fleshed cv. BF throughout fruit development. In addition, the peach CCD4 is closely related to apple MdCCD4 (Fig. 4). It has been reported that MdCCD4 degrades β-carotene to yield β-ionone (Huang et al. 2009). Collectively, all these results strongly suggest that CCD4 is likely to utilize β-carotene as the substrate in peach. In fruits of white-fleshed peach, carotenoids are synthesized but are subsequently degraded into colorless compounds, leading to the formation of white color.
On the other hand, the transcript level of HYB in fruits of white-fleshed cv. BF decreases severely in late stage of fruit development. HYB utilizes β-carotene as a substrate. We have analyzed the amino acid sequences of HYB and CCD4 using ChloroP 1.1 Prediction Server (http://www.cbs.dtu.dk/services/ChloroP/). The result indicates that HYB and CCD4 proteins both contain chloroplastic transit peptides (cTP), allowing them to obtain access to plastid carotenoids. It seems likely that CCD4 competes with HYB to utilize the same substrate of β-carotene, and β-carotene is preferably involved in the reaction catalyzed by CCD4 enzyme. This may be responsible for the low expression of HYB in fruits of white-fleshed cv. BF in late stage of fruit development.
Unlike CmCCD4a that is exclusively expressed in a specific organ of chrysanthemum flower petals (Ohmiya et al. 2006), the peach CCD4 is expressed in both fruits and leaves of white-fleshed cv. BF. The transcript level of CCD4 in leaves (no midveins) is ~3-fold higher than that in midveins. However, the concentration of total carotenoids in leaves (no midveins) is up to three-fold higher than that in midveins. It is important to note that LCYE is highly expressed in leaves of both white- and yellow-fleshed cultivars (Fig. 9), whereas its transcript is not detectable in fruits. This result clearly demonstrates that the biosynthesis of carotenoids is different between fruits and leaves of peach (Fig. 1). LCYE is involved in the biosynthesis of lutein, a dihydroxy xanthophyll. It is well known that lutein is the predominant carotenoid in plant leaves, and plays vital roles in light-harvesting complex II (LHCII) such as photosystem stability maintenance and photoprotection (Dall'Osto et al. 2006; DellaPenna and Pogson 2006). Thus, it seems that high concentration of total carotenoids in peach leaves may be attributed to the activation of HYB, and CCD4 cannot utilize lutein as a substrate.
Color Development of White and Yellow Fruit Flesh of Peach
Our study indicates that young fruits of both white- and yellow-fleshed cultivars have very high concentrations of total carotenoids. However, the flesh color of young fruits is green due to the presence of chlorophyll. In fruits of yellow-fleshed cultivars, the chlorophyll breaks down in late stage of fruit development, the green color disappears, and the yellow color becomes visible. In fruits of white-fleshed cultivar, carotenoid degradation further occurs in late stage of fruit development, resulting in the formation of white color. This color development of white and yellow fruit flesh of peach is briefly summarized in Fig. 10.
In summary, our study demonstrates CCD4 controls white and yellow coloration of fruit flesh and leaf midvein in peach. The midvein coloration can serves as a morphological marker for selection of new peach cultivars with desirable white or yellow flesh colors in future peach breeding programs.
References
Adami M, Franceschi PD, Brandi F, Liverani A, Giovannini D, Rosati C, Dondini L, Tartarini S (2013) Identifying a carotenoid cleavage dioxygenase (ccd4) gene controlling yellow/white fruit flesh color of peach. Plant Mol Biol Rep 31:1166–1175. doi:10.1007/s11105-013-0628-6
Auldridge ME, McCarty DR, Klee HJ (2006) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9:315–321
Bliss FA, Arulsekar S, Foolad MR, Becerra V, Gillen AM, Warburton ML, Dandekar AM, Kocsisne GM, Mydin KK (2002) An expanded genetic linkage map of Prunus based on an interspecific cross between almond and peach. Genome 45:520–529
Bouvier F, Dogbo O, Camara B (2003) Biosynthesis of the food and cosmetic plant pigment bixin. Science 300:2089–2091
Bramley PM (2002) Regulation of carotenoid formation during tomato fruit. J Exp Bot 53:2107–2113
Brandi F, Bar E, Mourgues F, Horváth G, Turcsi E, Giuliano G, Liverani A, Tartarini S, Lewinsohn E, Rosati C (2011) Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol 11:24
Chen Y, Li F, Wurtzel ET (2010) Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol 153:66–79
Dall'Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol 6:32
DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57:711–738
Dirlewanger E, Graziano E, Joobeur T, Garriga-Calderé F, Cosson P, Howad W, Arús P (2004) Comparative mapping and marker-assisted selection in Rosaceae fruit crops. Proc Natl Acad Sci 101:9891–9896
Egea I, Barsan C, Bian W, Purgatto E, Latché A, Chervin C, Bouzayen M, Pech JC (2010) Chromoplast differentiation: current status and perspectives. Plant Cell Physiol 51:1601–1611
Falchi R, Vendramin E, Zanon L, Scalabrin S, Cipriani G, Verde I, Vizzotto G, Morgante M (2013) Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J. doi:10.1111/tpj.12283
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Hirschberg J (2001) carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4:210–218
Huang FC, Molnár P, Schwab W (2009) Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J Exp Bot 60:3011–3022
Isaacson T (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 14:333–342
Kato M, Ikoma Y, Matsumoto H, Sugiura M, Hyodo H, Yano M (2004) Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol 134:824–837
Kita M, Kato M, Ban Y, Honda C, Yaegaki H, Ikoma Y, Moriguchi T (2007) Carotenoid accumulation in Japanese apricot (Prunus mume Siebold & Zucc.): molecular analysis of carotenogenic gene expression and ethylene regulation. J Agric Food Chem 55:3414–3420
Li F, Murillo C, Wurtzel ET (2007) Maize Y9 encodes a product essential for 15-cis-zeta-carotene isomerization. Plant Physiol 144:1181–1189
Lu S, Van Eck J, Zhou X, Lopez AB, O'Halloran DM, Cosman KM, Conlin BJ, Paolillo DJ, Garvin DF, Vrebalov J, Kochian LV, Küpper H, Earle ED, Cao J, Li L (2006) The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 18:3594–3605
Maass D, Arango J, Wüst F, Beyer P, Welsch R (2009) Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS One 4:e6373
Marty I, Bureau S, Sarkissian G, Gouble B, Audergon JM, Albagnac G (2005) Ethylene regulation of carotenoid accumulation and carotenogenic gene expression in colour-contrasted apricot varieties (Prunus armeniaca). J Exp Bot 56:1877–1886
Ogundiwin EA, Peace CP, Gradziel TM, Parfitt DE, Bliss FA, Crisosto CH (2009) A fruit quality gene map of Prunus. BMC Genomics 10:587
Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K (2006) Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol 142:1193–1201
Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321–332
Rubio A, Rambla JL, Santaella M, Gómez MD, Orzaez D, Granell A, Gómez-Gómez L (2008) Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. J Biol Chem 283:24816–24825
Schwartz SH, Qin X, Zeevaart JA (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131:1591–1601
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
The International Peach Genome Initiative (2013) The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet 45:487–494
Toledo-Ortiz G, Huq E, Rodríguez-Concepción M (2010) From the cover: direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc Natl Acad Sci 107:11626–11631
Van Ooijen JW (2006) JoinMap® 4, software for the calculation of genetic linkage maps in experimental populations. Kyazma B.V., Wageningen
Wang L, Zhao S, Gu C, Zhou Y, Zhou H, Ma J, Cheng J, Han Y (2013) Deep RNA-Seq uncovers the peach transcriptome landscape. Plant Mol Biol. doi:10.1007/s11103-013-0093-5
Welsch R, Maass D, Voegel T, Dellapenna D, Beyer P (2007) Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol 145:1073–1085
Williamson JD, Peace CP, Bliss FA, Garner DT, Crisosto CH (2006) Evidence for a single locus controlling flesh color, senescent leaf color, and hypanthium color in peach. J Am Soc Hortic Sci 131:256–260
Zhang Q, Ma B, Li H, Chang Y, Han Y, Li J, Wei G, Zhao S, Khan MA, Zhou Y, Gu C, Zhang X, Han Z, Korban SS, Li S, Han Y (2012) Identification, characterization, and utilization of genome-wide simple sequence repeats to identify a QTL for acidity in apple. BMC Genomics 13:537
Acknowledgments
This project was supported by funds received from the National Program on Key Basic Research Project of China (973 Program) under Grant No. 2011CB100600, the National 863 Program of China (No. 2011AA100206), and the National 948 Project from the Ministry of Agriculture of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Ma, J., Li, J., Zhao, J. et al. Inactivation of a Gene Encoding Carotenoid Cleavage Dioxygenase (CCD4) Leads to Carotenoid-Based Yellow Coloration of Fruit Flesh and Leaf Midvein in Peach. Plant Mol Biol Rep 32, 246–257 (2014). https://doi.org/10.1007/s11105-013-0650-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-013-0650-8